Abstract
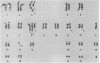
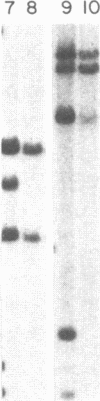
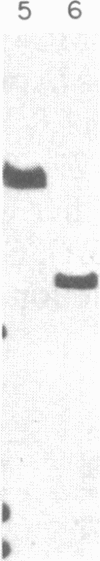
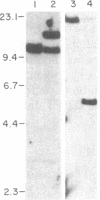
The SKW-3 cell line, which was established from the malignant cells of a patient with T-cell chronic lymphocytic leukemia, is characterized by a translocation involving chromosome 8 (band q24) and chromosome 14 (band q11) [t(8;14)(q24;q11)]. To determine the position of the gene encoding the alpha chain of the T-cell receptor and of the human protooncogene myc (c-myc) in relation to the breakpoint junctions and to evaluate their possible role in the pathogenesis of T-cell neoplasia, we applied the techniques of in situ chromosomal hybridization and Southern blot analysis to SKW-3 cells. Our results indicate that the breakpoint on chromosome 14 at band q11 occurs close to a joining sequence of the gene encoding the alpha chain of the T-cell receptor. Additional rearrangements within the alpha-chain locus appear to split the variable region cluster. As a result of the rearrangements, the constant region of this gene, as well as some variable region segments, are translocated to chromosome 8, to the 3' side of the c-myc-coding exons. The identification of a breakpoint to the 3' side of c-myc suggests that this translocation is analogous to the variant (2;8) and t(8;22) translocations observed in the B-cell malignancies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield C. D., Arthur D. C., Frizzera G., Levine E. G., Peterson B. A., Gajl-Peczalska K. J. Nonrandom chromosome abnormalities in lymphoma. Cancer Res. 1983 Jun;43(6):2975–2984. [PubMed] [Google Scholar]
- Caccia N., Bruns G. A., Kirsch I. R., Hollis G. F., Bertness V., Mak T. W. T cell receptor alpha chain genes are located on chromosome 14 at 14q11-14q12 in humans. J Exp Med. 1985 May 1;161(5):1255–1260. doi: 10.1084/jem.161.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubet J. F., Mathieu-Mahul D., Bernheim A., Larsen C. J., Berger R. Remaniement du locus du proto-oncogène c-myc dans une lignée cellulaire d'origine lymphoblastique. C R Acad Sci III. 1985;300(5):171–176. [PubMed] [Google Scholar]
- Croce C. M., Isobe M., Palumbo A., Puck J., Ming J., Tweardy D., Erikson J., Davis M., Rovera G. Gene for alpha-chain of human T-cell receptor: location on chromosome 14 region involved in T-cell neoplasms. Science. 1985 Mar 1;227(4690):1044–1047. doi: 10.1126/science.3919442. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Thierfelder W., Erikson J., Nishikura K., Finan J., Lenoir G. M., Nowell P. C. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6922–6926. doi: 10.1073/pnas.80.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Emanuel B. S., Cannizzaro L. A., Magrath I., Tsujimoto Y., Nowell P. C., Croce C. M. Chromosomal orientation of the lambda light chain locus: V lambda is proximal to C lambda in 22q11. Nucleic Acids Res. 1985 Jan 25;13(2):381–387. doi: 10.1093/nar/13.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel B. S., Selden J. R., Chaganti R. S., Jhanwar S., Nowell P. C., Croce C. M. The 2p breakpoint of a 2;8 translocation in Burkitt lymphoma interrupts the V kappa locus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2444–2446. doi: 10.1073/pnas.81.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Williams D. L., Finan J., Nowell P. C., Croce C. M. Locus of the alpha-chain of the T-cell receptor is split by chromosome translocation in T-cell leukemias. Science. 1985 Aug 23;229(4715):784–786. doi: 10.1126/science.3875152. [DOI] [PubMed] [Google Scholar]
- Gaeke M. E., Vardiman J. W., Miller W., Medenica M., Hopper J. E., Rowley J. D. Human T-cell lymphoma with suppressor effects on the mixed lymphocyte reaction (MLR). I. Morphological and cytogenetic analysis. Blood. 1981 Apr;57(4):634–641. [PubMed] [Google Scholar]
- Hecht F., Morgan R., Hecht B. K., Smith S. D. Common region on chromosome 14 in T-cell leukemia and lymphoma. Science. 1984 Dec 21;226(4681):1445–1447. doi: 10.1126/science.6438800. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Mitchell K. F., Battey J., Potter H., Taub R., Lenoir G. M., Leder P. A variant translocation places the lambda immunoglobulin genes 3' to the c-myc oncogene in Burkitt's lymphoma. Nature. 1984 Feb 23;307(5953):752–755. doi: 10.1038/307752a0. [DOI] [PubMed] [Google Scholar]
- Höchtl J., Zachau H. G. A novel type of aberrant recombination in immunoglobulin genes and its implications for V-J joining mechanism. Nature. 1983 Mar 17;302(5905):260–263. doi: 10.1038/302260a0. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Diaz M. O., Rowley J. D., Mak T. W. Chromosomal localization of the human T cell receptor beta-chain genes. Cell. 1985 May;41(1):335–335. doi: 10.1016/0092-8674(85)90086-8. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Rowley J. D. c-src is consistently conserved in the chromosomal deletion (20q) observed in myeloid disorders. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6692–6696. doi: 10.1073/pnas.82.19.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Michalopoulos E. E., Williams D. L., Minden M. D., Mak T. W. Breakpoints in the human T-cell antigen receptor alpha-chain locus in two T-cell leukaemia patients with chromosomal translocations. Nature. 1985 Oct 10;317(6037):544–546. doi: 10.1038/317544a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., McCoy C., Blanc D., Trucy J., Devaux C., Schmitt-Verhulst A. M., Fitch F., Hood L., Malissen B. Direct evidence for chromosomal inversion during T-cell receptor beta-gene rearrangements. Nature. 1986 Jan 2;319(6048):28–33. doi: 10.1038/319028a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., Waldmann R. A., Morton C. C., Bongiovanni K. F., Waldmann T. A., Shows T. B., Seidman J. G. Human gamma-chain genes are rearranged in leukaemic T cells and map to the short arm of chromosome 7. Nature. 1985 Aug 8;316(6028):549–552. doi: 10.1038/316549a0. [DOI] [PubMed] [Google Scholar]
- Rappold G. A., Hameister H., Cremer T., Adolph S., Henglein B., Freese U. K., Lenoire G. M., Bornkamm G. W. c-myc and immunoglobulin kappa light chain constant genes are on the 8q+ chromosome of three Burkitt lymphoma lines with t(2;8) translocations. EMBO J. 1984 Dec 1;3(12):2951–2955. doi: 10.1002/j.1460-2075.1984.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamori N., Kusano M., Nishino K., Tagawa M., Yao E., Yamada Y., Amagasaki T., Kinoshita K., Ichimaru M. Abnormalities of chromosome 14 at band 14q11 in Japanese patients with adult T-cell leukemia. Cancer Genet Cytogenet. 1985 Jul;17(3):279–282. doi: 10.1016/0165-4608(85)90019-6. [DOI] [PubMed] [Google Scholar]
- Showe L. C., Ballantine M., Nishikura K., Erikson J., Kaji H., Croce C. M. Cloning and sequencing of a c-myc oncogene in a Burkitt's lymphoma cell line that is translocated to a germ line alpha switch region. Mol Cell Biol. 1985 Mar;5(3):501–509. doi: 10.1128/mcb.5.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taub R., Kelly K., Battey J., Latt S., Lenoir G. M., Tantravahi U., Tu Z., Leder P. A novel alteration in the structure of an activated c-myc gene in a variant t(2;8) Burkitt lymphoma. Cell. 1984 Jun;37(2):511–520. doi: 10.1016/0092-8674(84)90381-7. [DOI] [PubMed] [Google Scholar]
- Ueshima Y., Rowley J. D., Variakojis D., Winter J., Gordon L. Cytogenetic studies on patients with chronic T cell leukemia/lymphoma. Blood. 1984 May;63(5):1028–1038. [PubMed] [Google Scholar]
- Williams D. L., Look A. T., Melvin S. L., Roberson P. K., Dahl G., Flake T., Stass S. New chromosomal translocations correlate with specific immunophenotypes of childhood acute lymphoblastic leukemia. Cell. 1984 Jan;36(1):101–109. doi: 10.1016/0092-8674(84)90078-3. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Chan A., Chin B., Minden M., Mak T. W. Analysis of cDNA clones specific for human T cells and the alpha and beta chains of the T-cell receptor heterodimer from a human T-cell line. Proc Natl Acad Sci U S A. 1985 May;82(10):3430–3434. doi: 10.1073/pnas.82.10.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]
- Zech L., Gahrton G., Hammarström L., Juliusson G., Mellstedt H., Robèrt K. H., Smith C. I. Inversion of chromosome 14 marks human T-cell chronic lymphocytic leukaemia. 1984 Apr 26-May 2Nature. 308(5962):858–860. doi: 10.1038/308858a0. [DOI] [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]