Abstract
Cocaine addiction involves an escalation in drug intake which alters many brain functions. The present study documented cocaine-induced changes in brain metabolic activity as a function of cocaine self-administration history. Experimentally naive rhesus monkeys (N=6) were given increasing access to cocaine under a fixed-ratio schedule of i.v. drug self-administration. PET imaging with F-18 labeled fluorodeoxyglucose (FDG) was used to measure acute i.m. cocaine-induced changes in brain metabolism in the cocaine-naïve state, following 60 sessions under limited-access conditions (1hr/day), following 60 sessions under extended-access conditions (4hr/day), and following 4 weeks of drug withdrawal. In the cocaine-naïve state, cocaine-induced increases in brain metabolism were restricted to the prefrontal cortex. As cocaine exposure increased from limited to extended access, metabolic effects expanded throughout the frontal cortex and were induced within the striatum. Conversely, cocaine-induced activation was far less robust following withdrawal. The results highlight a progressive expansion of the metabolic effects of cocaine to include previously unaffected dopamine innervated brain regions as a consequence of cocaine self-administration history. The identification of brain regions progressively influenced by drug exposure may be highly relevant toward efforts to develop treatments for cocaine addiction.
Keywords: nonhuman primates, cocaine, self-administration, dopamine, fluorodeoxyglucose (FDG), positron emission tomography (PET)
Introduction
Studies employing neuroimaging techniques have begun to define neurobiological substrates underlying the acute effects of cocaine in cocaine-dependent subjects. For example, acute cocaine administration increased cerebral blood flow mainly in the frontal and parietal regions (Mathew et al. 1996). A BOLD fMRI study reported dynamic patterns of brain activation following cocaine administration (Breiter et al. 1997). Regions including cingulate and lateral prefrontal cortex, showed short duration activations that were correlated with ratings of “rush”. Other regions including the nucleus accumbens showed sustained activation associated with ratings of “craving”. Cocaine also activated mesolimbic and mesocortical regions that receive dopaminergic afferents (Kufahl et al. 2005). The administration of methylphenidate, a dopamine transport blocker, induced increases in dopamine release, activated orbitofrontal cortex, and increased craving for cocaine despite the decrease in dopamine system function (Volkow et al. 1999; Volkow et al. 2005). In a study utilizing fMRI, cocaine-induced euphoria and craving was associated with increases in brain activity throughout the mesolimbic and mesocortical dopamine pathways in cocaine-dependent subjects including the prefrontal cortex and the anterior cingulate (Breiter et al. 1997; Kufahl et al. 2005). PET studies in detoxified cocaine abusers have revealed disruptions in brain metabolic activity throughout the dopamine system including reductions in glucose utilization in both the striatum and orbitofrontal cortex indicating persistent changes in dopamine function (London et al. 1990; Volkow et al. 1992). Taken together, imaging studies demonstrate that addiction to cocaine disrupts prefrontal brain activity, a region considered critical for cognitive function, which may compromise control over drug use.
Functional changes in brain activity following cocaine administration have also been demonstrated in nonhuman primates. The capability to conduct parallel neuroimaging studies in nonhuman primates and human subjects provides a powerful translational approach that can link findings from human and animal research. A significant advantage of nonhuman primate models is the use of initially drug-naïve subjects in longitudinal designs to characterize within-subject changes in aspects of the neurobiology associated with chronic drug use. In one series of studies, cocaine induced dose-dependent increases in functional activity in the prefrontal cortex determined by PET imaging of cerebral blood flow with O-15 labeled water in cocaine-naïve rhesus monkeys (Howell et al. 2001; Howell et al. 2002). Non-contingent administration of cocaine in cocaine-naïve subjects induced robust activation of prefrontal cortex localized primarily to the dorsolateral regions. More recently, the acute effects of cocaine on cerebral blood flow were evaluated during active drug self-administration and shown to be influenced by the history of drug exposure (Howell et al. 2010). In contrast to results obtained following non-contingent administration of cocaine in cocaine-naïve subjects, the pattern of brain activation induced by self-administered cocaine differed qualitatively and included anterior cingulate cortex. Collectively, PET imaging studies of cerebral blood flow demonstrate that cocaine induces a unique pattern of brain activation in nonhuman primates.
The present study characterized the progression of changes in brain metabolic activity in response to acute i.m. cocaine administration as a function of cocaine history in a well-controlled, within-subject design. Cocaine-naïve rhesus monkeys were given increasing access to cocaine under a fixed-ratio schedule of i.v. drug self-administration. PET imaging with the glucose analog, fluorodeoxyglucose (FDG), was used to measure drug-induced changes in brain metabolism first in the cocaine-naïve state, then following 60 sessions under both limited access conditions (1hr/day) and extended access conditions (6hrs/day), and again following 4 weeks of drug withdrawal. The results highlight a progressive expansion of cocaine-induced brain metabolic effects throughout the prefrontal cortex, the cingulate cortex and the striatum, as a result of self-administration history.
Materials and Methods
Subjects
Four male and two female adult (6–8 years old) rhesus monkeys (Macaca mulatta) weighing between 9 and 15 kg were used. All monkeys were experimentally naïve at the start of the present experiments. Between daily experimental sessions, monkeys were individually housed, provided access to food daily (Purina Monkey Chow, fresh fruit and vegetables), and ad libitum access to water. Animal use procedures were in strict accordance with the National Institutes of Heatlh’s “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of Emory University.
Self-Administration
Monkeys were surgically implanted with chronic indwelling intravenous catheters. Implantation was done under a combination of Telazol and isoflurane (1.0–2.0%) anesthesia using aseptic techniques. One end of a silicone catheter was inserted into either the femoral or jugular vein and advanced into the vena cava. The distal end of the catheter was routed subcutaneously and attached to a vascular access port (Access Technologies, Skokie, IL) in the interscapular region. Preoperative antibiotic (ceftriaxone) and postoperative analgesic (flunixin meglumine) were administered according to veterinary staff direction. Catheters were flushed daily with 1.0 ml of heparinized (100 U/ml) saline to maintain patency.
During self-administration sessions, each monkey was seated in a commercially-available primate chair (Primate Products, Miami, FL). A response panel equipped with a lever and stimulus lights was attached to the front of the chair. The skin over the vascular access port was cleaned with 95% ethanol and betadine, and then a special right-angle Huber needle (Access Technologies, Skokie, IL) was inserted into the port. The chair was then enclosed in a ventilated, sound-attenuating chamber (Med Associates, St. Albans, VT). Polyvinyl-chloride (PVC) tubing connected the Huber needle to a motor-driven syringe pump (Model PhD 2000; Harvard Apparatus, Holliston, MA) located outside the test chamber containing the drug solution. When activated, the pump delivered a unit dose of 0.1 mg/kg/infusion (−) cocaine HCl (National Institute on Drug Abuse, Bethesda, MD) in a volume of 2.0 ml during a 7.0 second infusion. Med-PC (Med Associates, St. Albans, VT) software systems controlled all experimental events and data collection.
Each monkey was trained on a fixed-ratio (FR) schedule of i.v. drug self-administration. Each test session began with the presentation of a red stimulus light. The completion of a FR 20 response requirement changed the stimulus light from red to white for 15 seconds and initiated a 0.1mg/kg infusion of cocaine. The infusion was followed by a 60-second timeout during which stimulus lights were extinguished and responding had no scheduled consequences. At the end of the timeout, the red light was presented again to initiate the next FR component. FR self-administration training began with an FR 1 and increased sequentially when at least 3 infusions were earned in three consecutive testing days at each FR until FR 20 was reached. Only 5 infusions were available for each session during FR self-administration training. It took on average about 30 sessions to complete FR self-administration training. Once the terminal schedule was reached, the sessions were limited to 1 hour each day, 6 days/week, but no limit was placed on the number of available infusions. Subjects were maintained on this limited-access schedule for 60 sessions. The limited-access condition was then followed by 60 sessions on an extended-access schedule during which the session length was increased to 4 hours each day with a limit of 60 available infusions to prevent adverse effects. Four weeks of withdrawal from cocaine self-administration followed the extended-access condition.
Imaging Procedures
[Fluorine 18]-fluorodeoxyglucose (FDG) was synthesized from fluorine 18 fluoride produced in a biomedical cyclotron 11MeV proton irradiation of 98% [oxygen 18] enriched water. FDG was used to measure the effects of acute cocaine administration on cerebral glucose metabolism in subjects with a long-term history of cocaine use. Each subject received five PET scans throughout their cocaine self-administration history – two before self-administration in the cocaine-naïve state, one following limited- and extended-access conditions, and one after 4 weeks of withdrawal (Figure 1). The first two PET scans were randomized and separated by at least two weeks. PET scans following limited- and extended-access conditions were obtained approximately 24 hours after the last self-administration session. Subjects received an i.m. injection of FDG (15 mCi) immediately followed by an i.m. injection of cocaine (1.0 mg/kg) in their home cages and remained undisturbed during the uptake phase. In the cocaine-naïve state, subjects also underwent an additional scan during which they received an i.m. injection of saline instead of cocaine as a baseline measure to which all cocaine-induced brain activation scans were compared. Immediately following the 45-min uptake phase, subjects were taken to the PET scanner for imaging under a combination of Telazol and isoflurane anesthesia. Whole brain, 3D imaging data were collected with a Siemens Focus 220 microPET scanner located at the Yerkes Imaging Center. The microPET scanner has a 26 cm transaxial field of view and an 8 cm axial field of view. The reconstructed resolution is 1.7 mm in all directions. The acquired data were corrected for random background events, scatter, attenuation, and dead-time. Registered PET images were normalized to mean whole brain activity using whole brain ratios of glucose metabolism.
Figure 1.
Study timeline for each drug-access condition including each FDG-PET scan. See Methods section for detailed explanation.
Data Analysis
All analyses were conducted within the Statistical Parametric Mapping (SPM5) toolbox (Wellcome Trust Centre for Neuroimaging, London, UK) within MATLAB (version 7.3; MathWorks, Natick, MA). Within-subject PET images were coregistered using a normalized mutual information algorithm and a six rigid body transformation. A custom-built MRI rhesus monkey brain template was used that had been previously generated by non-biased averaging of T1-weighted magnetic resonance images (MRIs) with a spatial resolution of 0.6 × 0.6 × 1.0mm of sixteen subjects. Coregistered PET images were then normalized to this template using the same algorithm. Spatial smoothing was applied using a 4mm kernel and generating a final pixel size of 1.2 × 1.2 × 2mm. Contrasts were generated between saline and cocaine treatment at each stage of cocaine access using a paired t-test. The results of these contrasts were expressed as color coded maps of statistical significance in units of the t statistic value. Analysis and uptake normalization was limited to voxels within gray matter. Scans were normalized for gain differences by proportional scaling. Between-subject differences in global cerebral blood flow and tracer uptake were accounted for using analysis of covariance (ANCOVA) by subject. Effects for each voxel were estimated using a general linear model with a minimum statistical threshold of p<0.0167 and a cluster size of 10 contiguous voxels. A p-value of 0.0167 yielded results corrected for the number of study conditions through Bonferroni methods but uncorrected for the number of voxels analyzed. However, data were further corrected for multiple comparisons via cluster size inference. A cluster size of 10 contiguous voxels was chosen on the basis of previous data showing a good correspondence between the predicted and underlying distribution when this size was matched to the applied smoothing kernel (Hayasaka and Nichols, 2003) and a priori estimates of size of regional activations resulting from cocaine administration in rhesus monkeys (Howell et al. 2002, 2010). The t-maps are from all six subjects for cocaine-naïve, limited, and extended access. Only five subjects were scanned following withdrawal, so the t-maps were recreated comparing cocaine naïve to withdrawal using only the remaining five subjects.
Results
Cocaine-Naïve State
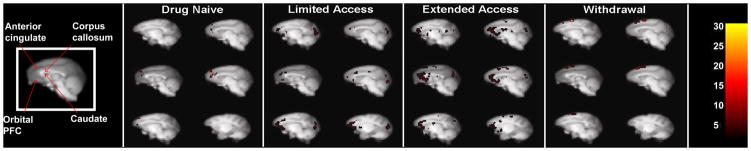
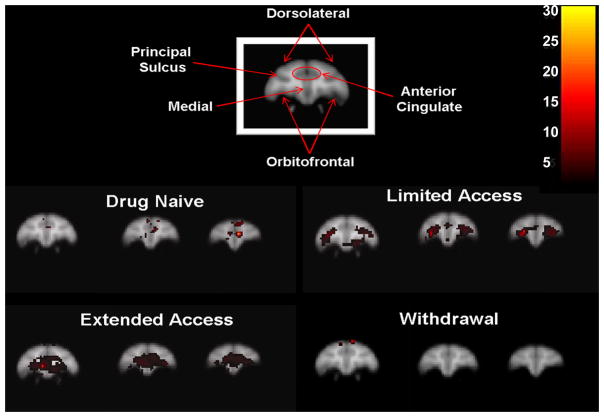
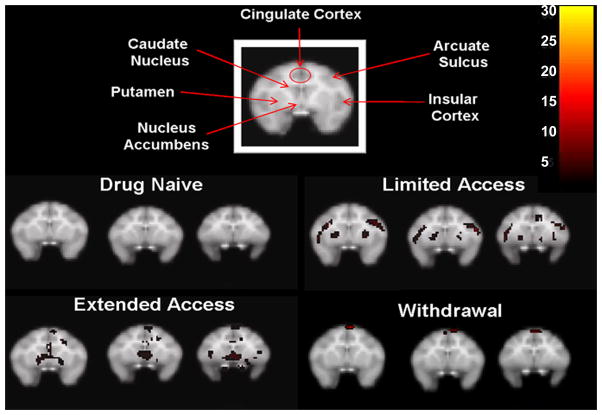
In the cocaine-naïve state, cocaine-induced increases in metabolism were localized only in the prefrontal cortex (Figure 2). Specifically, the medial prefrontal cortex was activated compared to glucose utilization following an injection of saline (Figure 3). There was no significant activation in the striatum (Figure 4).
Figure 2.
Cocaine-induced (1.0 mg/kg, i.m.) metabolic effects along midline sagittal brain sections following each cocaine access condition. Images are presented at 4 (top left) and 2 (top right) mm left of midline, midline (middle left), and 2 (middle right), 4 (bottom left), and 6 (bottom right) mm right of midline. Inset color scale represents the degree of statistical significance expressed in multiples of the t statistic value.
Figure 3.
Cocaine-induced (1.0 mg/kg, i.m.) activation within the prefrontal cortex following each cocaine access condition. Images for each condition represent 2mm coronal sections anterior (left to right) from the first appearance of the orbitofrontal cortex. Inset color scale represents the degree of statistical significance expressed in multiples of the t statistic value.
Figure 4.
Cocaine-induced (1.0 mg/kg, i.m.) activation within the striatum following each cocaine access condition. Images for each condition represent 2mm coronal sections anterior (left to right) from the first appearance of the head of the caudate nucleus. Inset color scale represents the degree of statistical significance expressed in multiples of the t statistic value.
Limited-Access Condition
Table 1 lists the total levels of drug intake (in milligrams per kilogram) in individual animals for each phase of i.v. cocaine access. Self-administration training sessions allowed for a maximum of 5 cocaine infusions per session (0.1 mg/kg/infusion). Training generally took about 30 sessions and total cocaine intake for all subjects averaged 9.3±1.3 mg/kg. Subsequently, cocaine became available under limited-access conditions. Self-administration sessions were performed 6 days per week for a total of 60 sessions and during limited access, cocaine intake averaged 82.4±13.3 mg/kg for the group. During this early stage of cocaine self-administration under limited-access conditions, acute administration of cocaine markedly increased metabolic activity throughout the prefrontal cortex (Figure 2). In addition, there was robust activation of the orbitofrontal cortex (Figure 3). Recruitment of activity was also noted in the striatum, particularly bilateral activation of the putamen and more lateral regions of the frontal cortex including primary areas of sensorimotor processing (Figure 4).
Table 1.
Total Cocaine Intake (mg/kg) during FR 20 Self-Administration Training and Limited and Extended Access Conditions for Individual Monkeys
| Monkey | Training (~30 days) | Limited Access (10 weeks) | Extended Access (10 weeks) |
|---|---|---|---|
| RBl6 (F) | 13.80 | 113.70 | 345.80 |
| RDn8 (F) | 4.47 | 131.30 | 315.10 |
| RJl8 (M) | 8.90 | 74.40 | 225.60 |
| RKn8 (M) | 8.30 | 50.80 | 186.90 |
| ROf8 (M) | 10.90 | 65.70 | 202.20 |
| RYt8 (M) | 9.40 | 58.50 | 194.10 |
| Mean | 9.3±1.3 | 82.4±13.3 | 245.0±27.84 |
The letter in parentheses (F or M) indicates sex. Mean ± SEM.
Extended-Access Conditions
Total i.v. cocaine intake increased considerably above limited-access levels when cocaine access was increased to 4hr/day during the extended-access condition, averaging 245.0±27.8 mg/kg for the entire group. During the longer duration of cocaine self-administration under extended-access conditions, the pattern of metabolic activity following acute i.m. administration of cocaine resembled that seen during limited-access conditions except activity was now more extensive (Figure 2). Cocaine induced even broader activation of the prefrontal cortex to encompass most of the orbitofrontal and medial cortices, as well as the anterior cingulate cortex. Cocaine also induced a small area of metabolic activity in a more superior area of the frontal cortex involved in primary motor processing (Figures 2 & 3). In the striatum, a concentrated area of enhanced metabolic activity was now more medial to the putamen, encompassing ventral portions of the striatum including the nucleus accumbens (Figure 4).
Drug Withdrawal
Finally, after 4 weeks of drug withdrawal during which subjects remained undisturbed in their homecages, a final set of FDG-PET scans revealed that i.m. cocaine no longer induced the same robust pattern of metabolic activity. Surprisingly, there was no activation of the prefrontal cortex or within the striatum (Figure 3 & 4). Only the small area of superior activation of the frontal cortex first seen during extended access remained activated during withdrawal (Figure 2).
Discussion
Functional changes in glucose metabolism were characterized in six rhesus monkeys with the positron-emitting tracer FDG following acute i.m. administration of cocaine at various phases of i.v. cocaine self-administration history. In a series of within-subject, repeated-measures studies, the FDG-PET method allowed for the visualization of the metabolic effects of cocaine under conditions in which duration of cocaine exposure had been systematically varied. The present metabolic mapping demonstrates that acute i.m. administration of cocaine to cocaine-naïve rhesus monkeys induced a discrete pattern of enhanced metabolic activity that was restricted to areas within the medial prefrontal cortex. As cocaine exposure increased from limited to extended access, cocaine-induced activation expanded throughout the cortex. It appears that cocaine exposure resulted in recruitment of cortical regions beyond the prefrontal cortex to encompass regions of sensorimotor processing and the anterior cingulate cortex. Enhanced metabolic activity also emerged in the striatum, first in the putamen then in more ventral medial regions. This array of gradual increases in metabolic activity suggests that the initial subjective effects of cocaine may be mediated by activity in the prefrontal cortex but as history with cocaine self-administration increases more higher-order processes may become affected, such as compulsivity, attentiveness, impulsivity, and emotion as cortical regions become activated in response to acute cocaine administration. Similarly, cortical regions of increased metabolic activity also send projections within the striatum to regions that demonstrated enhanced cocaine-induced metabolic activity following cocaine self-administration. Finally, after 4 weeks of drug withdrawal, cocaine no longer induced the same robust pattern of metabolic activity.
A corresponding pattern of resting metabolic activity has likewise been associated with cocaine dependence in diagnosed cocaine abusers measured for regional brain metabolism with FDG-PET (Volkow et al. 1991). Within a week of cocaine withdrawal, metabolism in the basal ganglia and orbitofrontal cortex was markedly higher in subjects with a history of cocaine abuse than levels measured in normal comparison subjects. This increased resting activity in the striatum and prefrontal cortex parallels the enhanced acute effects of cocaine on metabolic activity seen following limited- and extended-access conditions in the present study. Imaging human cocaine abusers, drug free, in the withdrawal state is, however, a major caveat when comparing resting-state regional brain metabolism to the direct metabolic effects of cocaine as determined in this study. Moreover, it should be acknowledged that the acute cocaine challenges in the present PET experiments were administered i.m. and non-contingently. Self-administration of cocaine via the i.v. route could induce a different pattern of brain activation. The acute effects of cocaine on brain activity have otherwise been investigated using functional MRI imaging methods in cocaine-dependent subjects, and cocaine does in fact induce activation in several regions including the nuclueus accumbens, caudate, putamen, as well as cingulate and prefrontal cortices when compared to infusions of saline in those same subjects (Breiter et al. 1997). Cocaine-dependent subjects also show increases in metabolism in the medial and orbitofrontal cortices following an infusion of methylphenidate (a stimulant pharmacologically similar to cocaine) compared to normal control subjects when determined with FDG-PET (Volkow et al. 2005). In both the MRI and FDG-PET studies, administration of drug and subsequent changes in functional activation was positively correlated with drug craving (Breiter et al. 1997; Volkow et al. 2005). Here, we compared cocaine-induced metabolic effects following limited and extended cocaine self-administration to a saline baseline in the cocaine-naïve state. As the history of cocaine self-administration increased, an acute infusion of cocaine induced progressively enhanced metabolic activity within both the prefrontal cortex and striatum. This was in contrast to the more restricted regions of activation in response to cocaine that was noted when the animals were cocaine-naïve.
Additionally, this progressive involvement of cortical and striatal domains as a function of cocaine exposure has also been previously demonstrated in macaque monkeys utilizing the 2-[14C]deoxyglucose (2-DG) method (Lyons et al. 1996; Porrino et al. 2002; Porrino et al. 2004). In a series of studies, different groups of subjects were evaluated for changes in functional responses to cocaine as assessed by autoradiography following different durations of cocaine self-administration (cocaine-naïve, 5 days, and 3.3 months) (Porrino et al. 2002; Porrino et al. 2004). Initial exposures to cocaine resulted in metabolic effects of cocaine contained primarily in the ventral medial regions of the prefrontal cortex compared to saline treated subjects. Changes in activity were also noted in the ventral striatum and small areas of the dorsal striatum. Following chronic exposure to cocaine self-administration, activity intensified within the striatum to encompass both dorsal and ventral regions. The gradual expansion of the metabolic effects of cocaine is similar to findings from the current study which also showed recruitment of metabolic activity in the cortex and striatum in response to cocaine following a history of cocaine self-administration. The main difference between these studies is that with the 2-DG method, changes in glucose utilization represented a decrease in activity rather than the increases in metabolic activity demonstrated in the current study. This discrepancy may be attributed to a number of procedural differences including, contingent versus non-contingent drug administration, i.v. versus i.m. drug administration, multiple within-session doses versus a single dose, total dose administered, and autoradiography versus PET neuroimaging. Despite the difference in the direction of cocaine-induced effects on brain activity, there is an obvious pattern in the recruitment of cortical and subcortical regions as the history of drug use progresses from the cocaine-naïve state to chronic drug use and withdrawal.
Functional changes in cerebral blood flow determined with PET imaging and 15-O water provide an additional measure to characterize drug-induced changes in brain activity (Howell et al. 2001). Brain activation normalized to global blood flow showed prominent drug-induced activation in the prefrontal cortex of cocaine-naïve rhesus monkeys that were conscious during image acquisition (Howell et al. 2002). Moreover, the pattern of activation induced by cocaine self-administration differed qualitatively compared to non-contingent cocaine administration with the majority of activation localized primarily in the medial region of the prefrontal cortex including the anterior cingulate cortex (Howell et al. 2010). In the present study, a history of cocaine self-administration also contributed to qualitative differences observed following acute cocaine administration compared to the cocaine-naive state. Limited- and extended-access conditions lead to more intense and widespread metabolic effects in the prefrontal cortex that also included anterior cingulate cortex. Cocaine-induced increases in metabolic activity within the prefrontal cortex of subjects with a history of drug use are relevant to the compulsive and obsessive behaviors that are key symptoms of cocaine addiction in humans.
In summary, the present study is the first to use functional brain imaging to document acute cocaine-induced changes in metabolic activity in vivo throughout the cocaine self-administration history of nonhuman primates. The longitudinal design of this study incorporated carefully controlled drug-access conditions that were uniform across subjects which allowed for the characterization of progressive metabolic changes within the same subjects. In the cocaine-naïve state, cocaine induced robust activation that was restricted to the prefrontal cortex. Following the limited- and extended-access conditions, the pattern of metabolic activity expanded to include more regions of the frontal cortex and induced activity within both the dorsal and ventral striatum. Interestingly, tolerance to cocaine-induced synaptic release of dopamine in the striatum has been reported for this same group of animals under both access conditions (Henry et al., 2009). However, there was no escalation in drug intake during the extended-access condition. Compared to the limited-access condition, drug intake increased markedly when subjects were given three extra hours of drug access but drug intake was remarkably stable over the 60 days of extended access. Moreover, drug intake during the first hour of extended access was virtually identical to drug intake during the one-hour limited-access condition. Despite the persistently diminished dopaminergic response, cocaine-induced increases in metabolic activity were enhanced with increased exposure to cocaine. Finally, the acute metabolic effects of cocaine were far less robust following a brief period of drug withdrawal. The results obtained here are consistent with the effects of cocaine history on brain activity in diagnosed cocaine abusers (Volkow et al. 1999). Altogether, it is apparent that a history of cocaine self-administration increases sensitivity to cocaine-induced metabolic effects in brain regions that are innervated by dopamine terminals. These brain regions create a network of connectivity that is associated with the reinforcing and subjective effects of cocaine. The pattern of activity characterized here in the rhesus monkey could lead to the creation of metabolic profiles of cocaine abuse that can be identified with FDG-PET and possibly lead to individualized treatment for human cocaine addicts. For example, the extent of metabolic effects may provide an index of the severity of drug dependence and indicate the need for aggressive treatment approaches, including pharmacotherapy when approved medications are available. Moreover, the progressive involvement of specific cortical domains could direct the focus of cognitive behavioral therapy to emphasize aspects of cognition that are influenced by cortical function identified through neuroimaging. Clearly, a better understanding of drug-induced changes in neurobiology will help direct effective treatment strategies.
Acknowledgments
Supported by USPHS grants DA016589, DA010344, DA00517, and RR00165
References
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JR, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20(4):2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Henry PK, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205(2):237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. Journal of Neuroscience Methods. 2001;106(2):161–169. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl) 2002;159(2):154–160. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 2010;208(2):191–199. doi: 10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28(4):904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN., Jr Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Archives of General Psychiatry. 1990;47(6):567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. The Journal of Neuroscience. 1996;16(3):1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Lowe JV, Humphries D. Acute changes in cranial blood flow after cocaine hydrochloride. Biol Psychiatry. 1996;40:609–616. doi: 10.1016/0006-3223(95)00033-x. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. The Journal of Neuroscience. 2002;22(17):7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. The Journal of Neuroscience. 2004;24(14):3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. The American Journal of Psychiatry. 1991;148(5):621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. The American Journal of Psychiatry. 1999;156(1):19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. The Journal of Neuroscience. 2005;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]