Abstract
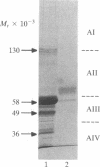
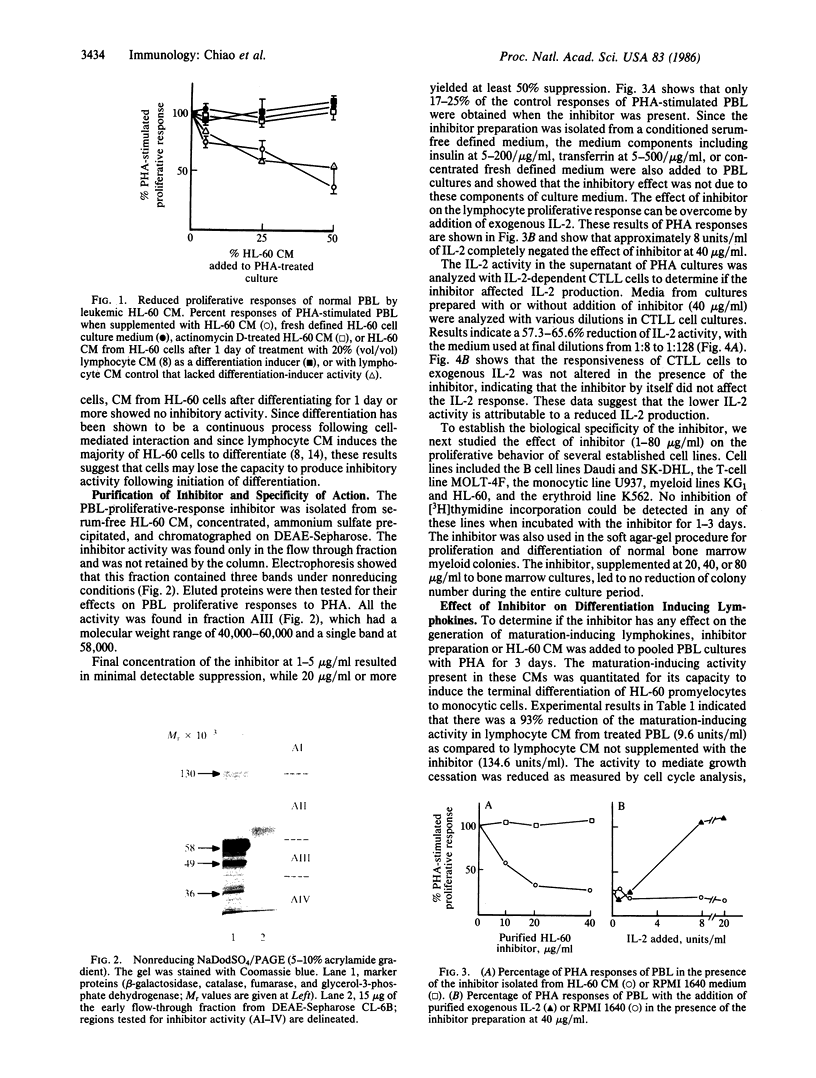
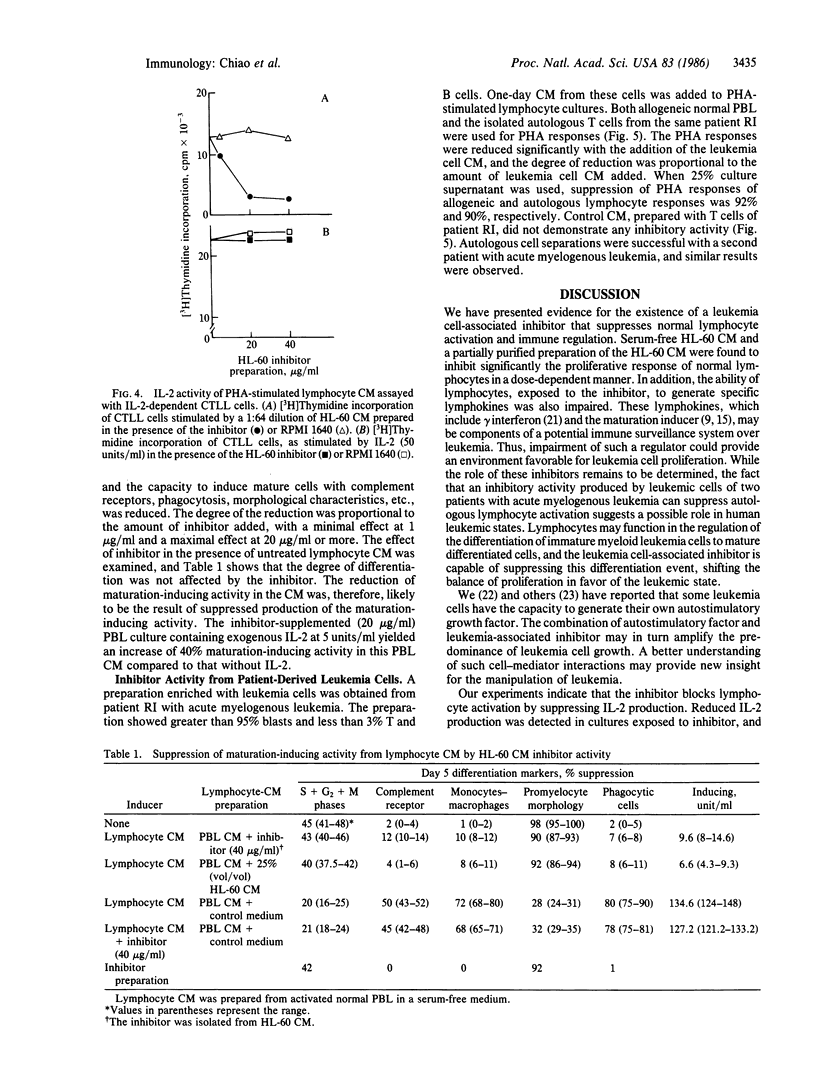
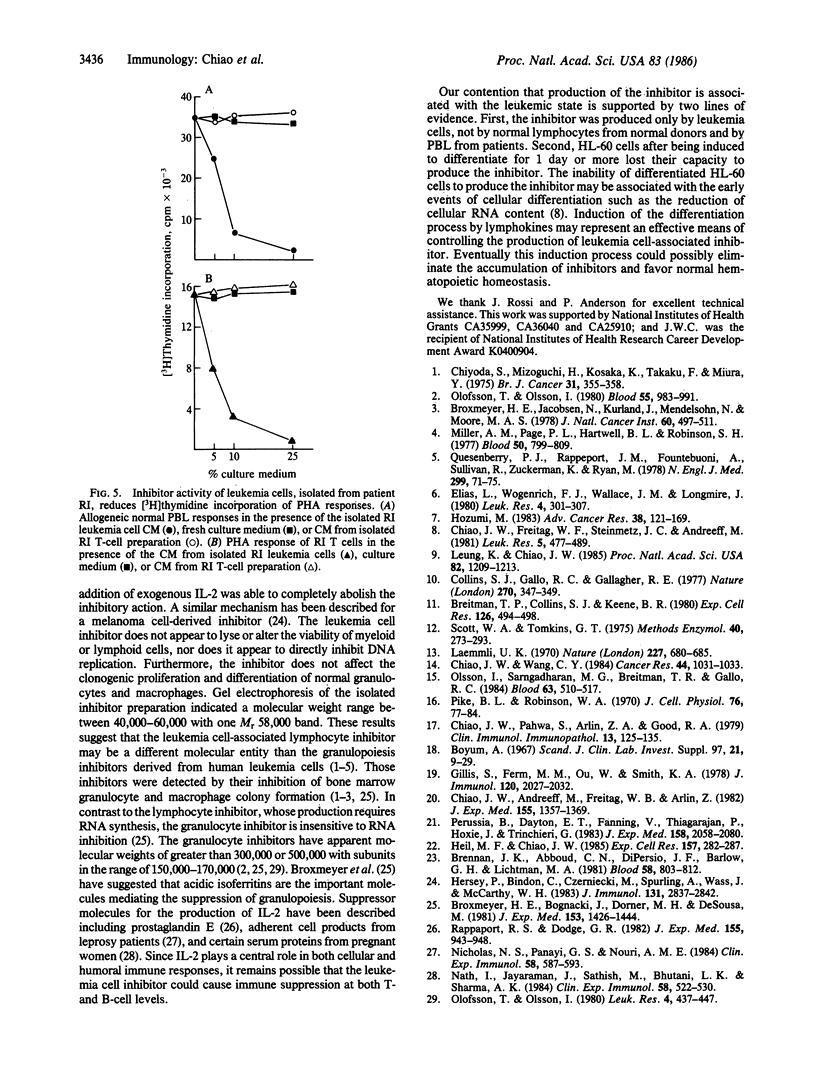
An inhibitor isolated from the serum-free culture medium of human myeloid leukemic HL-60 cells was able to suppress mitogen- and alloantigen-stimulated proliferative responses of normal lymphocytes in a dose-dependent manner. In vitro production, concentration, and purification by column chromatography and electrophoresis revealed that the inhibitor was produced constitutively, required RNA synthesis, and had a molecular weight in the range of 40,000-60,000. The inhibitor was also produced in vitro by myeloid leukemia cells isolated from patients with acute myelogenous leukemia. In a similar manner, the inhibitory material suppressed proliferative responses of allogeneic and autologous lymphocytes. Suppression was accompanied by drastically reduced production of interleukin 2 and lymphokines, which regulate differentiation of myeloid leukemia cells, and suppression was reversed by addition of exogenous interleukin 2. The inhibitor did not suppress clonogenic proliferation of normal granulocytes and macrophages suggesting that inhibition of production or interference with interleukin 2 activity as a possible mechanism. These interactions between leukemia cells and lymphocytes have shed new light on the immunosuppression and growth advantage of leukemia cells. Inhibitory activity of HL-60 cells was diminished after they were induced to differentiate, indicating that differentiation induced by lymphokines may be an effective means of controlling leukemia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitman T. R., Collins S. J., Keene B. R. Replacement of serum by insulin and transferrin supports growth and differentiation of the human promyelocytic cell line, HL-60. Exp Cell Res. 1980 Apr;126(2):494–498. doi: 10.1016/0014-4827(80)90296-7. [DOI] [PubMed] [Google Scholar]
- Brennan J. K., Abboud C. N., DiPersio J. F., Barlow G. H., Lichtman M. A. Autostimulation of growth by human myelogenous leukemia cells (HL-60). Blood. 1981 Oct;58(4):803–812. [PubMed] [Google Scholar]
- Broxmeyer H. E., Bognacki J., Dorner M. H., de Sousa M. Identification of leukemia-associated inhibitory activity as acidic isoferritins. A regulatory role for acidic isoferritins in the production of granulocytes and macrophages. J Exp Med. 1981 Jun 1;153(6):1426–1444. doi: 10.1084/jem.153.6.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Jacobsen N., Kurland J., Mendelsohn N., Moore A. S. In vitro suppression of normal granulocytic stem cells by inhibitory activity derived from human leukemia cells. J Natl Cancer Inst. 1978 Mar;60(3):497–511. doi: 10.1093/jnci/60.3.497. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. A two-phase system for removal of red cells with methylcellulose as erythrocyte-aggregating agent. Scand J Clin Lab Invest Suppl. 1968;97:9–29. [PubMed] [Google Scholar]
- Chiao J. W., Andreeff M., Freitag W. B., Arlin Z. Induction of in vitro proliferation and maturation of human aneuploid myelogenous leukemic cells. J Exp Med. 1982 May 1;155(5):1357–1369. doi: 10.1084/jem.155.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J. W., Freitag W. F., Steinmetz J. C., Andreeff M. Changes of cellular markers during differentiation of HL-60 promyelocytes to macrophages as induced by T lymphocyte conditioned medium. Leuk Res. 1981;5(6):477–489. doi: 10.1016/0145-2126(81)90118-1. [DOI] [PubMed] [Google Scholar]
- Chiao J. W., Pahwa S., Arlin Z. A., Good R. A. Association of helper activity for B-lymphocyte differentiation with lymphocytes having both SRBC and complement receptors in a patient with lymphoproliferative disease. Clin Immunol Immunopathol. 1979 Jun;13(2):125–135. doi: 10.1016/0090-1229(79)90056-4. [DOI] [PubMed] [Google Scholar]
- Chiao J. W., Wang C. Y. Differentiation antigens of HL-60 promyelocytes during induced maturation. Cancer Res. 1984 Mar;44(3):1031–1033. [PubMed] [Google Scholar]
- Chiyoda S., Mizoguchi H., Kosaka K., Takaku F., Miura Y. Influence of leukaemic cells on the colony formation of human bone marrow cells in vitro. Br J Cancer. 1975 Mar;31(3):355–358. doi: 10.1038/bjc.1975.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Elias L., Wogenrich F. J., Wallace J. M., Longmire J. Altered pattern of differentiation and proliferation of HL-60 promyelocytic leukemia cells in the presence of leucocyte conditioned medium. Leuk Res. 1980;4(3):301–307. doi: 10.1016/0145-2126(80)90037-5. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Heil M. F., Chiao J. W. Cell cycle effects of an autologous growth promoter from human leukemia cells. Exp Cell Res. 1985 Mar;157(1):282–287. doi: 10.1016/0014-4827(85)90171-5. [DOI] [PubMed] [Google Scholar]
- Hersey P., Bindon C., Czerniecki M., Spurling A., Wass J., McCarthy W. H. Inhibition of interleukin 2 production by factors released from tumor cells. J Immunol. 1983 Dec;131(6):2837–2842. [PubMed] [Google Scholar]
- Hozumi M. Fundamentals of chemotherapy of myeloid leukemia by induction of leukemia cell differentiation. Adv Cancer Res. 1983;38:121–169. doi: 10.1016/s0065-230x(08)60189-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung K., Chiao J. W. Human leukemia cell maturation induced by a T-cell lymphokine isolated from medium conditioned by normal lymphocytes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1209–1213. doi: 10.1073/pnas.82.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Page P. L., Hartwell B. L., Robinson S. M. Inhibition of growth of normal murine granulocytes by cocultured acute leukemic cells. Blood. 1977 Nov;50(5):799–809. [PubMed] [Google Scholar]
- Nath I., Sathish M., Jayaraman T., Bhutani L. K., Sharma A. K. Evidence for the presence of M. leprae reactive T lymphocytes in patients with lepromatous leprosy. Clin Exp Immunol. 1984 Dec;58(3):522–530. [PMC free article] [PubMed] [Google Scholar]
- Nicholas N. S., Panayi G. S., Nouri A. M. Human pregnancy serum inhibits interleukin-2 production. Clin Exp Immunol. 1984 Dec;58(3):587–595. [PMC free article] [PubMed] [Google Scholar]
- Olofsson T., Olsson I. Biochemical characterization of a leukemia-associated inhibitor (LAI) suppressing normal granulopoiesis in vitro. Blood. 1980 Jun;55(6):983–991. [PubMed] [Google Scholar]
- Olofsson T., Olsson I. Suppression of normal granulopoiesis in vitro by a leukemia associated inhibitor (LAI) derived from a human promyelocytic cell line [HL-60]. Leuk Res. 1980;4(5):437–447. doi: 10.1016/0145-2126(80)90026-0. [DOI] [PubMed] [Google Scholar]
- Olsson I. L., Sarngadharan M. G., Breitman T. R., Gallo R. C. Isolation and characterization of a T lymphocyte-derived differentiation inducing factor for the myeloid leukemic cell line HL-60. Blood. 1984 Mar;63(3):510–517. [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Fanning V., Thiagarajan P., Hoxie J., Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983 Dec 1;158(6):2058–2080. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Quesenberry P. J., Rappeport J. M., Fountebouni A., Sullivan R., Zuckerman K., Ryan M. Inhibition of normal murine hematopoiesis by leukemic cells. N Engl J Med. 1978 Jul 13;299(2):71–75. doi: 10.1056/NEJM197807132990204. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Tomkins G. M. The use of inhibitors in the study of hormone mechanisms in cell culture. Methods Enzymol. 1975;40:273–293. doi: 10.1016/s0076-6879(75)40022-2. [DOI] [PubMed] [Google Scholar]