Abstract
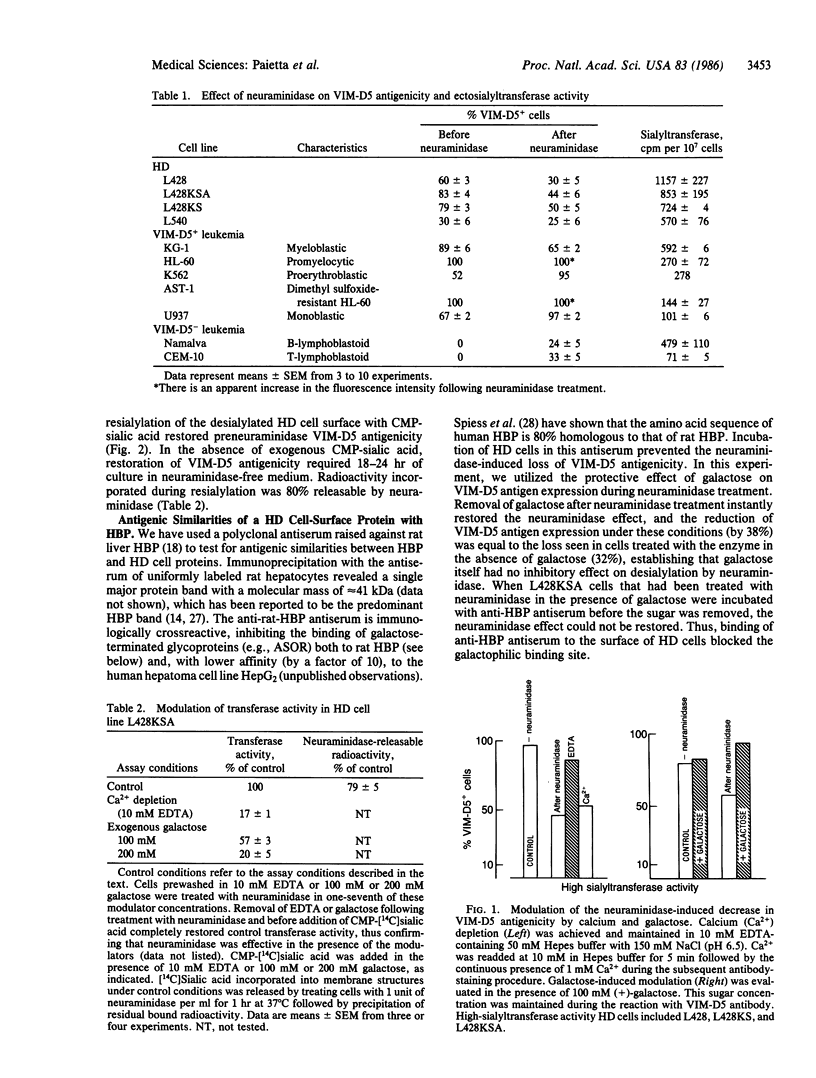
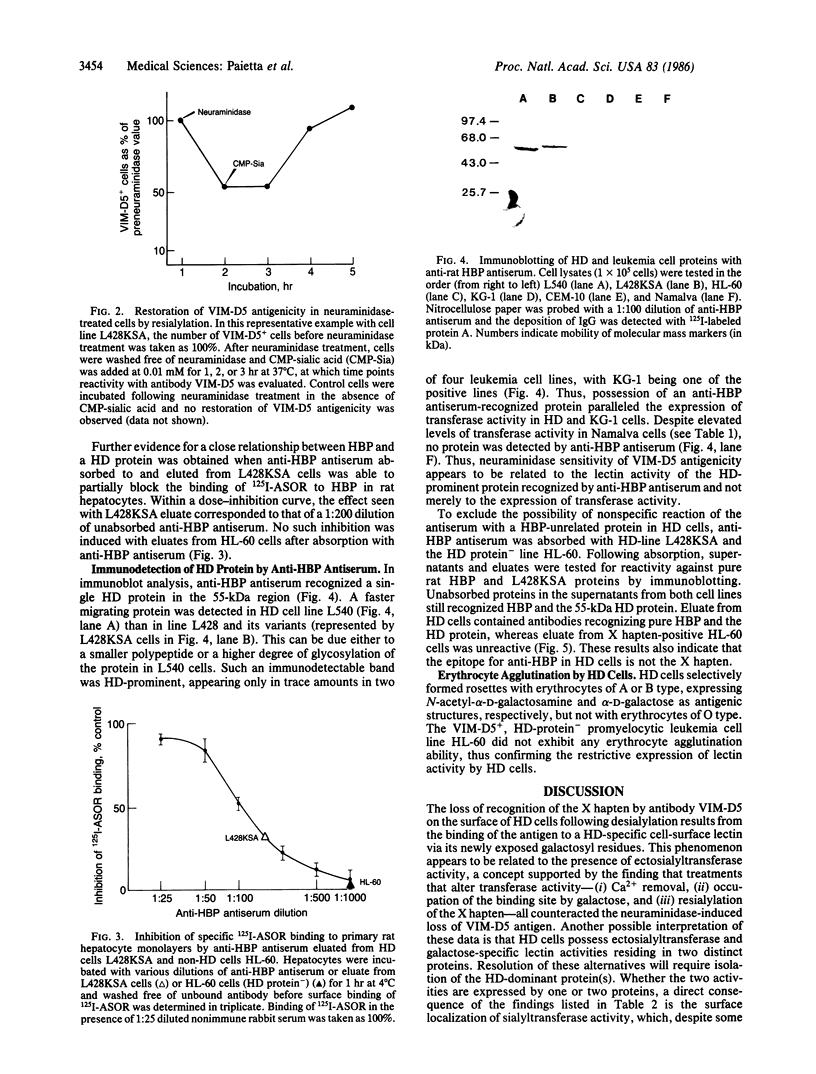
Treatment of cultured Hodgkin disease (HD) cells with neuraminidase results in decreased reactivity of monoclonal antibody VIM-D5 with its antigen, the X hapten, a fucosyl-N-acetyllactosamine. The other feature characteristic of HD cells is the expression of high levels of ectosialyltransferase activity. We present evidence for a cause-effect relationship between these two findings in that VIM-D5 antigenicity can be restored on neuraminidase-treated HD cells by modulating transferase activity. This can be interpreted in terms of a lectin activity of the ectosialyltransferase that binds the X hapten's desialylated galactosyl residues, thereby preventing antigen recognition by VIM-D5 antibody. This proposed mechanism is indistinguishable from the autoinhibition phenomenon described for another galactophilic binding protein, the hepatic binding protein (HBP), which binds its own terminal galactosyl residues following neuraminidase treatment. We establish a close relationship between the HD galactophilic binding site and HBP in that antiserum to HBP (i) inhibits the neuraminidase-induced loss of VIM-D5 antigenicity, (ii) blocks the binding of asialoglycoprotein to hepatocytes after being absorbed by and eluted from HD cells, and (iii) recognizes a single HD protein, which in its high level of expression is unique to HD cells. The presence of lectin activity in its classic sense on the surface of HD cells is confirmed by the erythrocyte-agglutinating ability of these cells. This lectin activity, which appears to be related to an ectosialyltransferase on the surface of HD cells, may serve as a marker for the abnormal cells characteristic of HD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulaziz Z., Mason D. Y., Stein H., Gatter K. C., Nash J. R. An immunohistological study of the cellular constituents of Hodgkin's disease using a monoclonal antibody panel. Histopathology. 1984 Jan;8(1):1–25. doi: 10.1111/j.1365-2559.1984.tb02318.x. [DOI] [PubMed] [Google Scholar]
- Aronson N. N., Jr, Tan L. Y., Peters B. P. Galactosyl transferase-the liver plasma membrane binding-site for asialo-glycoproteins. Biochem Biophys Res Commun. 1973 Jul 2;53(1):112–118. doi: 10.1016/0006-291x(73)91408-3. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Maynard Y. Human hepatic lectin. Physiochemical properties and specificity. J Biol Chem. 1980 May 25;255(10):4607–4613. [PubMed] [Google Scholar]
- Bosmann H. B. Cell surface glycosyl transferases and acceptors in normal and RNA- and DNA-virus transformed fibroblasts. Biochem Biophys Res Commun. 1972 Aug 7;48(3):523–529. doi: 10.1016/0006-291x(72)90379-8. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Rup D., Schwartz A. L. Influence of the N-linked oligosaccharides on the biosynthesis, intracellular routing, and function of the human asialoglycoprotein receptor. J Biol Chem. 1984 Aug 25;259(16):10414–10421. [PubMed] [Google Scholar]
- Bridges K., Harford J., Ashwell G., Klausner R. D. Fate of receptor and ligand during endocytosis of asialoglycoproteins by isolated hepatocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):350–354. doi: 10.1073/pnas.79.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C., Stanley P. The Chinese hamster ovary glycosylation mutants LEC11 and LEC12 express two novel GDP-fucose:N-acetylglucosaminide 3-alpha-L-fucosyltransferase enzymes. J Biol Chem. 1984 Sep 25;259(18):11208–11214. [PubMed] [Google Scholar]
- Diehl V., Kirchner H. H., Burrichter H., Stein H., Fonatsch C., Gerdes J., Schaadt M., Heit W., Uchanska-Ziegler B., Ziegler A. Characteristics of Hodgkin's disease-derived cell lines. Cancer Treat Rep. 1982 Apr;66(4):615–632. [PubMed] [Google Scholar]
- Dobrossy L., Pavelic Z. P., Bernacki R. J. A correlation between cell surface sialyltransferase, sialic acid, and glycosidase activities and the implantability of B16 murine melanoma. Cancer Res. 1981 Jun;41(6):2262–2266. [PubMed] [Google Scholar]
- Dorreen M. S., Habeshaw J. A., Stansfeld A. G., Wrigley P. F., Lister T. A. Characteristics of Sternberg-Reed, and related cells in Hodgkin's disease: an immunohistological study. Br J Cancer. 1984 Apr;49(4):465–476. doi: 10.1038/bjc.1984.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F., Binns G., Leung J. O. Primary structure of the rat liver asialoglycoprotein receptor. Structural evidence for multiple polypeptide species. J Biol Chem. 1984 Jan 25;259(2):770–778. [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Krusius T., Finne J. Characterization of a novel sugar sequence from rat-brain glycoproteins containing fucose and sialic acid. Eur J Biochem. 1978 Mar 15;84(2):395–403. doi: 10.1111/j.1432-1033.1978.tb12180.x. [DOI] [PubMed] [Google Scholar]
- Maca R. D., Hakes A. D. Differences in sialyl transferase activity and in concanavalin-A agglutination between T and B lymphoblastoid cell lines. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1124–1130. doi: 10.1016/0006-291x(78)91253-6. [DOI] [PubMed] [Google Scholar]
- Majdic O., Bettelheim P., Stockinger H., Aberer W., Liszka K., Lutz D., Knapp W. M2, a novel myelomonocytic cell surface antigen and its distribution on leukemic cells. Int J Cancer. 1984 May 15;33(5):617–623. doi: 10.1002/ijc.2910330511. [DOI] [PubMed] [Google Scholar]
- Majdic O., Liszka K., Lutz D., Knapp W. Myeloid differentiation antigen defined by a monoclonal antibody. Blood. 1981 Dec;58(6):1127–1133. [PubMed] [Google Scholar]
- Paulson J. C., Hill R. L., Tanabe T., Ashwell G. Reactivation of asialo-rabbit liver binding protein by resialylation with beta-D-galactoside alpha2 leads to 6 sialyltransferase. J Biol Chem. 1977 Dec 10;252(23):8624–8628. [PubMed] [Google Scholar]
- Paulson J. C., Prieels J. P., Glasgow L. R., Hill R. L. Sialyl- and fucosyltransferases in the biosynthesis of asparaginyl-linked oligosaccharides in glycoproteins. Mutually exclusive glycosylation by beta-galactoside alpha2 goes to 6 sialyltransferase and N-acetylglucosaminide alpha1 goes to 3 fucosyltransferase. J Biol Chem. 1978 Aug 25;253(16):5617–5624. [PubMed] [Google Scholar]
- Pizzolo G., Chilosi M., Semenzato G., Caligaris-Cappio F., Fiore-Donati L., Perona G., Janossy G. Immunohistological analysis of Tac antigen expression in tissues involved by Hodgkin's disease. Br J Cancer. 1984 Sep;50(3):415–417. doi: 10.1038/bjc.1984.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Bernacki R. J. Ultrastructural evidence for ectoglycosyltransferase systems. Nature. 1975 Aug 21;256(5519):648–650. doi: 10.1038/256648a0. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Rauvala H. Gangliosides of human kidney. J Biol Chem. 1976 Dec 10;251(23):7517–7520. [PubMed] [Google Scholar]
- Romagnani S., Ferrini P. L., Ricci M. The immune derangement in Hodgkin's disease. Semin Hematol. 1985 Jan;22(1):41–55. [PubMed] [Google Scholar]
- Rossowski W., Sahai Srivastava B. I. Ectosialyltransferase activity: a marker for certain human haematopoietic cells. Br J Cancer. 1983 Jan;47(1):158–160. doi: 10.1038/bjc.1983.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Schwartz A. L., Lodish H. F. Sequence of human asialoglycoprotein receptor cDNA. An internal signal sequence for membrane insertion. J Biol Chem. 1985 Feb 25;260(4):1979–1982. [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Stockert R. J., Gärtner U., Morell A. G., Wolkoff A. W. Effects of receptor-specific antibody on the uptake of desialylated glycoproteins in the isolated perfused rat liver. J Biol Chem. 1980 May 10;255(9):3830–3831. [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Hepatic binding protein: the protective role of its sialic acid residues. Science. 1977 Aug 12;197(4304):667–668. doi: 10.1126/science.877581. [DOI] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Mammalian hepatic lectin. Science. 1974 Oct 25;186(4161):365–366. doi: 10.1126/science.186.4161.365. [DOI] [PubMed] [Google Scholar]
- Stockinger H., Majdic O., Liszka K., Aberer W., Bettelheim P., Lutz D., Knapp W. Exposure by desialylation of myeloid antigens on acute lymphoblastic leukemia cells. J Natl Cancer Inst. 1984 Jul;73(1):7–11. [PubMed] [Google Scholar]
- Symington F. W., Hedges D. L., Hakomori S. Glycolipid antigens of human polymorphonuclear neutrophils and the inducible HL-60 myeloid leukemia line. J Immunol. 1985 Apr;134(4):2498–2506. [PubMed] [Google Scholar]
- Tabilio A., Del Canizo M. C., Henri A., Guichard J., Mannoni P., Civin C. I., Testa U., Rochant H., Vainchenker W., Breton-Gorius J. Expression of SSEA-I antigen (3-fucosyl-N-acetyl-lactosamine) on normal and leukaemic human haemopoietic cells: modulation by neuraminidase treatment. Br J Haematol. 1984 Dec;58(4):697–710. doi: 10.1111/j.1365-2141.1984.tb06117.x. [DOI] [PubMed] [Google Scholar]
- Tetteroo P. A., Mulder A., Lansdorp P. M., Zola H., Baker D. A., Visser F. J., von dem Borne A. E. Myeloid-associated antigen 3-alpha-fucosyl-N-acetyllactosamine (FAL): location on various granulocyte membrane glycoproteins and masking upon monocytic differentiation. Eur J Immunol. 1984 Dec;14(12):1089–1095. doi: 10.1002/eji.1830141205. [DOI] [PubMed] [Google Scholar]
- Tetteroo P. A., van't Veer M. B., Tromp J. F., von dem Borne A. E. Detection of the granulocyte-specific antigen 3-fucosyl-N-acetyl-lactosamine on leukemic cells after neuraminidase treatment. Int J Cancer. 1984 Mar 15;33(3):355–358. doi: 10.1002/ijc.2910330312. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]