Abstract
Objectives
To investigate the effect and molecular mechanisms of action of Vitamin D3 (VD3) as a neo-adjunctive agent before cryosurgery in an effort to increase treatment efficacy for prostate cancer (CaP).
To eliminate the potential for disease recurrence that exists at the periphery of the freeze lesion, where temperatures may be insufficient to destroy both androgen-sensitive (AS) and androgen-insensitive (AI) CaP.
Methods
Human CaP cells, LNCaP, were each genetically altered to express the AS and AI phenotypes and subjected to VD3 treatment and freezing in an in vitro and tissue-engineered model.
Cell viability, caspase inhibitor and western blot studies were used to determine the basis of the different responses of AI and AS cells to VD3 cryosensitization.
Results
VD3 was found to be a highly effective cryosensitizer, resulting in a >50% overall increase in cell death after -15°C freezing.
Fluorescence microscopy, western blot analysis and caspase protease assays confirmed that the increased activation of apoptosis was modulated through a mitochondrial-mediated pathway.
Caspase inhibition studies showed that apoptosis played an integral role in cell death, with VD3 cryosensitivation-induced apoptotic events responsible for > 30% of the overall cell death after -15°C freezing.
Conclusions
The present study suggests that the use of VD3 as a cryosensitizer increases cryoablation efficacy through the increased activity of apoptosis as well as through necrosis.
The data show that through VD3 treatment the overall level of AI CaP cell tolerance to freezing is reduced to a level similar to that of AS CaP.
VD3 pre-treatment in conjunction with cryoablation may increase treatment efficacy and reduce disease recurrence for CaP patients.
Keywords: cryosurgery, apoptosis, prostate cancer, vitamin D, adjunctive, cryosensitization, cryoablation
Introduction
In 2009 ≈ 250 000 new cases of prostate cancer (CaP) were diagnosed and nearly 40 000 deaths from CaP were reported in the USA [1, 2]. While the outlook for patients with early-stage CaP has markedly improved, the prognosis for advanced stage disease remains poor. Cryosurgery is now one of the many tools available for the treatment of CaP [3--8]. In addition to being highly effective, cryoablation reduces hospitalization time, postoperative morbidity, the interval before return to daily activities, and overall treatment cost compared with some conventional treatments [9--15]. In 2008, the AUA released a best practice statement recommending cryoablation of the prostate as both a primary and salvage therapy[16]. That same year, Cohen et al.[17] published the first 10-year study to report cryoablation efficacy equivalent to that of other therapies. Although cryoablation is highly effective, there remains a degree of concern about potential cell survival at the periphery of the frozen volume of tissue where lethal temperatures are not achieved, leading to possible disease recurrence. In addition, there is the need to avoid injury to adjacent anatomical structures which may limit the aggressiveness of freezing. Strategies to improve primary and salvage cryosurgical options are therefore necessary to improve this form of CaP treatment and prevent recurrence [11, 13].
To improve efficacy, research has focused on the freeze zone periphery and the use of agents to sensitize cells. Some studies have reported the synergistic effects of administering low-dose 5-fluorouracil, Taxotere®, or cisplatinum before cryotherapy, which results in improved cell ablation for the treatment of CaP in in vitro and in vivo models [18--26]. These studies have shown that sensitizing cell populations before freezing achieves enhanced cryoablative efficacy through the induction of apoptosis and secondary necrosis. The aim of this approach is to increase the volume of tissue destroyed by bringing the temperature that is lethal to cells closer to 0°C. Despite the improved performance, cells treated with chemotherapy-based sensitizers cause problematic patient toxicity, side effects and drug resistance, especially for hormone-refractory tumours [27].
Among the agents that have been considered to increase cell sensitivity to freezing is cholecalciferol, or vitamin D3 (VD3), which is thought to have a beneficial effect attributable to the induction of apoptosis, regulation of cell growth and antiangiogenesis [3, 7]. These properties have been recognized as having potential for use in breast, pancreatic and ovarian cancer, as well as in CaP therapy[21, 28--32]. Kimura et al. [33] recently reported on the benefit of VD3 cryosensitization in a murine CaP model. This report, along with the range of cellular effects of VD3, supports the potential of VD3 to increase the efficacy of cryotherapy, thereby reducing associated morbidity and risk of recurrence.
The ability of VD3 to inhibit growth factor signalling pathways is believed to underlie the potential of this agent as a cryosensitizer. VD3 inhibits the mitochondrial protein Bcl-2 thereby activating the apoptotic caspase cascade [29]. The Bcl-2 family of proteins is responsible for maintaining mitochondrial membrane potential via the mitochondrial transition pore. A reduction in Bcl-2 can result in the opening of the pore, the release of cytochrome c and the activation of apoptosis. Agents that reduce Bcl-2 levels increase cell susceptibility to apoptotic induction, thereby increasing treatment efficacy [34--36]. We therefore hypothesized that VD3 activates mitochondrial-based apoptosis, resulting in increased cell death (apoptotic and necrotic) in response to a mild freeze insult, such as that experienced at the periphery a cryogenic lesion. As such, we investigated the use of VD3 as a cryosensitizer to increase treatment efficacy for late [androgen-insensitive (AI)] and early stage [androgen-sensitive (AS)] CaP.
Materials and Methods
Cell culture
The human CaP cell line, LNCaP, was obtained from the American Type Culture Collection (Manassas, VA, USA). The AI LNCaP high passage (HP) cell line was derived by repeated culture (> 60 passages) of the AS LNCaP low passage (LP) cell line in low-hormone medium (RPMI-1640, supplemented with 10% charcoal stripped serum [Biomeda, Foster City, CA, USA] and 1% Penicillin-Streptomycin [Life Technologies, Carlsbad, CA, USA]) as previously described [37]. Cultures were maintained at 37°C, 5% CO2/95% air in RPMI-1640 growth medium (Caisson Laboratories, Inc., North Logan, UT, USA) supplemented with 10% FCS (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% Penicillin-Streptomycin (Mediatech, Manassas, VA, USA). Cultures were grown in Falcon 75 cm2 T-flasks with medium exchange every 3 days. Subcultures were prepared in Costar 96-well, strip plates at 18 000 cells/well, and experiments were performed 2 days after subculture.
For tissue-engineered prostate cell (pTEM) studies, rat tail type I collagen solution (BD Bioscience, Bedford, MA, USA) was used to form gel matrices. Cells, 2.5 × 106 cells/mL, were suspended in the collagen solution before gel solidification in 35-mm Petri dishes. Matrices were cultured 24 h before freezing, and media were replenished each day.
Freezing protocol
Strip wells were placed into an aluminum block in a cooling bath pre-set at -15°C. The sample temperature was monitored at regular intervals using a type-T thermocouple. Ice nucleation was initiated by contact with a cold metallic probe when the sample temperature reached -2°C (± 1°C). After nucleation, samples were held for 12 min (15 min total) to allow for thermal equilibration. Samples were thawed at room temperature and then returned to normothermic culture. Where indicated, cells were treated as follows: (1) frozen to -15°C alone; (2) exposed to 50nM VD3 (1,25-dihydroxycholecalciferol; Calbiochem, San Diego, CA, USA) for 2 days before freezing; or (3) exposed to caspase 3,8 or 9 inhibitors (Calbiochem) immediately before freezing.
The pTEMs were frozen using a cryosurgical system (Galil) with a 1.6mm needle cryoprobe. Briefly, a cryoprobe was placed into the centre of the model and a single or double freeze cycle was initiated, each cycle consisting of a 10-min freeze followed by 20 min of thawing at 37°C. The temperature profiles were recorded with an array of type T thermocouples extending radially from the probe tip (Omega TempScan 1100, Omega, Stamford, CT, USA). Once thawed, samples were returned to culture for further assessment.
Cell viability
Cell viability was assessed using the alamarBlue™ assay (Invitrogen, Carlsbad, CA, USA) in HBSS (1:20 dilution) every other day following the freezing insult. Samples were exposed to alamarBlue™ for 1 h at 37°C and then analysed using a Tecan SPECTRAFluorPlus plate reader (TECAN GmbH, Grödig Austria) with an excitation of 530nm and emission of 590nm. Subsequently, cell culture media were replenished and returned to normal culture.
Fluorescence imaging
To determine the mode of cell death, samples were frozen then assessed via triple labelling using the fluorescent probes (Molecular Probes, Invitrogen) Hoechst (blue fluorescence, 0.06μg/μl), propidium iodide (red fluorescence, 0.007μg/μl), and YO-PRO-1 (green fluorescence, 0.8μM) to detect living, necrotic (freeze-ruptured), and apoptotic cells, respectively. Probes were added to each sample and incubated in the dark for 20 min. Samples were then visualized via fluorescence microscopy using a Zeiss Axiovert 200M microscope (Carl Zeiss,) at 240× magnification and quantified via automated cell counts using the Axiovision software (Carl Zeiss).
Western blot
Samples were cultured in Petri dishes and frozen. Cell lysates were extracted on ice at 1, 3, 6, 12, and 24 h after thawing using ice-cold radio-immunoprecipitation assay cell lysis buffer with phosphatase inhibitor (sodium fluoride 1mM, sodium orthovanadate 1mM, sodium pyrophosphate 1mM), leupeptin (1ug/ml), PMSF (1mM), and 1× Halt Protease Cocktail Inhibitor (Pierce, Rockford, IL, USA). Samples were homogenized by vortex mixing and centrifuged at 16 000 × g for 15 min at 4°C. Protein concentrations were determined using the BCA (Pierce) and quantified using a Tecan spectrophotometer (TECAN GmbH). Equal amounts of protein (25μg) for each sample were separated on a 10% SDS-PAGE gel (Bio-Rad, USA). Proteins were transferred to PVDF membranes (Bio-Rad), blocked with 3% BSA 0.05% Tween-20 solution, and incubated at 4°C overnight in the presence of 1μg/ml of each antibody (β-tubulin [BD Pharmingen,], pro-caspase-3 [Cell Signaling (CST)], pro-caspase-8 [CST], or pro-caspase-9 [CST]). Membranes were then washed three times with 0.05% Tween-20 in PBS and exposed with HRP-conjugated secondary antibodies.
Membranes were visualized using a Fujifilm LAS-3000 luminescent image analyser. All protein levels were compared with tubulin (loading control) and quantitative assessment was conducted via densometric analysis using the Fuji software.
Caspase activity assays
Cell samples for caspase activity assays were frozen at -15°C for 15 min. Cell lysates were collected on ice at 1, 3, 6, 12, and 24 h after thawing using ice-cold radio-immunoprecipitation assay cell lysis buffer without protease or phosphatase inhibitors. Protein concentrations were quantified and 50μg of protein were assessed for caspase activity using the BD ApoAlert™ Caspase Fluorescent Assay Kits for Caspase-3, -8, and -9 (BD Pharmingen). Sample fluorescence (caspase activity) was quantified using the Tecan spectrophotometer and converted to fold-change in activity based on non-frozen controls.
Data analysis
Fluorescence units were converted to percent survival based on non-frozen controls (37°C). Viability experiments were repeated a minimum of three times with an intra-experiment repeat of 8. Western Blot, fluorescence imaging and protease activity assays were conducted on a minimum of three separate experiments. Standard error calculations were performed and statistical significance was determined by single-factor ANOVA.
Results
VD3 pretreatment increases cell sensitivity to freezing
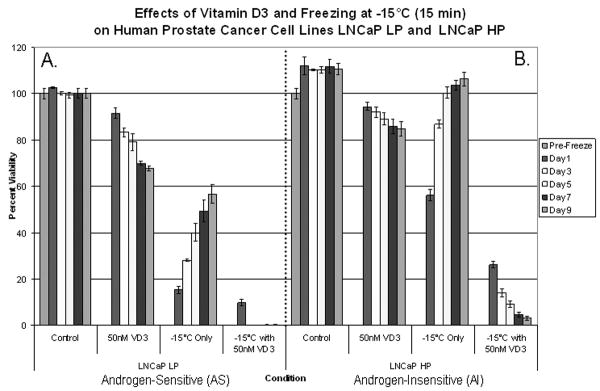
Cryochemo approaches have been shown to increase cell death, but at higher temperatures (such as -15°C) there is substantial cell survival. The use of VD3 as a neo-adjunctive agent was investigated to determine whether improved CaP ablation could be achieved. Accordingly, AS and AI samples (LNCaP LP and LNCaP HP) were treated with VD3 for 2 days before freezing (Fig. 1). In AS samples, a combination of VD3 cryosensitization and freezing resulted in increased cell death compared with freezing alone. With the combination treatment at 1-day after freezing, AS samples showed an increase in cell death compared with freezing alone and total cell loss by 3 days after freezing to -15°C. | In the AI sample, the combination of VD3 cryosensitization and freezing to -15°C had better efficacy compared with either treatment alone, with a 30% overall reduction in cell viability after freezing that was 2.2 times greater than freezing alone (P < 0.01; Fig. 1). Interestingly, the cell viability in the AI sample continued to decline during recovery after the combination treatment and consequently the cells were unable to re-populate. These data suggest that cryosensitization using VD3 in combination with cryotherapy achieved complete ablation for both AS and AI cells after freezing to -15°C.
Fig. 1.
AS samples (A) treated with VD3 showed continuous cell death over the 9-day assessment period, while VD3 and freezing combined treatments achieved total cell ablation with lack of re-growth. Similarly, VD3-treated AI samples (B) showed continuous cell death over the 9-day assessment period. The combination of VD3 and freezing treatment achieved total cell ablation (P < 0.05) with lack of re-growth for AI samples.
VD3 treatment increased freeze-induced apoptosis and necrosis levels
The enhanced cell death resulting from VD3 cryosensitization for both AS and AI CaP cells prompted our investigation into the mechanism of cell death. Total levels of apoptotic and necrotic cell death were evaluated in the various conditions using fluorescence microscopy (Fig. 2). AS samples (Fig. 2A) showed little apoptosis and necrosis in controls (unfrozen), but after freezing at −15°C, apoptosis was found to increase, peaking after 3 h and remaining high up to 24 h after freezing. VD3-treated AS samples had higher levels of apoptosis and necrosis in controls (unfrozen) compared with untreated controls. The combination of VD3 cryosensitization and freezing yielded a slight increase in apoptosis and necrosis compared with freezing alone in AS samples at all time points evaluated (Fig. 2B). Quantitative analysis of the 3-h time point showed the greatest difference between freezing and freezing with VD3. After freezing alone, AS samples showed 22.1 (± 1.2) % viability, 58.2 (± 0.8) % necrosis, and 19.7(± 1.5) % apoptosis, and after the combination of VD3 and freezing the AS samples showed 14.2 (± 1.1) % viability, 60.2 (± 0.7) % necrosis, and 25.6 (± 0.9) % apoptosis. Compared with AS samples, the AI (Fig. 2C) samples subjected to freezing alone showed less overall apoptosis and necrosis. After freezing at −15°C, apoptosis peaked after 6 h and rapidly declined by 24 h. VD3-treated AI samples had higher levels of apoptosis and necrosis vs freezing alone and vs AS samples subjected to the combination treatment (P<0.01; Fig. 2D). Post-freezing analysis showed higher levels of apoptosis and necrosis in the combination-treated AI samples, with peak levels occurring at 3 h after freezing. This represented an acceleration in cell death progression compared with freezing alone samples. Quantitative analysis of freezing alone AI samples at 3 h after freezing showed 63.7 (± 1.4) % viability, 23.4 (± 0.6) % necrosis and 12.9 (± 1.1) % apoptosis, whereas samples treated with VD3 showed 27.3 (± 0.6) % viability, 50.6 (± 1.2) % necrosis, and 22.1 (± 1.4) % apoptosis (Fig. 2C vs 2D). These data indicated that VD3 treatment before freezing significantly increased both necrotic and apoptotic cell death, thereby reducing overall cell survival by more than half (27.3% vs 63.7%, respectively, [P<0.01]). Interestingly, the AI samples showed a greater increase in apoptosis and necrosis (and thus cryosensitization) than the AS samples.
Fig. 2.
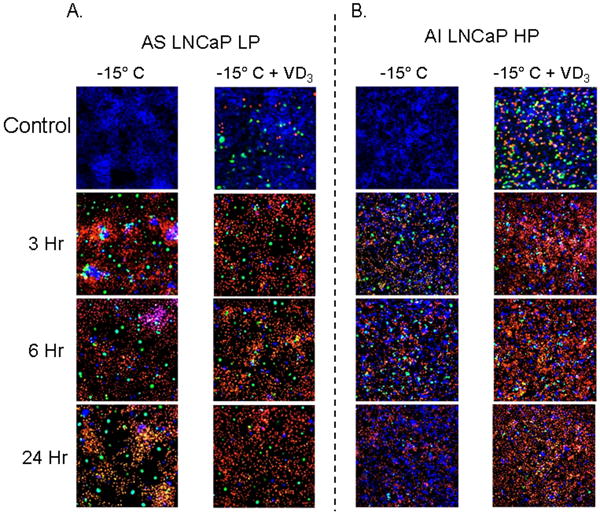
Levels of necrotic and apoptotic cell death were evaluated in AS (A) and AI (B) cells after freezing or VD3 cryosensitization. Samples were frozen at -15°C and triple-probe fluorescent micrographs were taken after 3h, 6h, and 24h using Hoechst (blue-viable), propidium iodide (red- necrotic), and YO-PRO-1 (green-apoptotic). Compared with freezing alone, AS samples (LNCaP LP) treated with VD3 showed only slight increases in necrotic and apoptotic cells. VD3 treated AI samples exhibited significant increases (P<0.05) in necrotic and apoptotic cell death at the 3h and 6h time points, indicating that AI cell lines experienced greater activation of apoptotic cascades resulting from VD3 treatment before freezing.
VD3 increases mitochondrial-mediated apoptosis
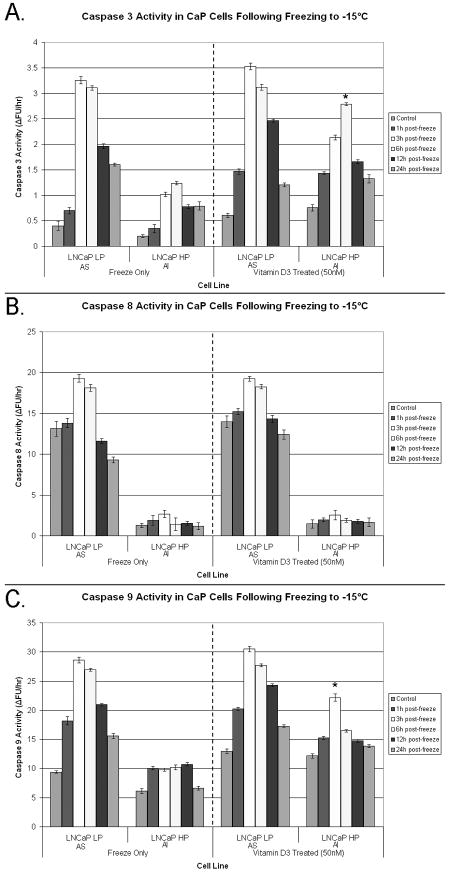
In the present study, we investigated the involvement of apoptosis in CaP cellular response to VD3 cryosensitization and freezing. Western blot and protease activity analyses were performed to assess the alterations and activations in the apoptotic proteins caspase-3, -8 and -9 after freezing and VD3 cryosensitization (Figs 3 and 4). Analysis after freezing showed that the AS samples maintained higher levels of pro-caspase-3 and -8, compared with the AI samples (Figs 3A and B). This suggested a greater overall potential for apoptotic involvement in AS systems. AS samples had substantial decreases in pro-caspase-3, -8, and -9 levels by 3 h after freezing, indicating protein cleavage to an active form (Fig. 3A). Interestingly, compared with AS samples, AI samples had lower pro-caspase levels, and revealed little cleavage after freezing (Figs 3A vs 3B).
Fig. 3.
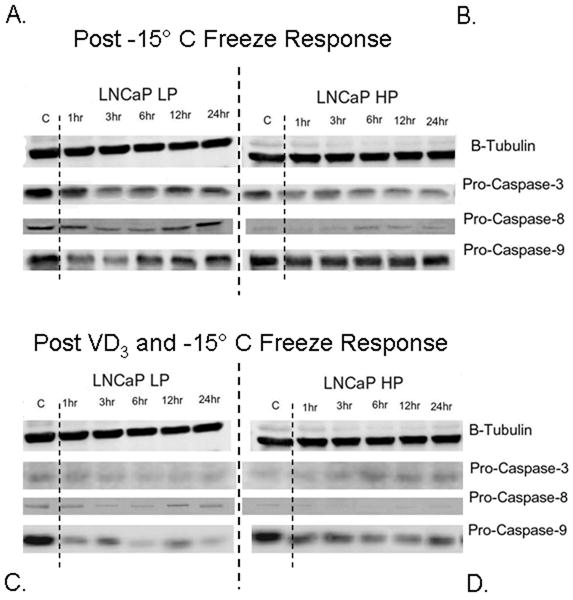
Levels of pro-caspase-3, pro-caspase-8, and pro-caspase-9 levels were assessed via western blots after freezing alone (A and B) or VD3 and freeze treatment combination (C and D) in AS and AI samples for a 24-h period after freezing. The AS cells (A) exhibited greater control levels of each caspase than AI samples (B). Compared to freezing alone, levels of pro-caspase-3 and pro-caspase-8 after the combination of VD3 and freezing were found to be reduced in all cell lines evaluated, indicating increased caspase activation (C and D). AI samples exhibited decreases in pro-caspase expression (especially pro-caspase-9) which was similar to AS samples (D and A vs B) indicating that VD3 was able to induce similar apoptotic effects in response to freezing in all samples tested.
Fig. 4.
Caspase-3 (A), caspase-8 (B), and caspase-9 (C) activity was determined for AS and AI samples treated with VD3 before freezing. Samples were frozen and cell lysates were collected 24 h after freezing. Compared with freezing alone, VD3-treated AS exhibited slightly higher (P < 0.05) levels of caspase-3 (A) and caspase-9 (C) activity that peaked 3 h after freezing. Caspase-8 (B) activity was not significantly different from freeze alone. Interestingly, VD3 treated AI samples exhibited significantly (P < 0.05) greater levels of caspase-3 and caspase-9 activity (compared with frozen alone samples) that peaked after 3--6 h, while caspase-8 activity was not statistically different from that in frozen alone samples. The data indicated that VD3 treatment induced greater cell death responses in AI cells that primarily resulted in increased caspase-9 (and thus caspase-3) activity.
Compared with the freezing alone treatment, both AS and AI VD3-treated and frozen samples showed significantly lower levels of caspase proteins (Figs 3C and D). AS samples treated with VD3 and frozen had lower levels of pro-caspase-8 compared with AS samples frozen alone (Fig. 3C vs A). Similarly, AI VD3-treated and frozen samples showed lower levels of pro-caspase-8 compared with AI samples frozen alone (Fig. 3D). Analysis after VD3 cryosensitization and freezing showed that caspase-9 levels were significantly different from frozen alone samples. There were similar levels of pro-caspase-9 in VD3-treated and untreated (unfrozen) controls (Fig. 3D), however, after the combination of VD3 and freezing a significant decrease in pro-caspase-9 expression levels in both AS and AI samples was noted (Figs 3C and D vs 3A and B).
We further analysed alterations in pro-caspase levels to determine the extent of their proteolytic activity and involvement in the freezing responses after VD3 cryosensitization in AS and AI samples (Fig. 4). Analysis of caspase 3 activity showed that in frozen-only AS samples there was an overall increase in protease activity compared with AI samples, which peaked at 3 to 6 h after freezing, suggesting that freezing rapidly induced apoptotic death cascades (Fig. 4A). Conversely, frozen-only AI samples showed lower overall levels of caspase-3 activity, peaking at 6 h, indicating delayed activation. Freezing at -15°C resulted in peak caspase-3 activity in AS samples 2.8 times greater than in AI samples (3.25 vs. 1.2, respectively). In AI samples, the combination of VD3-treatment and freezing resulted in a significant increase (P < 0.01) in caspase-3 activity compared with freezing alone (1.2 vs. 2.75, respectively; Fig. 4A). In sharp contrast to freezing-alone, there was an increase in caspase-3 activity in VD3-treated AI samples throughout the 24-h interval after freezing (twofold increase, P<0.001). In VD3-treated samples, the peak caspase-3 activity in AS and AI samples was similar, indicating that VD3 treatment induced similar post-freezing responses. These data suggest that VD3 treatment before freezing increased the caspase-3 activity level and duration in AI cells, resulting in the greater level of cell death observed after freezing.
Assessment of caspase-8 activity in VD3-treated and frozen samples showed activity levels similar to freezing alone (Fig. 4B). AS samples showed greater caspase-8 activity after both freezing alone and VD3 treatment than did AI samples (7.1 fold increase, P<0.01). Overall caspase-8 activity after VD3 cryosensitization did not significantly differ in either the AS or AI samples compared with freezing alone (P≠ NA for both treatments). These data suggested that VD3 cryosensitization had little effect on caspase-8 activity.
Analysis of caspase-9 activity showed significant differences between frozen alone and VD3- treated samples (Fig. 4C). AS samples treated with VD3 had increased levels of caspase-9 activity, peaking at 3 h after freezing. Although overall caspase-9 activity was lower in the AI samples, the level of activity was markedly higher in VD3-treated samples than in the AI frozen-alone samples (22 vs. 10, respectively; P<0.01). Interestingly, AS samples treated with VD3 and frozen were found to have only a 1.7 fold difference (increase) in caspase-9 activity compared with the VD3-treated and frozen AI samples (31 vs. 22, respectively). The higher caspase-9 activity for the AI samples indicated that VD3 treatment before freezing was able to initiate a greater mitochondrial-mediated apoptotic response in the AI samples than freezing only.
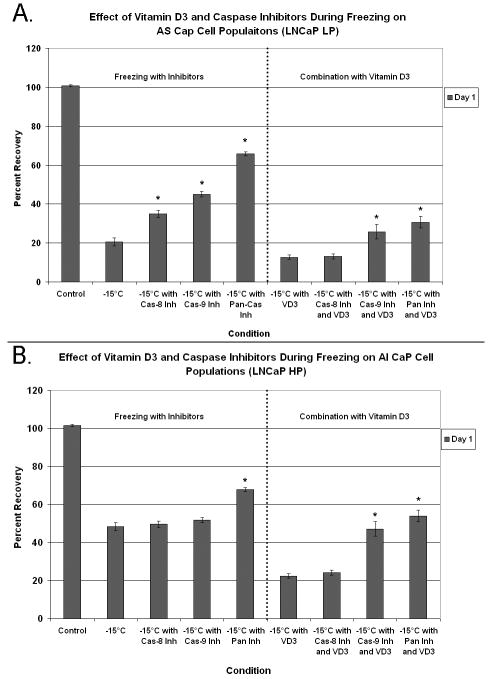
Caspase inhibition corroborates a mitochondrial-mediated apoptotic mechanism
After increased caspase activity as a result of VD3 cryosensitization had been identified, studies were conducted to assess the overall influence of caspase activation in the observed cell death. Accordingly, AS (Fig. 5A) and AI (Fig. 5B) samples were treated with VD3 and caspase inhibitors (caspase-8, caspase-9, or pan-caspase) before freezing. It was hypothesized that the modulation of caspase activity during cryosensitization would negate the beneficial effects of VD3 pretreatment.
Fig. 5.
The effect of apoptosis in VD3 cryosensitization assessed using caspase inhibition in combination with VD3 and freezing in AS (A) and AI (B) samples.
The AS samples showed greater cell viability after freezing for all caspase inhibitors tested (Fig. 5A). Compared with freezing alone, viability after freezing was increased by 14.4, 24.4, and 45.1% using caspase-8,-9, or pan-caspase inhibitors, respectively (P<0.01 for each vs freezing alone). These data showed that caspase activity played a substantial role in cell death associated with freezing to -15°C in the AS samples. Cryosensitization with VD3 reduced viability after freezing in the AS samples by 2.6 times compared with freezing alone (P<0.01). Caspase-8 inhibition with VD3 had little effect on viability after freezing, compared with VD3-treatment combined with freezing. The addition of caspase-9 and pan-caspase inhibitors, however, increased cell viability after freezing in the AS samples by 1.7- and 2.3 fold, respectively (P<0.01 for each compared with freezing alone).
Results for caspase inhibition in AI samples were similar to those in the AS samples (Fig. 5B). The inclusion of a pan-caspase inhibitor in frozen-alone samples resulted in a 19.5% increase in cell viability (P<0.01). Interestingly, caspase-8 and -9 inhibition in AI samples had no protective effect after freezing. As previously described, VD3-treatment combined with freezing of AI samples resulted in a 2.2 fold reduction in cell viability compared with freezing alone (21% vs. 48%, respectively). Caspase-8 inhibition with VD3-treatment combined with freezing resulted in no change in cell viability in the AI samples after freezing compared with non-inhibitor VD3-treatment combined with freezing. Compared with VD3-treatment combined with freezing, however, caspase-9 and pan-caspase inhibition significantly increased cell viability by 2.1 times (46 vs. 21%) and 2.4 times (52 vs. 21%), respectively (P < 0.01). The results from the caspase inhibition studies confirmed the involvement of the apoptotic cascade after freezing as well as the ability of VD3 cryosensitization to increase the level of mitochondrial-based apoptosis and, thereby, the overall level of cell death.
VD3 cryosensitization enhances cell death in a tissue-engineered model
To further assess the impact of VD3 cryosensitization, an in vivo-like tissue-engineered prostate model (pTEM)[38] was used. The pTEM, containing AS (Fig. 6A) and AI CaP cells (Fig. 6B), was frozen using a centrally placed cryoprobe and the zones of live (green) vs dead (red) cells were assessed 24 h after thawing. As in the in vitro studies, AI pTEM showed increased resistance to freezing alone, with extensive cell survival observed out to the -30°C and -40°C isotherm (Fig. 6B). In contrast, AS samples showed survival only to the 20°C isotherm (Fig. 6A). VD3 crysosensitization and freezing achieved two effects. First, the treatment reduced the density of surviving cells in both AS and AI samples. Second, the cell survival margin was reduced by half. VD3 cryosensitization and freezing of AI samples decreased the critical isotherm to about -15°C (Fig. 6B). These data showed that VD3 cryosensitization was able to elevate the lethal (critical) isotherm in both the AS and AI cell populations after a single freeze event.
Fig. 6.
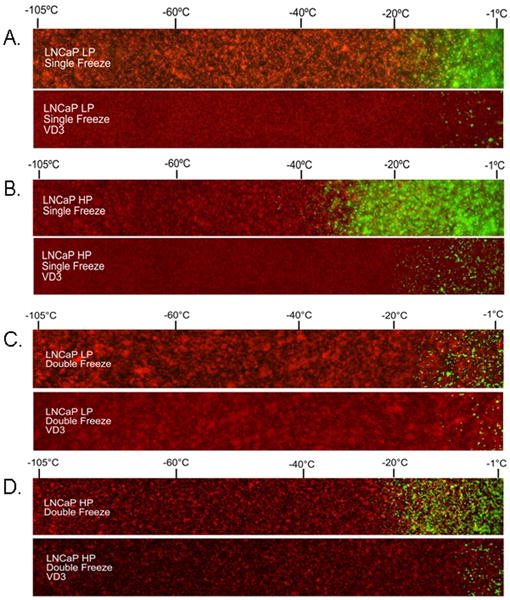
Tissue-engineered matrices containing untreated or VD3-treated AS and AI HP cells were frozen using a single freezing cycle (A and B) or a double freezing cycle (C and D). Matrices were frozen for a single or double freeze followed by return to 37°C. Twenty-four hours after thawing, matrices were probed with calcein AM (green, live cells) and propidium iodide (red, dead cells). A 50× panoramic series of fluorescent micrographs was taken extending from the centre near the cryoprobe tip (left of images) to the periphery of the ice sphere (right of image).
Studies evaluating the effect of the combination after a double freeze event, which is often used clinically, showed an even further increase in cell destruction through VD3 cryosensitization (Figs 6C and D). Overall, the double freeze cycle (untreated and VD3-treated) showed increased cell death compared with the single freeze cycle (Figs 6C & D vs. A & B). Similar to a single freeze, VD3 with a double freeze cycle reduced cell survival by 50% in both the AS and AI samples. Double-freeze-alone AI samples showed cell survival up to between -20°C and -25°C (Fig. 6D). The -20 to -25°C critical isotherm was increased from that of -35 to -40°C after a single freeze (Figs 6B vs D). The incorporation of VD3 cryosensitization and a double freeze cycle resulted in a further increase in the critical lethal isotherm to the -10°C region. The data from the pTEM studies for single and double freeze cycles correlated well with the in vitro data, suggesting the possible use of VD3 as a highly effective cryosensitization agent, when used in combination with freezing.
Discussion
Cryosurgery is an effective treatment option for both early and advanced CaP[11, 39] Despite successes in disease treatment, cryosurgery still results in low, yet noteworthy, recurrence rates, for advanced stage disease [11, 13]. Furthermore, the progression of CaP to an AI, treatment-resistant form [28] remains a therapeutic challenge. The identification of VD3 as a cryosensitizer with antineoplastic and antimetastatic properties that ‘cross-talk’ with androgen signalling makes it a promising candidate for the treatment of both AS and AI CaP. Interest in VD3 is attributable to observations that men have higher rates of CaP occurrence when they have low levels of VD3 [40--43]. These observations led to the concept that the administration of this agent might prevent or treat CaP. In this study, we investigated the effects of VD3 cryosensitization on cell death after cryoablation. The data showed that neo-adjunctive use of VD3 in cryotherapy was able to enhance delayed onset cell death (apoptotic and necrotic) in both AS and AI CaP models.
The increased ablative capacity of VD3 cryosensitization prompted the investigation into the cell death pathways activated, resulting in enhanced CaP destruction. Research has shown that VD3 can regulate apoptotic processes [29, 44], but its role in the freeze response of AS and AI CaP is unknown. Fluorescence microscopy revealed that levels of apoptosis and necrosis were significantly higher for AI cells treated with VD3 before freezing, while AS cells showed minimal changes (Fig. 2). Western blot analysis showed that pro-caspase levels were reduced after freezing with VD3 treatment compared with freezing alone (Fig. 3). Caspase activity analysis showed increases in proteolytic caspase activity after thawing (Fig. 4). Caspase-3 analysis revealed that VD3-treated AS cells had a lower relative increase in activity than AI cells. Furthermore, AI samples showed increased caspase-3 activation during the 24-h period after freezing, which contrasted with that of frozen-alone samples, which decreased during the 24-h period. This increased activity correlated with viability studies showing that AI cells had significantly greater reductions in viability after freezing compared with AS cells after VD3 treatment. Importantly, VD3-treated and frozen AI samples showed caspase-3 activity similar to that in AS samples, indicating that VD3 treatment achieved a similar total level of apoptosis in AS and AI samples. VD3 had little effect on caspase-8 activity for AS and AI samples, suggesting little involvement of the extrinsic apoptotic pathway. The increases in caspase-9 activity for both AS and AI samples indicated that VD3 cryosensitization is mediated through mitochondrial cell death pathways. Final corroboration of this VD3 mitochondrial-mediated apoptotic mechanism was provided in caspase inhibition studies. These data showed that caspase-9 inhibition, but not caspase-8, was able to reverse the effects of VD3 cryosensitization (Fig. 6). These results further confirmed that VD3 cryosensitization activated a mitochondrial-mediated apoptotic response vs an extrinsic pathway.
Several reports have detailed the advantages of using neo-adjunctive, low-dose chemotherapeutic agents to weaken prostate tumours before cryosurgery. These studies have shown a beneficial effect of this sensitization strategy greater than that of either treatment alone [18--20, 45]. This study supports the hypothesis that VD3 has potential for use as an effective neo-adjunctive agent before cryoablation. The data also suggest that VD3 may increase treatment efficacy for AI CaP compared with traditional agents. Importantly, the mechanism of VD3 cryosentiziation of CaP (AS and AI) is shown to be linked to increased mitochondrial-mediated apoptosis and secondary necrosis. These data provide the direction for further investigation into VD3 cryosensitization therapy to increase the efficacy of CaP treatment.
Acknowledgments
This study was funded in part by CPSI Biotech, Inc. (Owego, NY, USA), and The National Institutes of Health Grant # R43CA1123993-01A1 and R43CA118537-01A1.
Abbreviations
- CaP
prostate cancer
- AS
androgen-sensitive
- AI
androgen-insensitive
- VD3
vitamin D3
- LNCaP
human prostate cancer cell line
- HP
high passage
- LP
low passage
- pTEM
tissue-engineered prostate cells
Reference List
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Kendirci M, Bejma J, Hellstrom WJ. Update on erectile dysfunction in prostate cancer patients. Curr Opin Urol. 2006;16:186–95. doi: 10.1097/01.mou.0000193407.05285.d8. [DOI] [PubMed] [Google Scholar]
- 3.Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R. Cryosurgery--a putative approach to molecular-based optimization. Cryobiology. 2004;48(2):190–204. doi: 10.1016/j.cryobiol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Grubb R, 3rd, Vardi IY, Bhayani SB, Kibel AS. Minimally invasive approaches to localized prostate carcinoma. Hematol Oncol Clin North Am. 2006;20(4):879–95. doi: 10.1016/j.hoc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in 2006. Curr Opin Urol. 2006;16(3):152–6. doi: 10.1097/01.mou.0000193393.54598.9f. [DOI] [PubMed] [Google Scholar]
- 6.Onik GM, Cohen JK, Reyes GD, Rubinsky B, Chang Z, Baust J. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. Cancer. 1993;72(4):1291–9. doi: 10.1002/1097-0142(19930815)72:4<1291::aid-cncr2820720423>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Baust JG, Gage AA. Technol Cancer Res Treat. 2. Vol. 3. 2004. Progress toward optimization of cryosurgery; pp. 95–101. [DOI] [PubMed] [Google Scholar]
- 8.Gage AA, Baust JG. Cryosurgery for tumors - a clinical overview. Technol Cancer Res Treat. 2004;3(2):187–99. doi: 10.1177/153303460400300212. [DOI] [PubMed] [Google Scholar]
- 9.Wong WS, Chinn DO, Chinn M, Chinn J, Tom WL, Tom WL. Cryosurgery as a treatment for prostate carcinoma: results and complications. Cancer. 1997;79(5):963–74. [PubMed] [Google Scholar]
- 10.Long JP, Bahn D, Lee F, Shinohara K, Chinn DO, Macaluso JNJ. Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology. 2001;57(3):518–23. doi: 10.1016/s0090-4295(00)01060-8. [DOI] [PubMed] [Google Scholar]
- 11.Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60(2 Suppl 1):3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 12.Katz AE, Rukstalis DB. Recent scientific and technological advances have challenged the traditional treatment options for patients with localized prostate cancer. Urology. 2002;60(2 Suppl 1):1–2. doi: 10.1016/s0090-4295(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 13.Bahn DK, Lee F, Silverman P, et al. Salvage cryosurgery for recurrent prostate cancer after radiation therapy: a seven-year follow-up. Clin Prostate Cancer. 2003;2(2):111–4. doi: 10.3816/cgc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed S, Lindsey B, Davies J. Salvage cryosurgery for locally recurrent prostate cancer following radiotherapy. Prostate Cancer Prostatic Dis. 2005;8(1):31–5. doi: 10.1038/sj.pcan.4500774. [DOI] [PubMed] [Google Scholar]
- 15.Chin JL, Touma N. Current status of salvage cryoablation for prostate cancer following radiation failure. Technol Cancer Res Treat. 2005;4(2):211–6. doi: 10.1177/153303460500400210. [DOI] [PubMed] [Google Scholar]
- 16.Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180(5):1993–2004. doi: 10.1016/j.juro.2008.07.108. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JK, Miller RJJ, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008;71(3):515–8. doi: 10.1016/j.urology.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 18.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42(4):274–85. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 19.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004;49(1):45–61. doi: 10.1016/j.cryobiol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Le Pivert P, Haddad RS, Aller A, et al. Ultrasound guided combined cryoablation and microencapsulated 5-Fluorouracil inhibits growth of human prostate tumors in xenogenic mouse model assessed by luminescence imaging. Technol Cancer Res Treat. 2004;3(2):135–42. doi: 10.1177/153303460400300206. [DOI] [PubMed] [Google Scholar]
- 21.Bonneterre J, Roche H, Monnier A, et al. Docetaxel vs 5-fluorouracil plus vinorelbine in metastatic breast cancer after anthracycline therapy failure. Br J Cancer. 2002;87(11):1210–5. doi: 10.1038/sj.bjc.6600645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Airoldi M, Cattel L, Pedani F, et al. Clinical data and pharmacokinetics of a docetaxel-vinorelbine combination in anthracycline resistant/ relapsed metastatic breast cancer. Acta Oncol. 2003;42(3):186–94. doi: 10.1080/02841860310010709. [DOI] [PubMed] [Google Scholar]
- 23.Mavroudis D, Alexopoulos A, Malamos N, et al. Salvage treatment of metastatic breast cancer with docetaxel and carboplatin. A multicenter phase II trial. Oncology. 2003;64(3):207–12. doi: 10.1159/000069306. [DOI] [PubMed] [Google Scholar]
- 24.Petrioli R, Pozzessere D, Messinese S, et al. Weekly low-dose docetaxel in advanced hormone-resistant prostate cancer patients previously exposed to chemotherapy. Oncology. 2003;64(4):300–5. doi: 10.1159/000070285. [DOI] [PubMed] [Google Scholar]
- 25.Ferraro JM, Foa C, Thezenas S, et al. A weekly schedule of docetaxel for metastatic hormone-refractory prostate cancer. Oncology. 2004;66(4):281–7. doi: 10.1159/000078328. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Hong X, Hussain M, Sarkar SH, Li R, Sarkar FH. Gene expression profiling revealed novel molecular targets of docetaxel and estramustine combination treatment in prostate cancer cells. Mol Cancer Ther. 2005;4(3):389–98. doi: 10.1158/1535-7163.MCT-04-0244. [DOI] [PubMed] [Google Scholar]
- 27.Ferlini C, Ojima I, Distefano M, et al. Second generation taxanes: from the natural framework to the challenge of drug resistance. Curr Med Chem Anticancer Agents. 2003;3(2):133–8. doi: 10.2174/1568011033353489. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari MS, Petrylak DP, Hussain M. Clinical trials in metastatic prostate cancer--has there been real progress in the past decade? Eur J Cancer. 2005;41(6):941–53. doi: 10.1016/j.ejca.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141(1):10–7. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 30.Bonaccorsi L, Marchiani S, Ferruzzi P, et al. Non-genomic effects of the androgen receptor and vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells. Steroids. 2006;71(4):304–9. doi: 10.1016/j.steroids.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20(50):7342–51. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 32.Catz SD, Johnson JL. BCL-2 in prostate cancer: a minireview. Apoptosis. 2003;8(1):29–37. doi: 10.1023/a:1021692801278. [DOI] [PubMed] [Google Scholar]
- 33.Kimura M, Rabbani Z, Mouraviev V, et al. Role of Vitamin D3 as a Sensitizer to Cryoablation in a Murine Prostate Cancer Model: Preliminary In Vivo Study. Urology. 2010 doi: 10.1016/j.urology.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 34.Wang JL, Zhang ZJ, Choksi S, et al. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 2000;60(6):1498–502. [PubMed] [Google Scholar]
- 35.Scott SL, Higdon R, Beckett L, et al. BCL2 antisense reduces prostate cancer cell survival following irradiation. Cancer Biother Radiopharm. 2002;17(6):647–56. doi: 10.1089/108497802320970253. [DOI] [PubMed] [Google Scholar]
- 36.Chi KN. Targeting Bcl-2 with oblimersen for patients with hormone refractory prostate cancer. World J Urol. 2005;23(1):33–7. doi: 10.1007/s00345-004-0477-x. [DOI] [PubMed] [Google Scholar]
- 37.Klossner DP, Baust JM, VanBuskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008;101(10):1310–6. doi: 10.1111/j.1464-410X.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 38.Robilotto AT, Clarke D, Baust JM, Van Buskirk RG, Gage AA, Baust JG. Development of a tissue engineered human prostate tumor equivalent for use in the evaluation of cryoablative techniques. Technol Cancer Res Treat. 2007;6(2):81–9. doi: 10.1177/153303460700600204. [DOI] [PubMed] [Google Scholar]
- 39.Onik G. Image-guided prostate cryosurgery: state of the art. Cancer Control. 2001;8(6):522–31. doi: 10.1177/107327480100800607. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? Anticancer Res. 1990;10(5A):1307–11. [PubMed] [Google Scholar]
- 41.Mitra D, Bell NH. Racial, geographic, genetic, and body habitus effects on vitamin metabolism. In: Feldman D, Glorieux FH, Pike JW, editors. Vitamin D. 1st. San Diego, CA: Academic Press; 1997. pp. 521–32. [Google Scholar]
- 42.Giovannucci E, Rimm EB, Wolk A, et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58(3):442–7. [PubMed] [Google Scholar]
- 43.Peehl DM, Krishnan AV, Feldman D. Pathways mediating the growth-inhibitory actions of vitamin D in prostate cancer. J Nutr. 2003;133(7 Suppl):2461S–9S. doi: 10.1093/jn/133.7.2461S. [DOI] [PubMed] [Google Scholar]
- 44.Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26(4A):2551–6. [PubMed] [Google Scholar]
- 45.Clarke DM, Robilotto AT, VanBuskirk RG, Baust JG, Gage AA, Baust JM. Targeted induction of apoptosis via TRAIL and cryoablation: a novel strategy for the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(2):175–84. doi: 10.1038/sj.pcan.4500920. [DOI] [PubMed] [Google Scholar]