Background
Hepatitis C virus (HCV) is a global infection, with an estimated 170 million people affected.1 The geographical distribution is of some interest as it gives clues as to the history and transmission of the virus. It appears there are endemic strains, which have persisted in specific locations for many centuries. These can be readily identified by viral genotype—for example, Genotype 2 is a strain which evolved in West Africa, while Genotype 6 evolved in South East Asia.2 In the last century, there have been iatrogenic outbreaks leading to a massive spread of specific subtypes in countries such as Egypt (Genotype 4A).3 There was also global spread of certain strains, most notably Genotype 1 within Europe and North America, and more recently Genotype 3A in European intravenous drug user (IDU) populations.4
HCV heterogeneity is huge, based on its capacity to develop mutation through its error-prone polymerase, and its very long co-evolutionary history with man. This is a major challenge for drug design and vaccines. Clinically, genotypic information on the virus is of major importance in defining response to conventional as well as newer therapies.1,4
Chronic HCV infection is now a leading cause of hepatic failure requiring transplantation in the west. Cirrhosis associated with HCV is also linked to the development of hepatocellular carcinoma, although unlike hepatitis B virus (HBV) this does not occur in pre-cirrhotic states. Chronic HCV infection is also associated with a number of extra-hepatic diseases, linked to B cell hyper-activation and potentially also chronic antigenemia—most notably mixed essential cryoglobulinaemia.5
Acute infection and host defence
Acute hepatitis C is actually a rare clinical presentation, despite the widespread nature of the virus. Typically, acute hepatitis is mild and may be clinically very subtle, with a delay of several weeks before a rise in alanine transaminase (ALT) is seen. HCV infection may set up persistence in the majority of those infected; although interestingly, upto 30% may clear the virus and remain negative by polymerase chain reaction (PCR) tests of blood thereafter. This clearance typically occurs within the first 6 months of infection. Some patients show a ‘yo-yo’ course of infection, with partial control over this period, followed by persistence. This may be due in some cases to super-infection or infection with multiple strains.6
A great deal of effort has been spent trying to define the mechanisms by which this robust immunity is mediated. Studies of cohorts such as women in Ireland and Germany, who were infected with contaminated blood products in Rhesus disease prevention programmes have been particularly useful here as the viral sequence and timing of infection were very well defined.7 From such studies, as well as studies of prospectively followed cohorts, the following features have emerged:
Clearance of the virus is typically associated with a robust T cell response, comprising both CD4+ and CD8+ T cell responses, which is sustained over several weeks.8
A role of T lymphocytes in clearance of virus is supported by HLA association with outcome—including HLA B27, HLA B57 (both also protective in human immunodeficiency virus), HLA A3 and HLA DR11.7
There is a major role for innate responses. In particular, polymorphisms in the interferon lambda gene IL28B have been strongly associated with outcome, as they are with treatment response (see below).9
Antibody responses do develop, although effective neutralization is limited by the rapid emergence of escape mutants in the envelope gene.10 Escape mutation also limits the efficacy of T cell responses.11
Progression to chronicity is associated with a marked attenuation of circulating cellular immune responses, although within liver tissue these may be retained and contribute to immune-mediated pathology.
Viral replication
The development of successful treatments for HCV has relied upon efforts to understand the viral life cycle in vitro and in vivo. While there remain no intact small animal models for replicating HCV (although partial mouse models exist12), much progress has been made in in vitro culture model systems and with it has come a massive increase in our understanding of host–virus interactions. Current HCV culture systems arose from the subgenomic replicons developed a decade ago. These required specific hepatocyte cell lines (Huh-7 derived) and tissue culture adaptation of the subgenomic constructs. A major breakthrough in this area came with the development of strains derived from a Japanese genotype 2 virus (JFH-1), which can complete a full replication cycle and produce new infectious virus to good titres in vitro.13 These systems also rely on specific modified hepatocyte cell lines, which lack some innate signalling programmes.
These systems have revealed:
The key cell entry factors for HCV, which include CD81, scavenger receptor class B1 (SRB1), low-density lipoprotein receptor (LDLR) and the tight junction-associated proteins human occludin (OCLN) and claudin-1 (CLDN1).14
The tight linkage between viral replication and modification of the lipid handling of the hepatocyte, including the generation of lipid droplets.15
The ability of the virus to evade host defence, including the significant impact of non structural protein 3 and 4A (NS3 and NS4A) in cleavage of the host signalling molecule Cardif/mitochondrial anti-viral signalling proteins (MAVS), disrupting early events in virus recognition.16
The impact of antivirals (including interferons) in vitro, including the development of escape mutations in response to selection pressure.
Vaccines
No effective preventive vaccine for HCV exists currently.17 Given the problems of viral diversity described above, a conventional vaccine based on anti-envelope antibodies (as is effective in HBV infection) is unlikely to be easily generated. Much effort has gone into the development of vaccines to stimulate cellular immunity. These still need to overcome the problem of viral diversity, but the hope is that such responses may target a sufficient range of viral proteins to limit escape mutations. Such vaccines are still in the early phase of development both for prevention and also as potential therapies. In the latter case, the published data suggest only a limited or transient response to date, but diverse strategies are still under investigation.18,19
Therapy
Up until this year, standard therapy for HCV has been a combination of Pegylated Interferon alpha (PegIFNα) and Ribavirin. The actual mechanism of action of this combination is not clear, especially the role of Ribavirin, which has weak antiviral activity. Overall, treatment has incrementally improved over the last decade and PegIFNα/Ribavirin can lead to a sustained virologic response (SVR) i.e. undetectable virus 6 months beyond the end of therapy, in 60–70% of patients with Genotypes 2 and 3 (after 6 months therapy), but in <50% of patients with Genotype 1 (requiring 12 months therapy).
It has long been known that a variety of factors contribute to outcome, including host genetics. What was striking from recent studies using genome-wide scans is the specific role of polymorphisms in IL28B.9,20 The actual role of this cytokine in vivo is not yet clear, indeed the impact of the polymorphism is also not fully understood. However, genotyping for IL28B can provide additional information (along with other clinical factors such as viral load, fibrosis, co-morbidities), which can aid stratification for therapy. Trials of lambda interferon—which has a more restricted side-effect profile than the alpha form—are underway (reviewed in Ref. 21).
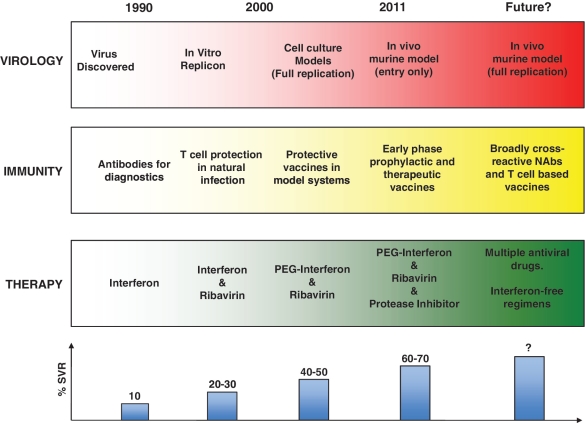
New therapies with agents focusing on specific viral proteins such as protease and polymerase are now emerging. Two protease inhibitors, Telapravir and Bocepravir, have now undergone extensive trials and have recently been licensed.22–25 Current treatment strategies require co-administration with PegIFNα/Ribavirin—monotherapy is associated with the rapid emergence of drug resistance. These regimens have shown improved efficacy compared with current standard of care (60–75% SVR), with potentially shorter treatment courses, although these are restricted to Genotype 1. Interestingly, they also show good efficacy in previous unsuccessfully treated patients (60–65% SVR). Combination therapy will be of major interest in the future and the possibility of interferon-free regimens has been raised, if multiple specific antivirals are sufficiently potent (Figure 1). Once again the circulating viral diversity and the capacity of the virus to adapt rapidly to new selective forces are major hurdles to overcome.
Figure 1.
Timeline for advances in understanding HCV. The coloured blocks represent rough timelines for the key steps in the fields of virology, immunology and therapy. The graph below indicates the approximate success rates for treatment success using the regimens indicated (for Genotype 1). Success rates vary with genotype as well as a number of other factors.22–24
Future directions
Further host genetics, so fruitful so far in this infection, may give important clues as to viral clearance mechanisms, while further viral genetics will remain important to define mechanisms of resistance to therapy and vaccines. The explosion in next-generation sequencing should help both endeavours. The efforts to optimize new treatment regimes as new antivirals emerge will occupy many in clinical research programmes. It will be important to learn from the HIV experience, where combination therapies have proven so successful—regimens which only emerged as a result of substantial collaborative effort. This may be aided by the further development of small animal models. The future does look bright for new therapies for HCV although in this wave of enthusiasm it should not be forgotten that many in the global epidemic—both in the west and in the developing world—will not have ready access to such treatments in their current forms.
Funding
Wellcome Trust Fellowship awards (WT091663MA and WT096212AIA to P.K. and P.G.); NIHR Biomedical Research Centre (Oxford); The Oxford Martin School; National Institutes of Health (NIAID 1U19AI082630-01).
Conflict of interest: P.K.G. has acted as a consultant on immune responses to HCV to Tibotec.
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Pybus OG, Barnes E, Taggart R, Lemey P, Markov PV, Rasachak B, et al. Genetic history of hepatitis C virus in East Asia. J Virol. 2009;83:1071–82. doi: 10.1128/JVI.01501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pybus OG, Charleston MA, Gupta S, Rambaut A, Holmes EC, Harvey PH. The epidemic behavior of the hepatitis C virus. Science. 2001;292:2323–5. doi: 10.1126/science.1058321. [DOI] [PubMed] [Google Scholar]
- 4.Simmonds P. Genetic diversity and evolution of hepatitis C virus–15 years on. J Gen Virol. 2004;85:3173–88. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 5.Charles ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, et al. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–56. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60:837–45. doi: 10.1136/gut.2010.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKiernan SM, Hagan R, Curry M, McDonald GS, Kelly A, Nolan N, et al. Distinct MHC class I and II alleles are associated with hepatitis C viral clearance, originating from a single source. Hepatology. 2004;40:108–14. doi: 10.1002/hep.20261. [DOI] [PubMed] [Google Scholar]
- 8.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–49. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–44. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 11.Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–52. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–11. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–6. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–6. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DM, McLauchlan J. Hepatitis C virus: assembly and release of virus particles. J Biol Chem. 2010;285:22733–9. doi: 10.1074/jbc.R110.133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 17.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–6. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 18.Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, et al. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology. 2008;134:1385–95. doi: 10.1053/j.gastro.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Habersetzer F, Honnet G, Bain C, Maynard-Muet M, Leroy V, Zarski JP, et al. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology. 2011;141:890–899. doi: 10.1053/j.gastro.2011.06.009. e891–894. [DOI] [PubMed] [Google Scholar]
- 20.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 21.Kelly C, Klenerman P, Barnes E. Interferon lambdas: the next cytokine storm. Gut. 2011;60:1284–93. doi: 10.1136/gut.2010.222976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 23.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]