Abstract
G protein-coupled receptors (GPCRs) are cell membrane proteins that recognize specific chemical signals such as drugs and hormones and transduce these signals into cellular responses by activating G-proteins. As is the case for all newly synthesized proteins, GPCRs are subjected to conformational scrutiny at the endoplasmic reticulum prior to processing and trafficking to the cell surface membrane. Because of this stringent quality control screening mechanism, mutations that result in protein misfolding frequently lead to retention in the endoplasmic reticulum, or other misrouting and, eventually, to disease. This article reviews some patents and new therapeutic opportunities based on the misfolding and retention of otherwise functional GPCRs that represent promising approaches to correct conformational abnormalities leading to distinct disease states.
Keywords: G protein-coupled receptor, pharmacological chaperones, pharmacoperones, gonadotropin-releasing hormone receptor, mutation, protein targeting, protein misrouting
INTRODUCTION
Pharmacological chaperones or “pharmacoperones,” are small, often lipophilic compounds that enter cells, bind selectively to biosynthetic intermediates or conformationally defective proteins to influence folding and allow correct routing to their final destination in the cell [1–6]. Frequently, such molecules were initially identified as peptidomimetics from high throughput screens for antagonists or agonists and may come from diverse chemical classes. Because such peptidomimetics interact with proteins to which they are selectively targeted, it has been the first place where many investigators have started in the search for agents that bind to and stabilize G protein-coupled receptors (GPCRs). In vitro and in vivo studies have shown that pharmacoperone rescue may apply to a number of diseases, including inherited metabolic disorders (e.g. Pompe disease), cystic fibrosis, hypercholesterolemia, cataracts, phenylketonuria, neurodegenerative diseases (e.g. Alzheimer’s, Parkinson’s, and Huntington’s), and cancer as well as to GPCRs-related diseases such as retinitis pigmentosa, nephrogenic diabetes insipidus, and hypogonadotropic hypogonadism, among others [2, 3, 7–9].
G protein-coupled receptors are a large and functionally diverse superfamily of membrane proteins whose primary function is to transduce extracellular stimuli into the intracellular environment through the activation of one or more signal transduction pathways [10, 11]. This particular type of receptors is the largest and most important group of targets for therapeutics and they currently are a focus of intensive research efforts. The ligands that recognize and activate these receptors are highly variable in chemical structure and include sensory stimuli (e.g. photons and odorants), lipid mediators, hormones and neurotransmitters. As a result, GPCRs play a key role in regulating a number of biological functions, including cell growth and differentiation, immune responses and cellular metabolism [12]. G protein-coupled receptors vary considerably in molecular size yet share a common molecular topology consisting of a single polypeptide chain that traverses the lipid bilayer seven times, forming characteristic transmembrane (TM) hydrophobic α-helices connected by alternating extracellular and intracellular loops, with an extracellular amino-terminus and an intracellular carboxyl-terminal sequence [10, 11, 13, 14]. Upon agonist binding, GPCRs undergo conformational changes that allow exposure of particular sequences to G proteins, which are signal-transducing molecules regulated by guanine nucleotides and that act as mediators of receptor-evoked effector (enzymes and ion channels) activation [15, 16]. Activated (agonist-bound) GPCRs are rapidly desensitized and internalized, which leads to termination of signaling [17].
Structural alterations provoked by mutations in the gene sequence of GPCRs may lead to disease. Loss-of-function mutations may alter sequences involved in specific functions of the receptor (e.g. ligand binding or interaction with coupled effectors) or domains important for proper folding and intracellular transport of the receptor from its site of synthesis in the endoplasmic reticulum (ER) to the plasma membrane (PM) or internalization [4, 17–19]. Mutant GPCRs with defects in folding and delivery to the cell surface membrane are retained in the ER and eventually degraded, leading to disease [18]. Nevertheless, misfolding can result in proteins that retain function but that for reasons of mislocation alone cannot function normally [18, 20]. This contrasts with the prior presumption that mutational inactivation always reflects loss of intrinsic function (i.e. a receptor that either fails to recognize ligand or activate upon agonist binding, or that does not couple productively to its effectors).
As mentioned above, mutations in GPCRs may cause misrouting of otherwise functional proteins and lead to disease, Table 1 [21–45]. This is the case with the X-linked nephrogenic diabetes insipidus, the autosomal dominant form of retinitis pigmentosa, and hypogonadotropic hypogonadism due to mutations in the gonadotropin-releasing hormone (GnRH) receptor (GnRHR). Mutations in the vassopresin V2 receptor (V2R) gene cause X-linked nephrogenic diabetes insipidus, a disease characterized by an inability to concentrate urine despite normal or elevated plasma concentrations of the antidiuretic hormone arginine vasopressin [46–49]. Nearly 70% of V2R mutants causing X-linked diabetes insipidus are unable to reach the cell surface membrane and respond to agonist stimulation [47–49]. In retinitis pigmentosa, ER trapping of misfolded mutant rhodopsin eventually leads to rod photoreceptor degeneration [50, 51] followed by cone degeneration. Mutations leading to receptor misfolding of the human GnRHR cause congenital hypogonadotropic hypogonadism (HH), a disease characterized by delayed puberty and reproductive failure due to partial or complete inability of the pituitary gonadotropes to respond to GnRH [52, 53]. The majority ( 90%) of the GnRHR mutants whose function has been examined to date are trafficking-defective receptors as disclosed by response to genetic maneuvers or pharmacological chaperones [3, 54–56]. Trafficking-defective mutants of the glycoprotein hormone receptors (luteinizing hormone, follicle-stimulating hormone, and thyrotropin receptors) have been described as a cause of Leydig cell hypoplasia [57, 58]. ovarian failure [59, 60] and congenital hypothyroidism [61, 62] respectively. The melanocortin-1 receptor has been found to be mutated in patients with skin and hair abnormalities, and increased susceptibility to skin cancers [63, 64]; among the 60 or so mutants described, at least four display decreased PM expression [63]. Misfolding and intracellular retention of mutants from two other melanocortin receptors, the melanocortin-3 and melanocortin-4 receptors associated with regulation of fat deposition and energy homeostasis, have been detected in patients with morbid obesity [65, 66].
Table 1.
Loss of function abnormalities caused by GPCR misfolding.
| Disease or Abnormality | GPCR | Examples of pharmacoperones tested in vitro and/or in vivo | Refs |
|---|---|---|---|
| Hypogonadotropic hypogonadism | GnRHR | IN3, IN30, Q89, A177775, TAK-013. | [21–27] |
| Nephrogenic diabetes insipidus | V2R | Satavaptan, relcovaptan, VPA-985, YM087, tolvaptan, OPC31260 | [28–34] |
| Retinitis pigmentosa | Rhodopsin | Retinoids (9-cis-retinal, 11-cis-retinal, 11-cis-7-ring retinal, vitamin A palmitate). | [35–40] |
| Congenital hypothyroidism | TSHR | ||
| Obesity | MC3R, MC4R | ML00253764 and compounds described in ref. | [41, 42, 65] |
| Ovarian failure | FSHR | Org41841 | [43] |
| Male pseudohermaphroditism | LHR | ||
| Familial hypocalciuric hypercalcemia | CaR | NPS R-568 | [44] |
| Red head color phenotype and propensity to skin cancer | MC1R | NBA-A | [45] |
| Familial glucocorticoid deficiency | MC2R | ||
| Hirschsprung’s disease | E-BR | ||
| Resistance to HIV-1 infection | CCR5 |
V2R: Vasopressin V2 receptor; GnRHR: Human gonadotropin-releasing hormone receptor; CaR: Calcium-sensing receptor; LHR: Luteinizing hormone receptor; FSHR: Follicle-stimulating hormone receptor; TSHR: Thyrotropin receptor; E-BR: Endothelin-B receptor; MC1R: Melanocortin-1 receptor; MC2R: Melanocortin-2 receptor [or adrenocorticotropin (ACTH) receptor]; MC3R: Melanocortin-3 receptor; MC4R: Melanocortin-4 receptor; CCR5: Chemokine receptor-5.
Mutations in the calcium-sensing receptor leading to intracellular retention of the abnormal receptor have been found in patients with familial hypocalciuric hypercalcemia [67], whereas mutations that provoke trapping of the endothelin-B receptor have been detected in a subset of patients with Hirschsprung’s disease or aganglionic megacolon [68, 69]. Intracellular retention of the chemokine receptor 5 at the ER has also been observed in a subset of subjects with resistance to HIV infection [70].
The present review focuses on how pharmacoperones interact with misfolded GPCRs to correct its outward export trafficking from the ER to the PM. Special emphasis is placed on the gonadotropin-releasing hormone receptor Fig. (1A), because of the great deal of experimental evidence available with this particular receptor [3, 7, 26, 71, 72].
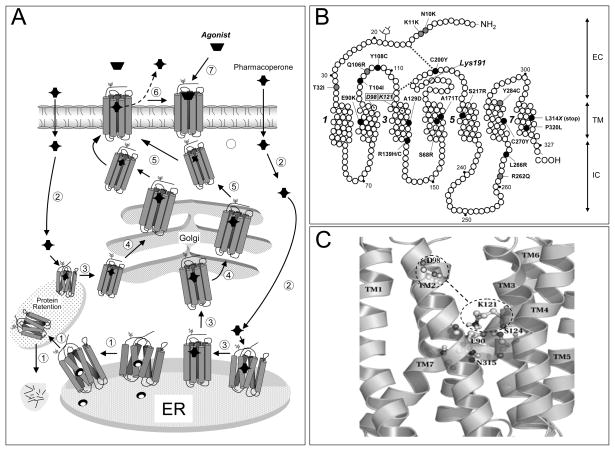
Fig. 1.
A: Quality control system in the endoplasmic reticulum (ER). Newly synthesized proteins (e.g. GPCRs) are translocated to the lumen of the ER where folding is facilitated by molecular chaperones (oval structures); if folding fails, misfolded proteins are retained in the ER and targeted for degradation by cytosolic proteasomes that cleaved the protein into fragments (short lines) (step1). Pharmacoperones diffuse into the cell (step 2) and selectively bind the misfolded protein to influence folding (step 3) and allow correct routing to the Golgi complex for further processing (e.g. glycosylation) (step 4). Previously synthesized misfolded proteins, retained by the QCS, may still be rescued by pharmacoperones (steps 2 and 3 at left). Mature proteins are then delivered to the cell surface membrane (step 5), where the pharmacoperone is dissociated from the receptor protein (step 6) allowing the receptor to interact with its cognate ligand (step 7). B: Location of the inactivating mutations in the human GnRHR. Grey ovals are mutations that lead to partial hypogonadotropic hypogonadism, whereas black ovals are mutations that provoke complete hypogonadotropic hypogonadism. Also shown is the location of K191 at the second extracellular loop, which destabilizes the formation of the C14-C200 bridge, as well as the location of D98 (in TM2 2) and K121 (in TM3) that form the surrogate D98-K121 salt bridge upon pharmacoperone action. Transmembrane domains 1, 3, 5, and 7 are numbered. EC: Extracellular domain; TM: Transmembrane domains; IC: Intracellular domains. C: Enlarged diagram showing specific interactions between E90 (in TM2), N315 (in TM7), K121 (in TM3) and S124 (in TM3), which form a microdomain important for GnRHR stability. D98 and K121 are shown within the dashed line ovals; the dashed line between the ovals represents the surrogate salt bridge that is formed upon pharmacoperone binding.
PHARMACOPERONES AND THE ER QUALITY CONTROL SYSTEM
Synthesis and processing of secretory and membrane proteins occurs in the ER and Golgi apparatus. The ER provides the specialized means necessary for folding, carbohydrates attachment and oligomeric assembly of thousands of proteins prior to their translocation to the Golgi where processing of the protein continues Fig. (1A). As proteins are synthesized in the ER, they fold and adopt distinct conformations that should be compatible with trafficking from the ER to other compartments of the cell (e.g. the plasma membrane) [8, 19, 73]. Newly synthesized proteins are monitored by a quality-control system (QCS) that guards against aberrant protein structures and checks for proper folding. By monitoring the structural correctness of newly synthesized proteins, the QCS prevents accumulation of defective (misfolded) proteins that may potentially accumulate, aggregate and interfere with normal cell function [73–76]. The scrutiny by the ER QCS relies on conformational features of the protein rather than on functional criteria, so even minor alterations in the secondary or tertiary structure of a protein may lead to intracellular retention and degradation [18, 77]. A variety of mechanisms operate at the ER to identify and sort proteins according to their maturation status. These mechanisms include specialized folding factors, escort proteins, retention factors, enzymes, and members of major molecular chaperone families [78–80]. Molecular chaperones are ER-resident proteins that bind to and stabilize unstable conformers of nascent polypeptides to assist in folding or assembly of the polypeptide for efficient ER export, preventing aggregation or incorrect interactions between misfolded proteins and other molecules. Although the steric character of the protein backbone restricts the spectrum of protein shapes that are recognized by the stringent quality control mechanisms, some features displayed by proteins, including hydrophobic shapes, unpaired cysteines, immature glycans, and particular sequence motifs, have been identified as important for chaperone-protein association [8, 77, 81, 82]. Proteins that do not fulfill the criteria of the ER QCS or whose aberrant structure (e.g. as a result of a mutation in their amino acid sequence) cannot be corrected by molecular chaperones, are submitted to degradation to the polyubiquitination/proteasome pathway Fig. (1) [18, 83].
In the last years, it has become evident that synthesis of certain membrane proteins is normally inefficient, which leads to restricted PM expression [84–89]. This is the case for example, of the olfatory receptors [86, 90], the human GnRHR [26, 87, 91], and the Δ-opioid receptor [89], whose efficiency of synthesis and trafficking to the PM is considerably reduced in normal conditions. Thus, the possibility that intracellularly retained misfolded or incompletely mature receptors may be rescued from ER trapping and degradation by drugs that act as chaperones (i.e. chemical chaperones and pharmacoperones) offers a unique opportunity for new therapeutic interventions in many diseases associated to protein misfolding.
Several in vitro approaches to correct folding and promote trafficking of the protein from the ER to the PM have been described. Studies on the biosynthesis of the cystic fibrosis transmembrane conductance regulator (CFTR) F508 deletion mutant (which leads to cystic fibrosis) showed that the mutation leads to subtle misfolding that does not grossly interfere with proper function but that leads to intracellular retention of the mutant protein [92]. Incubation of cells expressing this mutant at reduced temperatures (20–30° C) reverted processing of the CFTR mutant towards the wild-type (Wt) channel, allowing the cAMP-regulated Cl− channel to be expressed at the cell surface membrane [93]. Similarly, increased PM expression of several conformationally defective GnRHRs bearing different point mutations (see below), resulted from incubating transfected cells at lower (32° C) temperatures [18]. Thus, it appears that for certain temperature-sensitive, misfolded proteins, the use of physical methods (i.e. lowering incubation temperatures) prevent their retention by the ER QCS and facilitate the defective protein to traffic to their site of action. Another strategy to enhance PM expression of misfolded proteins is by introducing or deleting specific sequences into the conformationally abnormal protein (“genetic rescue”) [54, 82, 94]. This approach either over-expresses or stabilizes molecules rendered unstable by genetic defects and, in theory, does not provoke global changes in the ER secretory activity. For example, addition of carbohydrates [95] or amino-terminal sequences from the serotonin HT3 receptor [96] or rhodopsin [97], markedly enhanced PM targeting of inefficiently expressed odorant receptors. In the case of the mammalian GnRHR, which is unique among members of the GPCR superfamily in that it lacks the carboxyl-terminal extension [72], addition of this latter domain from other species (e.g. fish) or deletion of Lys191 (which restricts PM expression [98]), dramatically increased PM expression in both cases [99–101]. Genetic approaches, albeit effective, are probably impractical as therapeutic intervention because, if it were possible to access the gene sequence, the primary error could be directly corrected. Rescue of misfolded membrane receptors can also be achieved by manipulating ER and/or post-ER mechanisms that influence GPCR export, as is the case of the molecular chaperone calnexin [7, 102, 103]. In the GnRHR, calnexin seems to act as a quality control protein by retaining misfolded receptors and steering properly folded receptors to the PM. In fact, expression of Wt human GnRHR (whose maturation is normally inefficient [18, 104, 105]) with calnexin in COS-7 cells, decreased receptor expression and receptor-mediated second messenger production, an effect that was counteracted by using siRNA to knock down overexpressed calnexin [102]. In the case of the P23H mutation within the rhodopsin gene, which causes rhodopsin misfolding and autosomal dominant retinitis pigmentosa, overexpression of BiP/Grp78, which is an ER-localized molecular chaperone involved in the protective unfolded protein response (a cell stress program activated when misfolded proteins accumulate in the ER and that may later lead to apoptosis), led to reduction of photoreceptor apoptosis and retinal degeneration, and rescued retinal activity in rats expressing this rhodopsin mutant [106]. In this vein, Gebbink has described a method based on molecular chaperones (e.g. BiP) to decrease accumulation of misfolded proteins comprising precursors of crossbeta structures that eventually form amyloid deposits [107]. In another example, related to manipulation of ER mechanisms, butyric acid, which is a chemical chaperone (see below) known to reduce ER stress [18], has been proposed as a potentially useful agent to treat obesity and type 2 diabetes [108].
An additional strategy to manipulate the cellular QCS to enhance misfolded receptor PM expression is by employing cell-penetrating peptides that modify cytosolic Ca2+ stores, thereby affecting function of Ca2+-dependent chaperones as those involved in post-ER quality control [109]. Nevertheless, as in the case of chemical chaperones (see below), the major drawback of these maneuvers is their lack of specificity for the target protein.
Another effective strategy to rescue PM expression and function of misfolded membrane receptor is by incubating cells expressing the mutant protein with non-specific stabilizing agents (such as polyols and sugars) [5, 6, 33, 110]. Chemical chaperones are small molecular weight compounds that promote folding by stabilizing proteins without interacting with them or interfering with their function. Osmolites stabilize proteins by reducing the free movement of proteins [111], and by increasing their hydration [5, 112] preventing aggregation of incompletely folded conformers and promoting protein stabilization by modifying the free-energy difference between partially folded proteins and their more compact native structures [5]. For example, incubation of stable CFTR Fdes508 transfectants with the cellular osmolytes glycerol or trimethylamine N-oxide led to accumulation of functional Fdes508 protein and an increase in whole cell Cl− conductance [92, 93, 113].
In the case of temperature-sensitive mutants, such as the A135V mutant of the tumor suppressor protein p53 [114], when cells expressing this mutant were cultured at non-permissive temperatures, the mutant was localized in the cytoplasm and was biologically inactive unless cells were incubated in the presence of chemical chaperones, such as glycerol, trimethylamine N-oxide or heavy water (D2O) [92]. Chemical chaperones require high concentrations for effective folding of mutant proteins and hence are too toxic for in vivo applications. On the other hand, although chemical chaperones can rescue some misfolded proteins, they are nonspecific and might potentially increase secretion or intracellular retention of many different proteins in various cellular compartments leading to inappropriate changes in the levels and/or secretion of many proteins, thereby compromising cell function and/or local homeostatsis [2, 115]. Nevertheless, it has been observed that glycerol, 4-phenylbutyric acid and trimethylamine N-oxide may selectively increase the secretion efficiency of α-1-antitrypsin without influencing that of other proteins or decreasing proteasomal degradation [116, 117].
Pharmacoperones appear to be among the most promising therapeutic approaches to treat conformational diseases [1–7, 118, 119]. In contrast with chemical chaperones, pharmacoperones have the advantage of selective binding to the misfolded protein, which allows the beneficial (normal) degradation of other misfolded proteins that require to be eliminated from the cell as part of the normal process in protein biosynthesis. In transthyretin amyloidogenesis, for example, several small molecules bind with high affinity to binding sites within the transthyretin molecule leading to stabilization of the native state of the protein, decreasing the concentration of the intermediate species and thereby amyloid formation [120, 121]. Short β-sheet breaker peptides have been designed for blocking the aggregation undergone by β-amyloid [122]. These small molecules with a structure homologous to the central hydrophobic region of the fibril aggregate, inhibit and dissolve β-amyloid aggregates in vitro and in vivo [123–125]. Another example is the competitive inhibitor 1-deoxy-galactonojirimycin, which increases the activity of the R301Q mutant form of α-galactosidase A (whose accumulation in the ER leads to the lysosomal storage disease, Fabry’s disease in humans) and facilitates its transport from the ER to lysosomes [126–128].
It has been shown that agonists and antagonists of the GPCRs may promote cell surface delivery of the misfolded receptor from the ER [1–3, 7, 129, 130]. The efficiency of pharmacoperones to rescue PM expression and function will depend on the particular structure of the pharmacoperone (that determines selectivity toward the target protein), the severity of the folding defect present in the target protein and the particular location of the mutation (i.e. the mutation should not include critical residues involved in agonist binding, receptor activation or coupling to effectors). For example, in the case of the S168R and S217R human GnRHR mutants Fig. (1B), replacement of any of these serine residues (which in the three-dimensional structure of the receptor are located in the lipid membrane-contact phase of the helix) by the highly hydrophilic arginine involve a thermodynamically unfavorable exchange that rotates the TM4 and TM5, moving the EL2, and making formation of the Cys14-Cys200 bridge improbable [19]; in the human GnRHR, the Cys14-Cys200 bridge stabilizes the receptor in a conformation that is compatible with ER export[19, 131, 132]. Both mutants are completely resistant to pharmacoperone treatment in vitro [55, 132]. In the case of the P320L GnRHR mutant, the abnormal protein is unrescuable by genetic approaches because the peptide backbone of proline is constrained in a ring structure; in fact, occurrence of this amino acid is associated with a forced turn in the protein sequence of α-helices and its replacement may severely disturb the structure of the helix. Similarly, mutant human V2Rs displaying amino acid exchanges at the interface of the TM2 and TM4 (H80R, W164R, and S167L mutants), are resistant to pharmacoperone-mediated cell surface delivery, probably because the replacing residues lead to a severe folding defect [85]. Mutational defects that interfere with ligand binding would also be expected to show no rescue of second messenger coupling in response to pharmacoperones.
TREATMENT OF MISFOLDED GPCRS WITH PHARMACOPERONES
The Human GnRHR
Misfolded mutants from two distinct GPCRs, the GnRHR and the V2R, have been extensively tested for functional rescue with pharmacoperones. As shown in Fig. (1B), twenty-one inactivating mutations (including two leading to deletion of large sequences) in the human GnRHR have been described as a cause of HH [3, 52, 53]. Seven homozygous and 12 heterozygous combinations of GnRHR mutants are expressed by individuals exhibiting either partial or complete forms of HH [3, 53]. Expression of these GnRHR mutants in heterologous systems results in cells that neither bind GnRH agonists nor respond to GnRH stimulation by effector activation. These observations initially suggested that such mutations were associated with alterations in agonist binding, receptor activation or interaction with coupled effectors. However, the majority ( 90%) of GnRHR mutants, whose function has been examined to date (19 mutants), are trafficking-defective receptors as disclosed by mutational studies and/or response to pharmacoperones [53]. Such ER-retained mutants frequently show a change in residue charge compared with the Wt receptor (e.g. the E90K mutant receptor), or gain or loss of either cysteine (an amino acid known to form bridges associated with the formation of third order structure of proteins) (e.g. the Y108C and C200T mutants) or proline (e.g. the P320L mutant) Fig. (1B) residues [53].
The ability of different GnRHR peptidomimetics to rescue defective GnRHR mutants has been extensively analyzed [21–24, 55, 56, 133]. The peptidomimetics assessed as potential pharmacoperones came from four different chemical classes: indoles, quinolones, thienopyrimidinediones, and erythromycin-derived macrolides Fig. (2) and Table 1, which were originally developed as GnRH peptidomimetic antagonists [21–25]. These particular pharmacoperones were selected for study as potential pharmacoperones considering their predicted ability to permeate the cell membrane and specifically bind the GnRHR with a known affinity, rather than for their originally described actions as antagonists. In fact, rescue of misfolded GPCRs might also be achieved by agonists of the natural ligand [129, 130].
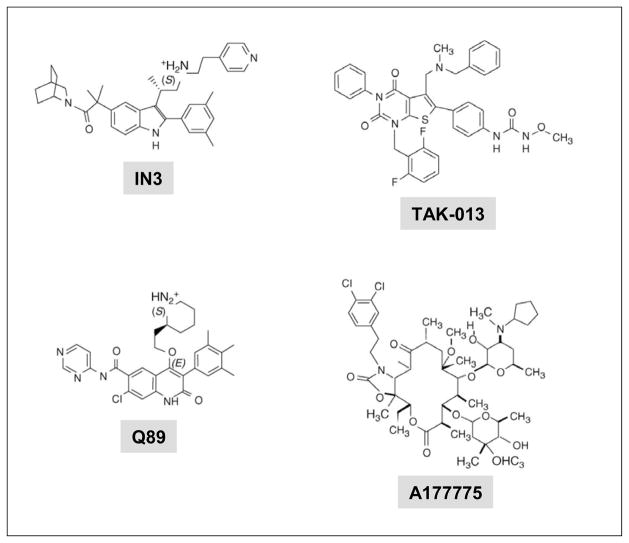
Fig. 2.
The structure of four representative pharmacoperones from different chemical classes. IN3 and Q89 (Merck and Company); TAK-013 (Takeda Chemical Industries, Ltd., Osaka 532–8686, Japan). and A177775 (Abbott Laboratories, Abbott Park, IL, USA) [24].
The first pharmacoperone tested, IN3 (((2S)-2-[5-[2-(2-azabicyclo[2.2.2]oct-2-yl)-1,1-dimethyl-2-oxoethyl] 2-(3,5-dimethylphenyl)-1H-indol-3-yl]-N-(2-pyridin-4-ylethyl) propan-1-amine (Merck and Company, Rahway, NJ, USA)) belongs to the indole class and provided the first proof of principle for rescuing misfolded human GnRHRs [55, 104, 134]. Further studies then examined the efficacy of chemically distinct drugs [indoles (e.g. IN3), quinolones (e.g. Q89: (7-chloro-2-oxo-4-{2-[(2S)-piperidin-2-yl]ethoxy}-N-pyrimidin-4-yl-3-(3,4,5-trimethylphenyl)-1,2-dihydroquinoline-6-carboxaminde) (Merck and Company)), and erythromycin-derived macrolides (e.g. A177775: [3′-N-desmethyl-3′-N-cyclopenty1–11-deoxy-11-[carboxy-(3,4-dichlorophenylethylamino)]-6-O-methyl-erythromycin A11,12-(cyclic carbamate)] (Abbott Laboratories, Abbott Park, IL, USA))] Fig. (2) as pharmacoperones for a palette of misfolded mutant GnRHRs [24]. These studies demonstrated that all but three [S168R, S217R, and L314X(stop)] of the 17 mutants tested were partially or completely rescued with pharmacoperones [26, 55, 118, 134]. The efficacy of these drugs (measured by the ability of a fixed dose of the pharmacoperone to rescue receptor function in terms of inositol phosphate production in response to agonist) was proportional to the binding affinity of the pharmacoperone for the Wt receptor and, in general, there was a lack of rescue specificity for the different drugs (i.e. all effective agents rescued virtually the same mutants) [24], an expected finding considering that these molecules were originally designed as GnRH peptidomimetics and thus would presumably compete with the natural ligand for receptor occupancy [135] (see below). All peptidomimetics studied with an IC50 value (for Wt GnRHR) ≤ 2.3 nM had measurable efficacy in rescuing GnRHR mutants, and within a single chemical class, this ability correlated to these IC50 values. Among the most effective pharmacoperones tested, the indole IN30 seems to be the most potent based on the IC50 value (0.2 nM) for the Wt GnRHR, followed by the quinolone Q89 (IC50 0.3 nM), IN3 (IC50 0.6 nM), and the erythromycin macrolides A177775 (IC50 17.7 nM) and A2222509 (IC50 20 nM) [24]. As mentioned above, the S168R and S217R GnRHRs are mutants in which the thermodynamic changes leading to receptor distortion are too severe to allow stabilization by pharmacoperones [19]. Accordingly, even though these two mutants are not rescued by these compounds, their failure to route correctly is attributable to misfolding, and probably not to an intrinsic inability to potentially participate in particular receptor functions.
Other GPCRs
In addition to the misfolded GnRHR mutants described above, for which non-peptide antagonists have proved to be useful as pharmacoperones, there are other conformationally defective GPCRs in which these drugs have been demonstrated to be efficacious in rescuing function or preventing abnormal accumulation of the defective molecule, Table 1. In the case of the V2R, it has been shown that distinct cell membrane permeable antagonists effectively rescue in vitro function of several misfolded, traffic-defective mutants that cause diabetes insipidus in humans [28–30, 46, 136]. These findings are important since the majority (~70%) of V2R mutations leading to disease are due to receptor misrouting [47]. Recently, the effect of the peptidomimetic V1AR/V2R antagonist SR49059 [30] to rescue function of R137H, W164S, and des185–193 V2R mutants in patients with nephrogenic diabetes insipidus has been examined [46]. This short trial, that had to be interrupted during the course of the study as a result of possible interference with the cytochrome P450 metabolic pathway, revealed a drop in urine production and water intake as well as a significant increase in urine osmolarity in response to this particular compound. In rhodopsin retinitis pigmentosa, the vast majority of mutations ( 89%) affects folding of the receptor protein leading to decreased PM expression, intracellular retention, aggregation, and eventually cell death (see above). Rescue of the T17M and P23H mutants associated with retinitis pigmentosa has been achieved by in vitro exposure of the cells to 11-cis-retinal or 11-cis-7-ring-retinal (a seven-membered ring variant of 11-cis-retinal), the chromophore of rhodopsin that plays a central role in the photoactivation process [35, 36, 39]. Further, in transgenic mice bearing the T17M opsin mutation, a high vitamin A diet given for 4 mo was followed by a significant decrease in retinal degeneration, without causing liver toxicity [40]. In the vast majority of melanocortin-4 receptor (MC4R)(a GPCR involved in energy homeostasis) mutants, which may lead to monogenic obesity, the mutant receptors are retained intracellularly due to misfolding [42, 65]. Recent studies on a palette of misfolded mutant MC4Rs demonstrated that the pharmacoperone ML00253764 [65, 137] and 4-phenylbutyric acid rescued PM expression and function of the mutant receptors to different extents [42, 65, 138]. In the case of PM expression-deficient μ-opioid receptors and melanin concentrating hormone receptor-1 mutants, different cell permeable agonists and antagonists also have been shown to effectively enhance cell surface expression of the mutant receptors [45, 139].
The overall data indicate that pharmacoperones represent a novel approach for the potential development of defined therapeutic strategies for an array of diseases caused by incorrectly routed GPCRs.
MECHANISM OF ACTION OF PHARMACOPERONES
In general, desirable characteristics of molecules that could function as pharmacoperones for misfolded proteins include: i. Ability to reach physiologically acceptable concentrations; ii. Cell permeability; iii. Ability to reach and intervene at the ER and/or post-ER compartments where the misfolded protein is retained; iv. Ability to remain undegraded long enough to stabilize the target mutant; v. Specificity for the target protein; and vi. To reversibly bind the misfolded protein so they may dissociate from the target molecule after its localization at the correct cellular destination (e.g. the plasma membrane) or, alternatively, not to compete with the natural ligand binding site. Pharmacoperones can correct folding of defective proteins allowing that the mutant could escape the ER QCS and traffic to the PM or interfering with its aggregation or degradation. The mechanism(s) by which pharmacoperones stabilize and rescue PM expression of the target receptor is still speculative and current information is mostly based on theoretical predictions of protein structure and drug interactions [85, 140, 141]. Two mechanisms have been proposed to explain the ability of pharmacoperones to stabilize misfolded proteins. Pharmacoperones may bind to and enhance the stability of the native or native-like state of the target protein for which they have higher affinity than for intermediate, immature forms (i.e. non-native structures) or, alternatively, they may bind to the less folded, non-native folding intermediates and act as a scaffold for subsequent folding, increasing the rate at which these intermediates are converted to the native form. This would prevent the protein from being recognized by the ER QCS as defective, allowing it to escape degradation and promoting its transport to the Golgi apparatus for further processing [5, 36].
In the case of the human GnRHR, we have shown that one particular mutant, the E90K mutant is completely recued by genetic or pharmacological maneuvers [54, 55]. Computational human GnRHR models showed that residue E90, in TM1, forms a salt bridge with K121 [72, 142, 143] Fig. (1C); as a consequence of the mutation, this bridge is lost in the E90K receptor. This particular residue is highly conserved in the GnRHR of virtually all mammals as well as in fish, birds and reptiles [72]. The finding that all pharmacoperones tested have been able to completely rescue function and PM expression of this mutant [24] led us to analyze the chemical relation between the E90-K121 bridge (which is lost in the E90K mutation) and these drugs. Mutational and computational methods led to identify that pharmacoperones appear to act by forming an alternative bridge between D98 (at the extracellular face of TM2) and K121, which in turn functions as a surrogate bridge for the original (E90-K121) bridge disrupted by the substitution [118, 140]. Since GnRH includes, as contact points, D98 and K121, it was not surprising that competitors of GnRH may interact at or near the ligand binding site, which resides in the lateral plane of the PM, a region having a high percentage of hydrophobic residues [142]. In fact, analysis of the linear sequences of both E90 and K121 show that they are hydrophobic regions with a modest number of ionic or polar groups. The observation of this conserved ionic site strongly suggests that the pharmacoperones tested [IN3, Q89, A177775, and TAK-013: (N-{4-[5-{[benzyl(methyl)amino]methyl}-1-(2,6-difluorobenzyl)-2,4-dioxo-3-phenyl-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-N′-methoxyurea) (Takeda Chemical Industries, Ltd. Osaka 532–8686, Japan)], Fig. (2) were all chosen on the basis of this preferential ion-pair and/or polar interaction with the charged residues. The observation that the pharmacoperones tested to date rescue most of the GnRHR mutants, no matter the distribution of the mutations along the sequence of the receptor Fig. (1B), suggests that the E90-K121 bridge represents an additional critical core that, once stabilized, forms a conformer that bypasses the scrutiny of the QCS. For the GnRHR this core might stabilize the orientation of, and relation between TMs 2 and 3, as the C14-C200 bridge does with the second extracellular loop and the amino-terminus, and indirectly the TMs 4 and 5. In fact, complete rescue of the human GnRHR Y108C mutant (which favors formation of C108-C200 incorrect disulfide bridge) with pharmacoperones cannot be achieved unless formation of the abnormal disulfide bridge is prevented (by replacing the cysteine residue at position 200 with alanine) and K191 is deleted (which obviates the need to form the C14-C200 bridge).
One interesting observation is that pharmacoperones do not necessarily need to be present at the time of mutant synthesis Fig. (1A) as shown by studies with ER-retained human GnRHR and murine V2R, whose PM expression was restored even in conditions where protein synthesis was abolished [85, 109, 144]. These observations are important in that they suggest that the time and pattern of pharmacoperone administration do not need to be considered when attempting to translate the concept of pharmacoperone rescue to clinical practice.
CURRENT AND FUTURE DEVELOPMENTS
The growing number of examples of misfolded GPCRs as a cause of genetic disease indicates that misfolding, retention, and/or aggregation of conformationally abnormal receptors may be more common than previously recognized. The various approaches described in this review to correct misrouting of trafficking defective, misfolded GPCRs have proven useful in enhancing the plasma membrane expression of various GPCRs in heterologous cell systems. Of these, pharmacoperones represent the optimal therapeutic strategy for treating genetic diseases due to GPCR misfolding since they potentially guarantee selective rescue of a target receptor. In fact, a number of compounds (agonists, antagonists, partial agonists and/or inverse agonists) obtained from high-throughput screening strategies currently are under study for their potential application as pharmacoperones to treat diseases caused by misfolded GPCRs, such as HH, retinitis pigmentosa, and obesity [27, 37, 144, 145].
One problem in translating the concept of pharmacoperone rescue from the laboratory to the clinic is that the majority of the pharmacoperones for GPCRs (see above examples and Table 1) tested are competitive antagonists or agonists of the natural ligand, which in most cases makes it necessary to remove the drug after rescue in order to allow the receptor to bind agonist and become activated. The use of pharmacoperones that behave as allosteric modulators and that can stabilize the misfolded receptor without inhibiting endogenous (or exogenous) agonists or altering, in a significant manner, the affinity of the rescued receptor for its cognate ligand and its capability to become activated, seems an attractive alternative to solving this problem [44]. In fact, one pharmacoperone drug has been recently identified that does not seem to compete for the agonist binding site at the follicle-stimulating hormone receptor; this pharmacoperone is a thienopyr(im)idine (Org41841) that activates the luteinizing hormone receptor but neither competes with the natural ligand binding site nor shows agonistic action on the follicle-stimulating hormone receptor at sub-millimolar concentrations. Incubation of this drug at sub-micromolar concentrations, with host cells expressing the human follicle-stimulating receptor, increased PM expression of the wild-type receptor and partially rescued function of the misfolded A189V mutant [43], which leads to subfertility in men and primary ovarian failure in women [59, 146]. On the other hand, a drawback presented by GPCR agonists as pharmacoperones is that they may potentially lead to desensitization and internalization of the receptor once rescue is achieved. This problem, however, may be solved by agonist molecules that display selective properties (i.e. biased agonism), including inability to recruit arrestins and trigger receptor internalization. In this vein, biased agonists that restored PM trafficking and promoted activity of nephrogenic diabetes insipidus-causing misfolded V2R mutants without stimulating receptor internalization have recently been described [147]. One additional advantage of this strategy is that the presence of the biased agonist also may potentially interfere with the internalization of the target receptor provoked by the endogenous ligand.
In addition to non-peptide agonists and antagonists, which specifically bind misfolded receptors and promote PM delivery, new classes of substances that allow controlling the QCS and enhancing PM expression of GPCRs have been described [33]. The compounds thapsigargin, curcumin, and ionomycin, for example, promote the release of Ca2+ into the cytosol thereby affecting molecular chaperone function and promoting PM rescue of misfolded V2Rs [33]. Likewise, peptide compounds that penetrate into the ER-Golgi intermediate compartment via the retrograde transport pathway but fail to reach the ER, promote PM trafficking of misfolded receptors retained in that particular location by selectively promoting Ca2+ release [49, 109]. However, since these particular compounds do not correct folding (i.e. they are not true pharmacoperones), trafficking to the PM might not always be accompanied by functional rescue of the misfolded receptor. These compounds, along with others that may interfere with dimerization of receptors at the ER and prevent the dominant negative effects of misfolded mutants on Wt receptor PM expression [148, 149], might still be used to rebalance proteostasis and synergize the effect of pharmacoperones in increasing the population of receptors that can engage the upward trafficking machinery.
Acknowledgments
Supported by grants (NIH DK-85040, TW/HD-00668, RR-00163, and HD-18185) to P. Michael Conn, and grant 86881 from CONACyT, Mexico (to Alfredo Ulloa-Aguirre). Alfredo Ulloa-Aguirre is a recipient of a Research Career Development Award from the Fundación IMSS, México.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Bernier V, et al. Pharmacological chaperone action on G-protein-coupled receptors. Curr Opin Pharmacol. 2004;4:528–533. doi: 10.1016/j.coph.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Bernier V, et al. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Conn PM, Ulloa-Aguirre A. Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs. Trends Endocrinol Metab. 2010;21:190–197. doi: 10.1016/j.tem.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulloa-Aguirre A, et al. Misrouted cell surface receptors as a novel disease aetiology and potential therapeutic target: the case of hypogonadotropic hypogonadism due to gonadotropin-releasing hormone resistance. Expert Opin Ther Targets. 2003;7:175–185. doi: 10.1517/14728222.7.2.175. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa T, et al. Small molecule pharmacological chaperones: From thermodynamic stabilization to pharmaceutical drugs. Biochim Biophys Acta. 2006;1764:1677–1687. doi: 10.1016/j.bbapap.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- 7.Conn PM, et al. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 8.Ulloa-Aguirre A, Conn PM. Targeting of G protein-coupled receptors to the plasma membrane in health and disease. Front Biosci. 2009;14:973–994. doi: 10.2741/3290. Review. [DOI] [PubMed] [Google Scholar]
- 9.Wustman B, Do HV. Treatment of Pompe Disease with Specific Pharmacological Chaperones and Monitoring Treatment Using Surrogate Markers. Amicus Therapeutics, Inc; 2009. [Google Scholar]
- 10.Ulloa-Aguirre A, Conn PM. G protein-coupled receptors and the G protein family. In: Conn PM, editor. Handbook of Physiology, Section 7. Oxford University Press; New York: 1998. pp. 87–124. [Google Scholar]
- 11.Ulloa-Aguirre A, et al. Structure-activity relationships of G protein-coupled receptors. Arch Med Res. 1999;30:420–435. doi: 10.1016/s0188-0128(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 12.Fredriksson R, et al. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 13.Gershengorn MC, Osman R. Minireview: Insights into G protein-coupled receptor function using molecular models. Endocrinology. 2001;142:2–10. doi: 10.1210/endo.142.1.7919. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum DM, et al. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 16.Karnik SS, et al. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol Metab. 2003;14:431–437. doi: 10.1016/j.tem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Ulloa-Aguirre A, et al. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic (Copenhagen, Denmark) 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 19.Ulloa-Aguirre A, et al. G-protein-coupled receptor trafficking: understanding the chemical basis of health and disease. ACS Chem Biol. 2006;1:631–638. doi: 10.1021/cb600360h. [DOI] [PubMed] [Google Scholar]
- 20.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 21.Ashton WT, et al. Orally bioavailable, indole-based nonpeptide GnRH receptor antagonists with high potency and functional activity. Bioorg Med Chem Lett. 2001;11:2597–2602. doi: 10.1016/s0960-894x(01)00512-1. [DOI] [PubMed] [Google Scholar]
- 22.Ashton WT, et al. Substituted indole-5-carboxamides and -acetamides as potent nonpeptide GnRH receptor antagonists. Bioorg Med Chem Lett. 2001;11:1723–1726. doi: 10.1016/s0960-894x(01)00274-8. [DOI] [PubMed] [Google Scholar]
- 23.Ashton WT, et al. Potent nonpeptide GnRH receptor antagonists derived from substituted indole-5-carboxamides and -acetamides bearing a pyridine side-chain terminus. Bioorg Med Chem Lett. 2001;11:1727–1731. doi: 10.1016/s0960-894x(01)00275-x. [DOI] [PubMed] [Google Scholar]
- 24.Janovick JA, et al. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–614. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki S, et al. Discovery of a thieno[2,3-d]pyrimidine-2,4-dione bearing a p-methoxyureidophenyl moiety at the 6-position: a highly potent and orally bioavailable non-peptide antagonist for the human luteinizing hormone-releasing hormone receptor. J Med Chem. 2003;46:113–124. doi: 10.1021/jm020180i. [DOI] [PubMed] [Google Scholar]
- 26.Conn PM, Janovick JA. Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol. 2009;299:137–145. doi: 10.1016/j.mce.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conn PM. Rescue of Gonadotropin Hormone Receptor Mutants. Oregon Health & Science University; United States: 2010. [Google Scholar]
- 28.Albright JD, et al. 5-Fluoro-2-methyl-N-[4-(5H-pyrrolo[2,1-c]-[1, 4]benzodiazepin-10(11H)-ylcarbonyl)-3-chlorophenyl]benzamide (VPA-985): an orally active arginine vasopressin antagonist with selectivity for V2 receptors. J Med Chem. 1998;41:2442–2444. doi: 10.1021/jm980179c. [DOI] [PubMed] [Google Scholar]
- 29.Morello JP, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serradeil-Le Gal C, et al. Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest. 1996;98:2729–2738. doi: 10.1172/JCI119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahara A, et al. Pharmacological profile of YM087, a novel potent nonpeptide vasopressin V1A and V2 receptor antagonist, in vitro and in vivo. J Pharmacol Exp Ther. 1997;282:301–308. [PubMed] [Google Scholar]
- 32.Hawtin SR. Pharmacological chaperone activity of SR49059 to functionally recover misfolded mutations of the vasopressin V1a receptor. J Biol Chem. 2006;281:14604–14614. doi: 10.1074/jbc.M511610200. [DOI] [PubMed] [Google Scholar]
- 33.Robben JH, et al. Rescue of vasopressin V2 receptor mutants by chemical chaperones: specificity and mechanism. Mol Biol Cell. 2006;17:379–386. doi: 10.1091/mbc.E05-06-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robben JH, et al. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2007;292:F253–260. doi: 10.1152/ajprenal.00247.2006. [DOI] [PubMed] [Google Scholar]
- 35.Noorwez SM, et al. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem. 2003;278:14442–14450. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noorwez SM, et al. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem. 2004;279:16278–16284. doi: 10.1074/jbc.M312101200. [DOI] [PubMed] [Google Scholar]
- 37.Ostrov DA, et al. Opsin Stabilizing compounds and Methods of Use. University of Florida Research Foundation, Inc; Gainesville, FL, US United States: 2009. [Google Scholar]
- 38.Sung CH, et al. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krebs MP, et al. Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J Mol Biol. 2010;395:1063–1078. doi: 10.1016/j.jmb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Li T, et al. Effect of vitamin A supplementation on rhodopsin mutants threonine-17 --> methionine and proline-347 --> serine in transgenic mice and in cell cultures. Proc Natl Acad Sci U S A. 1998;95:11933–11938. doi: 10.1073/pnas.95.20.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J-q, et al. Pharmacological Chaperones for Treating Obesity. Amicus Therapeutics, Inc; Cranbury, NJ, US: Universite de Montreal; (Montreal, QC, CA): United States: 2009. [Google Scholar]
- 42.Fan ZC, Tao YX. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J Cell Mol Med. 2009;13:3268–3282. doi: 10.1111/j.1582-4934.2009.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janovick JA, et al. Increased plasma membrane expression of human follicle-stimulating hormone receptor by a small molecule thienopyr(im)idine. Mol Cell Endocrinol. 2009;298:84–88. doi: 10.1016/j.mce.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. The Journal of biological chemistry. 2007;282:9517–9525. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 45.Fan J, et al. A point mutation in the human melanin concentrating hormone receptor 1 reveals an important domain for cellular trafficking. Mol Endocrinol. 2005;19:2579–2590. doi: 10.1210/me.2004-0301. [DOI] [PubMed] [Google Scholar]
- 46.Bernier V, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 47.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis. 2006;13:96–104. doi: 10.1053/j.ackd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Fujiwara TM, Bichet DG. Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol. 2005;16:2836–2846. doi: 10.1681/ASN.2005040371. [DOI] [PubMed] [Google Scholar]
- 49.Hermosilla R, et al. Disease-causing V(2) vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5:993–1005. doi: 10.1111/j.1600-0854.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 50.Mendes HF, et al. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Saliba RS, et al. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 52.Beranova M, et al. Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2001;86:1580–1588. doi: 10.1210/jcem.86.4.7395. [DOI] [PubMed] [Google Scholar]
- 53.Ulloa-Aguirre A, et al. Misrouted cell surface GnRH receptors as a disease aetiology for congenital isolated hypogonadotrophic hypogonadism. Hum Reprod Update. 2004;10:177–192. doi: 10.1093/humupd/dmh015. [DOI] [PubMed] [Google Scholar]
- 54.Maya-Nunez G, et al. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab. 2002;87:2144–2149. doi: 10.1210/jcem.87.5.8386. [DOI] [PubMed] [Google Scholar]
- 55.Leanos-Miranda A, et al. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–4828. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- 56.Leanos-Miranda A, et al. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–3008. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- 57.Gromoll J, et al. Homozygous mutation within the conserved Ala-Phe-Asn-Glu-Thr motif of exon 7 of the LH receptor causes male pseudohermaphroditism. Eur J Endocrinol. 2002;147:597–608. doi: 10.1530/eje.0.1470597. [DOI] [PubMed] [Google Scholar]
- 58.Martens JW, et al. Mutant luteinizing hormone receptors in a compound heterozygous patient with complete Leydig cell hypoplasia: abnormal processing causes signaling deficiency. J Clin Endocrinol Metab. 2002;87:2506–2513. doi: 10.1210/jcem.87.6.8523. [DOI] [PubMed] [Google Scholar]
- 59.Aittomaki K, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 60.Tranchant T, et al. Preferential beta-arrestin signalling at low receptor density revealed by functional characterization of the human FSH receptor A189 V mutation. Mol Cell Endocrinol. 2011;331:109–118. doi: 10.1016/j.mce.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Biebermann H, et al. Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. J Clin Endocrinol Metab. 1997;82:3471–3480. doi: 10.1210/jcem.82.10.4286. [DOI] [PubMed] [Google Scholar]
- 62.Calebiro D, et al. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- 63.Beaumont KA, et al. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- 64.Beaumont KA, et al. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- 65.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine reviews. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144:4544–4551. doi: 10.1210/en.2003-0524. [DOI] [PubMed] [Google Scholar]
- 67.D’Souza-Li L, et al. Identification and functional characterization of novel calcium-sensing receptor mutations in familial hypocalciuric hypercalcemia and autosomal dominant hypocalcemia. J Clin Endocrinol Metab. 2002;87:1309–1318. doi: 10.1210/jcem.87.3.8280. [DOI] [PubMed] [Google Scholar]
- 68.Fuchs S, et al. Functional characterization of three mutations of the endothelin B receptor gene in patients with Hirschsprung’s disease: evidence for selective loss of Gi coupling. Mol Med. 2001;7:115–124. [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka H, et al. Novel mutations of the endothelin B receptor gene in patients with Hirschsprung’s disease and their characterization. J Biol Chem. 1998;273:11378–11383. doi: 10.1074/jbc.273.18.11378. [DOI] [PubMed] [Google Scholar]
- 70.Rana S, et al. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the delta ccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maya-Nunez G, et al. Biochemical mechanism of pathogenesis of human gonadotropin-releasing hormone receptor mutants Thr104Ile and Tyr108Cys associated with familial hypogonadotropic hypogonadism. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.01.016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Millar RP, et al. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 73.Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu Rev Cell Dev Biol. 2003;19:649–676. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- 74.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 75.Helenius A. Quality control in the secretory assembly line. Philos Trans R Soc Lond B Biol Sci. 2001;356:147–150. doi: 10.1098/rstb.2000.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 77.Angelotti T, et al. Regulation of G-protein coupled receptor traffic by an evolutionary conserved hydrophobic signal. Traffic. 2010;11:560–578. doi: 10.1111/j.1600-0854.2010.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brooks DA. Introduction: molecular chaperones of the ER: their role in protein folding and genetic disease. Semin Cell Dev Biol. 1999;10:441–442. doi: 10.1006/scdb.1999.0314. [DOI] [PubMed] [Google Scholar]
- 79.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 80.Morello JP, et al. Pharmacological chaperones: a new twist on receptor folding. Trends Pharmacol Sci. 2000;21:466–469. doi: 10.1016/s0165-6147(00)01575-3. [DOI] [PubMed] [Google Scholar]
- 81.Dong C, et al. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dunham JH, Hall RA. Enhancement of the surface expression of G protein-coupled receptors. Trends Biotechnol. 2009;27:541–545. doi: 10.1016/j.tibtech.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Werner ED, et al. Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci U S A. 1996;93:13797–13801. doi: 10.1073/pnas.93.24.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schubert U, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 85.Wuller S, et al. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem. 2004;279:47254–47263. doi: 10.1074/jbc.M408154200. [DOI] [PubMed] [Google Scholar]
- 86.Kato A, Touhara K. Mammalian olfactory receptors: pharmacology, G protein coupling and desensitization. Cell Mol Life Sci. 2009;66:3743–3753. doi: 10.1007/s00018-009-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conn PM, et al. ‘Effective inefficiency’: cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol. 2006;190:13–16. doi: 10.1677/joe.1.06771. [DOI] [PubMed] [Google Scholar]
- 88.Conn PM, et al. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol. 2006;20:3035–3041. doi: 10.1210/me.2006-0066. [DOI] [PubMed] [Google Scholar]
- 89.Petaja-Repo UE, et al. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 90.Lu M, et al. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic. 2003;4:416–433. doi: 10.1034/j.1600-0854.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 91.Re M, et al. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One. 2010;5:e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown CR, et al. Correcting temperature-sensitive protein folding defects. J Clin Invest. 1997;99:1432–1444. doi: 10.1172/JCI119302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown CR, et al. Strategies for correcting the delta F508 CFTR protein-folding defect. J Bioenerg Biomembr. 1997;29:491–502. doi: 10.1023/a:1022491124939. [DOI] [PubMed] [Google Scholar]
- 94.Schulein R, et al. Functional rescue of the nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutants G185C and R202C by a second site suppressor mutation. J Biol Chem. 2001;276:8384–8392. doi: 10.1074/jbc.M007045200. [DOI] [PubMed] [Google Scholar]
- 95.Katada S, et al. Structural determinants for membrane trafficking and G protein selectivity of a mouse olfactory receptor. J Neurochem. 2004;90:1453–1463. doi: 10.1111/j.1471-4159.2004.02619.x. [DOI] [PubMed] [Google Scholar]
- 96.Wetzel CH, et al. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus Laevis oocytes. J Neurosci. 1999;19:7426–7433. doi: 10.1523/JNEUROSCI.19-17-07426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krautwurst D, et al. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell. 1998;95:917–926. doi: 10.1016/s0092-8674(00)81716-x. [DOI] [PubMed] [Google Scholar]
- 98.Arora KK, et al. Influence of a species-specific extracellular amino acid on expression and function of the human gonadotropin-releasing hormone receptor. Mol Endocrinol. 1999;13:890–896. doi: 10.1210/mend.13.6.0291. [DOI] [PubMed] [Google Scholar]
- 99.Lin X, et al. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998;12:161–171. doi: 10.1210/mend.12.2.0056. [DOI] [PubMed] [Google Scholar]
- 100.Maya-Nunez G, et al. Combined modification of intracellular and extracellular loci on human gonadotropin-releasing hormone receptor provides a mechanism for enhanced expression. Endocrine. 2000;13:401–407. doi: 10.1385/ENDO:13:3:401. [DOI] [PubMed] [Google Scholar]
- 101.Jardon-Valadez E, et al. Conformational effects of Lys191 in the human GnRHR: mutagenesis and molecular dynamics simulations studies. J Endocrinol. 2009;201:297–307. doi: 10.1677/JOE-08-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brothers SP, et al. Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression. J Mol Endocrinol. 2006;37:479–488. doi: 10.1677/jme.1.02142. [DOI] [PubMed] [Google Scholar]
- 103.Morello JP, et al. Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochemistry. 2001;40:6766–6775. doi: 10.1021/bi002699r. [DOI] [PubMed] [Google Scholar]
- 104.Janovick JA, et al. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 105.Janovick JA, et al. Evolved regulation of gonadotropin-releasing hormone receptor cell surface expression. Endocrine. 2003;22:317–327. doi: 10.1385/ENDO:22:3:317. [DOI] [PubMed] [Google Scholar]
- 106.Gorbatyuk MS, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gebbink MFBG, Bouma B. Methods of binding of Cross-Beta Structures by Chaperones. United States: 2010. [Google Scholar]
- 108.Hotamisligil GS, Ozcan U. Reducing ER Stress in the Treatment of Obesity and Diabetes. Harvard University; (Cambridge, MA, US): United States: 2010. [Google Scholar]
- 109.Oueslati M, et al. Rescue of a nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutant by cell-penetrating peptides. J Biol Chem. 2007;282:20676–20685. doi: 10.1074/jbc.M611530200. [DOI] [PubMed] [Google Scholar]
- 110.Leandro P, Gomes CM. Protein misfolding in conformational disorders: rescue of folding defects and chemical chaperoning. Mini Rev Med Chem. 2008;8:901–911. doi: 10.2174/138955708785132783. [DOI] [PubMed] [Google Scholar]
- 111.Sampedro JG, Uribe S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol Cell Biochem. 2004;256–257:319–327. doi: 10.1023/b:mcbi.0000009878.21929.eb. [DOI] [PubMed] [Google Scholar]
- 112.Gekko K, Timasheff SN. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry. 1981;20:4667–4676. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- 113.Brown CR, et al. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Michalovitz D, et al. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990;62:671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- 115.Castro-Fernandez C, et al. Beyond the signal sequence: protein routing in health and disease. Endocr Rev. 2005;26:479–503. doi: 10.1210/er.2004-0010. [DOI] [PubMed] [Google Scholar]
- 116.Burrows JA, et al. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 118.Conn PM, Janovick JA. Drug development and the cellular quality control system. Trends Pharmacol Sci. 2009;30:228–233. doi: 10.1016/j.tips.2009.02.002. Review. [DOI] [PubMed] [Google Scholar]
- 119.Nakamura M, et al. Specific ligands as pharmacological chaperones: The transport of misfolded G-protein coupled receptors to the cell surface. IUBMB Life. 2010;62:453–459. doi: 10.1002/iub.344. [DOI] [PubMed] [Google Scholar]
- 120.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 121.Hammarstrom P, et al. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 122.Soto C. Protein misfolding and disease; protein refolding and therapy. FEBS letters. 2001;498:204–207. doi: 10.1016/s0014-5793(01)02486-3. [DOI] [PubMed] [Google Scholar]
- 123.Sigurdsson EM, et al. In vivo reversal of amyloid-beta lesions in rat brain. J Neuropathol Exp Neurol. 2000;59:11–17. doi: 10.1093/jnen/59.1.11. [DOI] [PubMed] [Google Scholar]
- 124.Estrada LD, Soto C. Inhibition of protein misfolding and aggregation by small rationally-designed peptides. Curr Pharm Des. 2006;12:2557–2567. doi: 10.2174/138161206777698792. [DOI] [PubMed] [Google Scholar]
- 125.Estrada LD, Soto C. Disrupting beta-amyloid aggregation for Alzheimer disease treatment. Curr Top Med Chem. 2007;7:115–126. doi: 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- 126.Fan JQ, et al. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 127.Ishii S, et al. Mutant alpha-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J. 2007;406:285–295. doi: 10.1042/BJ20070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ishii S, et al. Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004;1690:250–257. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Leskela TT, et al. Opioid receptor pharmacological chaperones act by binding and stabilizing newly synthesized receptors in the endoplasmic reticulum. J Biol Chem. 2007;282:23171–23183. doi: 10.1074/jbc.M610896200. [DOI] [PubMed] [Google Scholar]
- 130.Petaja-Repo UE, et al. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jardon-Valadez E, et al. Conformational Effects of Lys191 in the Human Gonadotrophin-Releasing Hormone Receptor (hGnRHR). Mutagenesis and Molecular Dynamics Simulations Studies. J Endocrinol. 2009 doi: 10.1677/JOE-08-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janovick JA, et al. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–8425. doi: 10.1074/jbc.M510601200. [DOI] [PubMed] [Google Scholar]
- 133.Janovick JA, et al. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol. 2009;23:157–168. doi: 10.1210/me.2008-0384. PMC2646616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conn PM, et al. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv. 2002;2:308–316. doi: 10.1124/mi.2.5.308. [DOI] [PubMed] [Google Scholar]
- 135.Finch AR, et al. Trafficking and signalling of gonadotrophin-releasing hormone receptors: an automated imaging approach. Br J Pharmacol. 2010;159:751–760. doi: 10.1111/j.1476-5381.2009.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bernier V, et al. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol. 2004;18:2074–2084. doi: 10.1210/me.2004-0080. [DOI] [PubMed] [Google Scholar]
- 137.Vos TJ, et al. Identification of 2-[2-[2-(5-bromo-2- methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 138.Granell S, et al. Obesity-linked variants of melanocortin-4 receptor are misfolded in the endoplasmic reticulum and can be rescued to the cell surface by a chemical chaperone. Mol Endocrinol. 2010;24:1805–1821. doi: 10.1210/me.2010-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chaipatikul V, et al. Rescuing the traffic-deficient mutants of rat mu-opioid receptors with hydrophobic ligands. Mol Pharmacol. 2003;64:32–41. doi: 10.1124/mol.64.1.32. [DOI] [PubMed] [Google Scholar]
- 140.Janovick JA, et al. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Molec Endocrinol. 2009;23:157–168. doi: 10.1210/me.2008-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nowak RJ, et al. Improving binding specificity of pharmacological chaperones that target mutant superoxide dismutase-1 linked to familial amyotrophic lateral sclerosis using computational methods. J Med Chem. 2010;53:2709–2718. doi: 10.1021/jm901062p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jardon-Valadez E, et al. Modeling and molecular dynamics simulation of the human gonadotropin-releasing hormone receptor in a lipid bilayer. J Phys Chem B. 2008;112:10704–10713. doi: 10.1021/jp800544x. [DOI] [PubMed] [Google Scholar]
- 143.Soderhall JA, et al. Antagonist and agonist binding models of the human gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 2005;333:568–582. doi: 10.1016/j.bbrc.2005.05.142. [DOI] [PubMed] [Google Scholar]
- 144.Janovick JA, et al. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol. 2007;272:77–85. doi: 10.1016/j.mce.2007.04.012. PMC2169380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noorwez SM, et al. A high-throughput screening method for small-molecule pharmacologic chaperones of misfolded rhodopsin. Invest Ophthalmol Vis Sci. 2008;49:3224–3230. doi: 10.1167/iovs.07-1539. [DOI] [PubMed] [Google Scholar]
- 146.Tapanainen JS, et al. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–206. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- 147.Jean-Alphonse F, et al. Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J Am Soc Nephrol. 2009;20:2190–2203. doi: 10.1681/ASN.2008121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cormet-Boyaka E, et al. A truncated CFTR protein rescues endogenous DeltaF508-CFTR and corrects chloride transport in mice. FASEB J. 2009;23:3743–3751. doi: 10.1096/fj.08-127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zarinan T, et al. Dominant negative effects of human follicle-stimulating hormone receptor expression-deficient mutants on wild-type receptor cell surface expression. Rescue of oligomerization-dependent defective receptor expression by using cognate decoys. Mol Cell Endocrinol. 2010;321:112–122. doi: 10.1016/j.mce.2010.02.027. PMC2854281. [DOI] [PMC free article] [PubMed] [Google Scholar]