Abstract
Development of a small diameter (<6 mm) synthetic vascular graft with clinically acceptable patency must overcome the inherent thrombogenicity of polymers and the development of neointimal thickening. Establishment of an endothelial cell lining on the lumenal surface has been hypothesized as a mechanism to improve the function of vascular grafts. The major aim of this study was to evaluate the use of laminin type 1, covalently bound to all surfaces of ePTFE grafts on neovascularization of the interstices and lumenal surface endothelialization.
One mm i.d. vascular grafts were surface modified through covalent attachment of laminin type 1. Grafts were subsequently implanted as interpositional aortic grafts in rats. Following five weeks implantation the grafts were explanted and morphologically evaluated using scanning electron microscopy and light microscopy.
Scanning electron microscopy identified an extensive coverage of antithrombogenic cells on the lumenal flow surface of laminin type 1 modified grafts. Histological evaluation confirmed the presence of endothelial cells on the mid-graft lumenal surface of laminin 1 modified grafts. Extensive neovascularization of the interstices of the laminin modified grafts occurred as compared to control grafts. We conclude that surface modification using laminin type 1 accelerates both the neovascularization and endothelialization of porous ePTFE vascular grafts.
Keywords: ePTFE, vascular graft, endothelial cells, extracellular matrix, laminin
INTRODUCTION
Development of a small diameter (<6 mm) synthetic vascular graft with clinically acceptable patency must overcome the inherent thrombogenicity of polymers and the development of neointimal thickening. One approach for improving patency consists of modifying the grafts to promote transmural endothelialization. Endothelialization of vascular grafts is a multi-step process which involves an initial angiogenesis in peri-implant tissue, followed by the migration of microvascular endothelial cells, from surrounding tissue, into the wall of grafts. These cells then populate the lumenal surface resulting in endothelialization of the blood contacting surface of the implant. Previous studies have established that a variety of modifications of expanded polytetrafluoroethylene grafts (ePTFE) will support neovascularization of the porous interstices of this material (1-4). On the other hand, complete endothelialization of vascular grafts by transinterstitial cell migration has only been approached with highly porous experimental grafts and the addition of growth factors (5,6). The current studies were performed using ePTFE vascular grafts with inter-nodal distances identical to clinically used grafts. This internodal distance, 30 microns, has previously been considered a physical barrier to cell migration and endothelialization.
Extracellular matrix proteins play a critical role in several endothelial cell functions including cell proliferation, migration and modulation of cell phenotype (7-9). These functions are necessary for the initiation and completion of the angiogenic process. Although numerous soluble factors, such as growth factors and cytokines, are integral to the activation and stimulation of new blood vessel development, insoluble factors, and specifically extracellular matrix proteins may be of equal importance. Matrix proteins provide a wide variety of signals to cells mediated through the transmembrane integrin proteins (10).
The primary goal of this study was to determine whether extracellular matrix modification of ePTFE would promote transmural neovascularization and subsequent endothelialization of grafts in an aortic interpositional graft model. During this study 1 mm inner diameter ePTFE grafts with a 30μm internodal distance, were utilized. Control, non treated grafts were compared to grafts treated with laminin type 1.
MATERIALS AND METHODS
Graft Modification
Expanded polytetrafluoroethylene grafts, with an internal diameter of 1 mm, 30 micron internodal distance and a wall thickness of approximately 400 microns were utilized1. Mouse laminin type 12 was obtained and covalently bound to the ePTFE as described below and is detailed in previous publications (11, 12). A hetero-bifunctional crosslinking agent (BBA-EAC-NOS) was synthesized and used to photoderivatize each protein. The BBA-EAC-NOS has a benzophenone photoactivatible group on one end (benzoyl benzoic acid, BBA), a spacer in the middle (epsilon aminocaproic acid, EAC), and an amine reactive thermochemical coupling group on the other end (N-oxysuccinimide, “NOS”). BBA-EAC was synthesized from 4-benzoylbenzoyl chloride and 6-aminocaproic acid. Then the NOS ester of BBA-EAC was synthesized by esterifying the carboxy group of BBA-EAC by carbodiimide activation with N-hydroxysuccimide to yield BBA-EAC-NOS.
The laminin type 1 was photoderivatized by covalently coupling primary amines on the proteins via the NOS ester of BBA-EAC-NOS. Solutions of photoderivatized laminin 1 (25 micrograms /cm2 ePTFE) was added to ePTFE, allowed to adsorb for 2 hours at room temperature, and illuminated at 320 to 340 nm to activate the BBA moieties and produce covalent coupling. Following illumination, free proteins were removed by washing the samples overnight in phosphate buffered saline (PBS) containing 1% Tween 20. After the PBS/Tween 20 wash, the samples were sterilized by soaking 20 minutes in 70% ethanol. The residual Tween 20 and ethanol were then removed by washing in sterile PBS. The grafts were cut into 1 cm lengths, separated into the test group (laminin 1 modified) and a non-modified control group. Selected samples were evaluated using fluorescein isothiocyanate (FITC) staining of the immobilized laminin type 1 and evaluation of protein distribution using confocal microscopy.
Graft Implant
NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication #85-23 Rev. 1985) have been observed. This study was performed following approval by the University of Arizona IACUC. The surgical procedure for implantation of 1 mm grafts has been previously published (13). Briefly, male Sprague Dawley rats weighing 300 - 350 grams anesthetized with 50mg/kg pentobarbital, IP. A sterile field was established and a midline abdominal incision made. Under an operating microscope, the aorta was isolated from the renal arteries to the iliac bifurcation. The aorta was clamped and resected below the renal arteries to accommodate a 1 cm length of graft. Using 10-0 Nylon the graft-aorta anastomoses were made. Flow was established and a flow probe was placed around the aorta just distal to the graft. Flow was monitored and recorded for 30 minutes. The flow probe was removed and the abdomen closed in 3 layers. The animals were recovered on a heating pad and returned to the animal care facility. A total of 6 animal with control grafts and four animals with laminin 1 treated grafts were evaluated.
Graft Explant
At 5 weeks the rats were anesthetized as above and the aorta and graft isolated. The flow probe was placed around the distal aorta and flow evaluated. The graft and adjacent aorta (5mm proximal and distal to graft) were removed, fixed and processed for light and scanning electron microscopy.
Histology
Half of each sample was fixed, dehydrated and paraffin embedded. Samples were sectioned and stained with hematoxylin and eosin to examine gross morphology. Sections were also reacted with horse-radish conjugated Griffonia simplicifolia (Gs1) lectin which shows specificity for endothelial cells, activated monocytes and activated macrophages (14).
Samples for scanning electron microscopy were fixed with 3% glutaraldehyde. Samples were washed with PIPES buffer, dehydrated in a graded series of acetone and critical point dried. Dried samples were sputter coated by using a gold target. Samples were then observed with a Joel 820 scanning electron microscope.
RESULTS
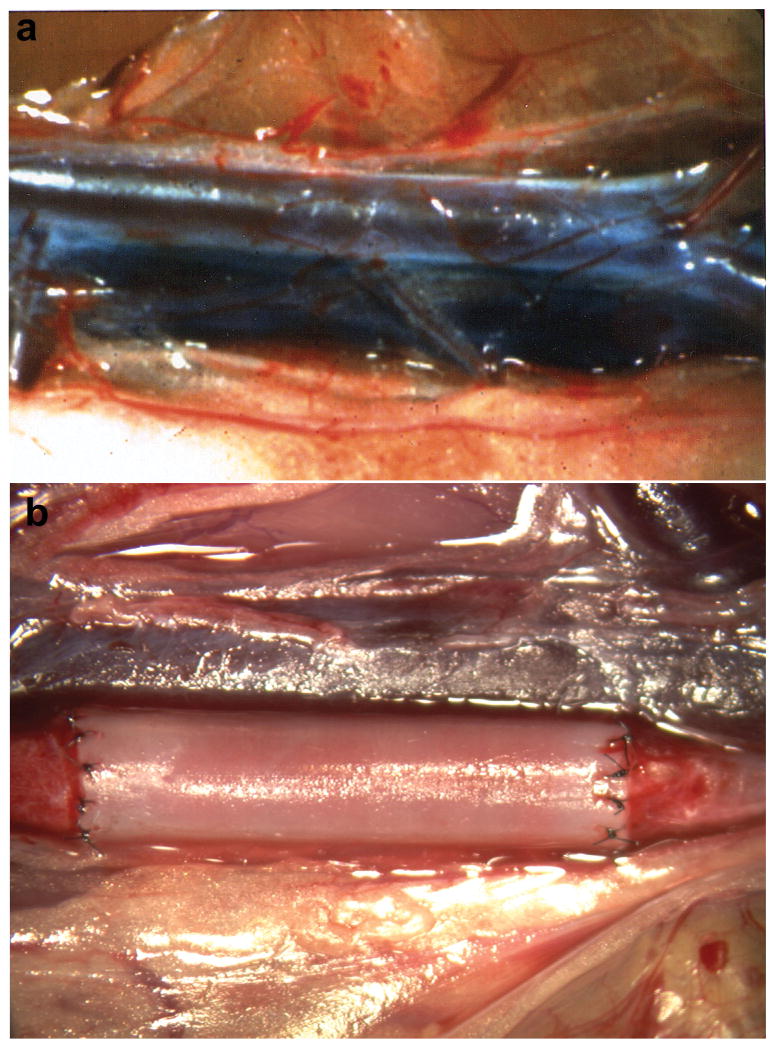
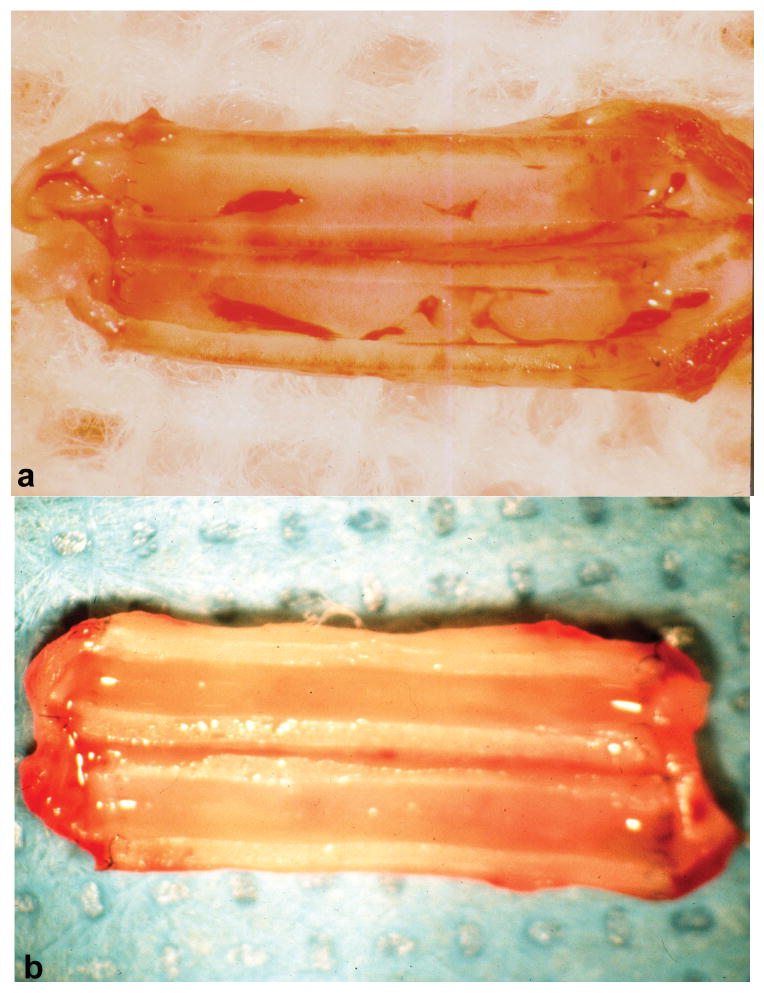
Figure 1 illustrates the surgical placement of 1 mm ePTFE grafts as interpositional conduits in the abdominal aorta. Treatment of grafts with laminin type 1 did not result in increased anastomotic or transinterstitial blood or plasma loss. Laminin treatment of the grafts did not cause increased graft occlusion, as all grafts remained patent. Figure 2 (a, b) compares the gross morphology of representative grafts at the time of explant. The control grafts (a) exhibited a significant red thrombus while the laminin type 1 modified grafts (b) exhibited a glistening white surface grossly devoid of red thrombus. Gross examination did not reveal the presence of intimal thickening in any of the grafts.
Figure 1.
Surgical placement of 1 mm ePTFE grafts as interpositional conduits in the abdominal aorta. a.) segment of the aorta dissected free of tissue; b.) completed graft placement with blood flow restored illustrating minimal anastomotic bleeding.
Figure 2.
Gross morphology of representative grafts at the time of explantation. a.) control graft illustrating the presence of red thrombus. b.) laminin type 1 treated graft illustrating lack of red thrombus on the lumenal blood contacting surface.
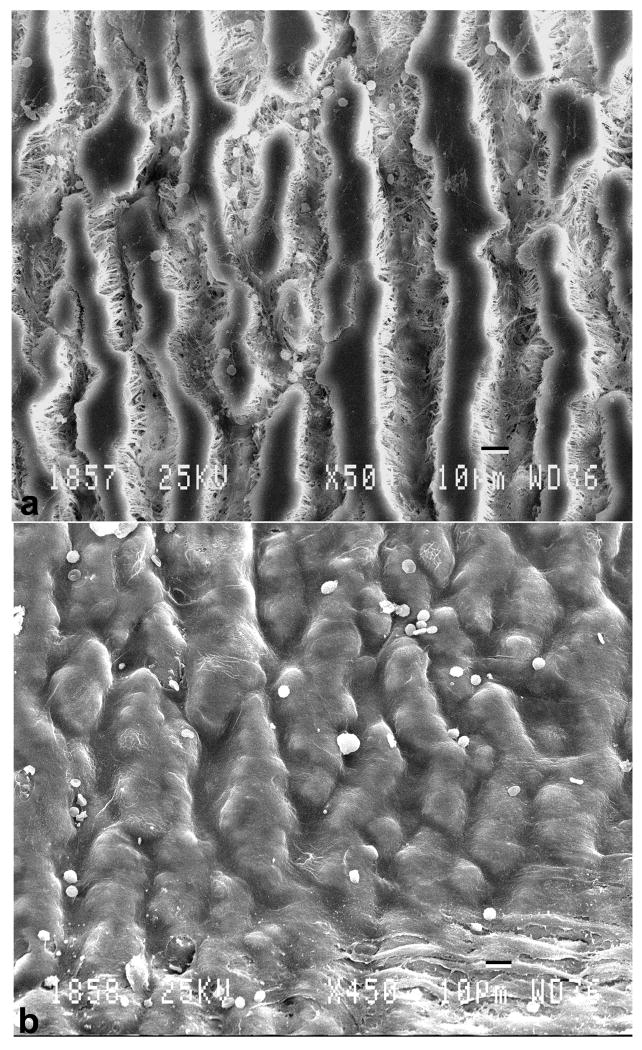
Figure 3 (a, b) illustrates the difference in surface morphology of the control and laminin type 1 treated grafts viewed by scanning electron microscopy. Control grafts (figure 3a) exhibited a highly thrombogenic surface with evidence of the deposition of red cells, leukocytes, fibrin and platelets. In contrast, the laminin type 1 treated grafts (figure 3b) exhibited a complete lining of highly attenuated cells consistent with the presence of endothelium. These cells were actively anti-thrombogenic as evidenced by the relative lack of adherent formed elements. There was no evidence of intimal thickening observed throughout the lumenal lining of these grafts.
Figure 3.
Scanning electron micrographs illustrating differences in surface morphology. a.) control graft - note absence of cellular lining and b.) laminin type 1 treated grafts that exhibited a confluent lining of cells.
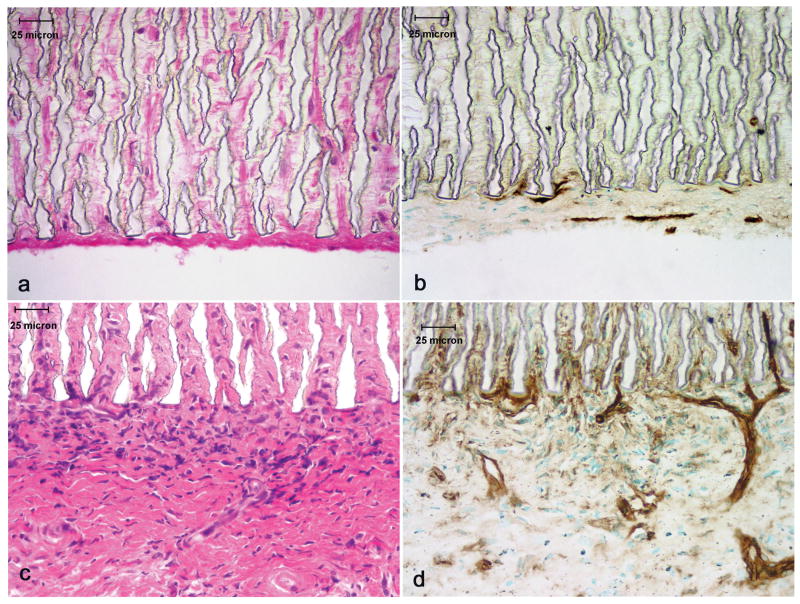
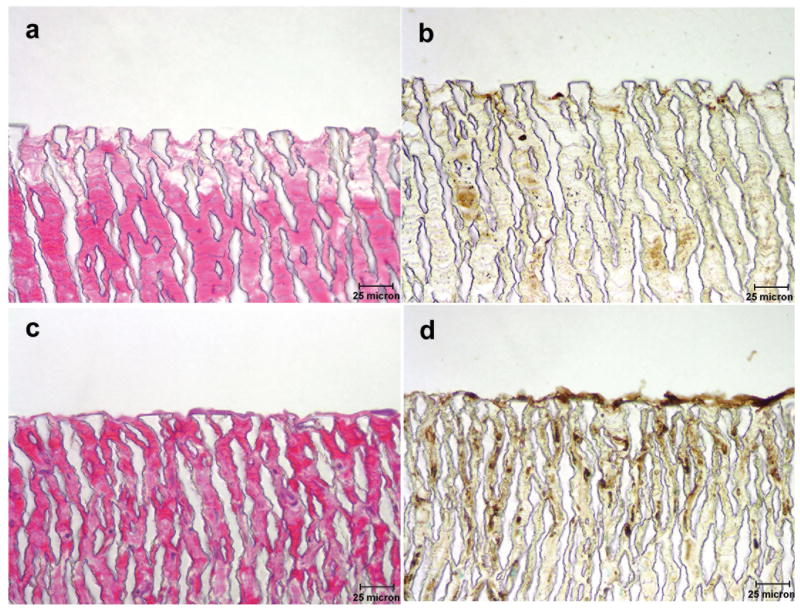
These grafts were subsequently subjected to histologic evaluation and the results are illustrated in Figures 4 (a-d) and 5 (a-d). Figure 4a (H&E) and b (Gs-1) illustrate a section of the abluminal surface of a control graft while figure 4c (H&E) and d (Gs-1) depicts the abluminal surface of a laminin type 1 treated graft. A remarkable difference was observed between control and laminin treated samples with respect to vascular response. As illustrated in figure 4 c and d the laminin type 1 treated grafts exhibited the development of microvascular networks which were observed penetrating into the interstices of the ePTFE while in control grafts (figure 4 a and b) endothelial cells were only observed in the tissue directly associated with the grafts. The luminal surfaces of these grafts were also evaluated and are illustrated in figure 5 (a-d). The control graft (figures 5a and b) exhibited minimal pseudointima on the luminal surface. Gs-1 staining revealed the absence of an endothelial cellular lining. On the other hand, the laminin type 1 treated grafts (figures 5c and d) exhibited a cellular lining on the lumenal surface which reacted actively with Gs-1.
Figure 4.
Light micrographs of the abluminal surface of grafts stained with H&E and Gs-1. a.) and b.) control graft; c.) and d.) laminin type 1 modified graft. Note microvascular networks penetrating into the graft interstices of the laminin type 1 treated grafts.
Figure 5.
Micrographs illustrating the lumenal surface of grafts stained with H&E and Gs-1. a.) and b.) control graft - note absence of a cellular lining; c.) and d.) laminin type 1 treated graft exhibiting a cellular lining which reacted actively with Gs-1.
CONCLUSIONS
This study establishes the ability to accelerate the in vivo formation of an endothelial cell lining on a synthetic vascular graft by treating the graft with an extracellular matrix protein (laminin type 1). Spontaneous formation of an endothelial cell lining on porous vascular grafts involves a series of processes which include the initial stimulation of a peri-implant tissue angiogenesis and the directed migration of endothelial cells to populate the lumenal surface. The results off this study indicate that spontaneous endothelialization of commercially available vascular grafts can be achieved using extracellular matrix modification of these polymers. While the mechanism underlying this spontaneous cellular response is not fully understood, laminin type 1 provides all of the signals necessary to accelerate graft neovascularization and endothelialization.
The spontaneous formation of an endothelium on the blood contacting surface of implanted medical devices has been hypothesized as a method to improve the long term function of these devices (5, 6). The poor performance of small diameter (< 6 mm) synthetic vascular grafts is attributed mainly to the thrombogenicity of bare polymer (15, 16). The antithrombogenic characteristics of endothelium would potentially mask the thrombogenicity of polymers, such as expanded polytetrafluoroethylene or polyethylene terephthalate. For this reason many investigations have evaluated methods to accelerate formation of a new endothelium using techniques such as endothelial cell transplantation (17-19), accelerated pannus ingrowth, and the focus of the present study, accelerated transinterstitial ingrowth (20,21). Stimulants for transinterstital ingrowth have concentrated previously on two major efforts, namely incorporation of soluble endothelial cell growth factors to stimulate angiogenesis (22), and physical changes in polymers to make them more porous and less restrictive to endothelial cell penetration (3,5,23,24). Our efforts in the present study have focused on the use of immobilized extracellular matrix proteins to accelerate spontaneous endothelialization.
The rationale for the use of extracellular matrix proteins in these studies is based on significant evidence that endothelial cell function is regulated by extracellular proteins through the trans membrane protein integrin system (7-9). Some of these matrix proteins have been extensively evaluated and shown to regulate endothelial cell proliferation and migration. These functions are integral parts of the angiogenic process and for this reason were evaluated for their effect on endothelialization. Laminin type 1 was chosen since it is a component of the sub-endothelial basement membrane and has been shown, through in vitro studies, to also effect cell function (25-27). These studies were performed with the realization that polymer associated healing response involves many cell types, and the effects of polymer matrix modification may not be endothelial cell specific.
The data supports the conclusion that laminin type 1 modification of ePTFE results in altered peri-implant angiogenic and polymer neovascularization responses as compared to control, unmodified ePTFE. The stimulation of peri-implant tissue angiogenesis was increased with laminin type 1 treated ePTFE as compared to control. Laminin also stimulated penetration of endothelial cells into the interstices of the porous ePTFE. Only the laminin type 1 treated vascular grafts exhibited endothelialization of the lumenal blood flow surface. The presence of laminin type 1 on surfaces throughout these grafts supports the formation of all the individual components of spontaneous endothelialization including peri-implant angiogenesis, polymer neovascularization and finally blood flow surface endothelialization. This final process is a unique cellular event since cells are required to move and self associate in the presence of a physical force, shear stress, generated by the flow of blood. Cells that formed this monolayer must balance the events of selective attachment and detachment necessary for cell migration and the maintenance of a critical attachment to the surface to avoid detachment and removal from the surface.
The endothelial cell identity of these lumenal cells was substantiated using an endothelial cell specific lectin, Griffonia simplicifolia. Further characterization of this monolayer is provided by scanning electron microcopy which established the anti-thrombogenic nature of this lining due to the lack of platelet adherence or fibrin deposition. It is also of interest that this lining is nearly a monolayer of cells at this explanation time point with no evidence of intimal thickening. While these results represent only one time point, intimal responses are progressive and may still occur. The lack of any sub-endothelial cell thickening in these laminin type 1-treated grafts indicates the lining is stable and not acutely hyper-proliferative.
These studies also provide evidence that the mechanism responsible for the observed inhibition of spontaneous endothelialization of commercially available ePTFE is not a physical barrier to cell penetration through the interstices of this material. Several investigations have evaluated the use of experimental ePTFE of increased porosity with the hypothesis that this increased porosity is necessary for endothelialization (3, 5, 23, 24). The current study was performed using ePTFE with internodal distances identical to clinically used ePTFE vascular grafts. The penetration of endothelial cells, and as illustrated, intact microvascular networks into the interstices of laminin type 1 treated ePTFE, provides evidence that porosity is not the critical element controlling polymer neovascularization. There is certainly a lower limit of porosity which will preclude cell penetration due to a physical barrier. The current study suggests this physical barrier to endothelialization does not exist in commercially available grafts and the barrier to endothelialization may lie in the biological response associated with ePTFE.
The mechanisms underlying the observed accelerated formation of an endothelial cell lining on laminin type 1-treated ePTFE vascular grafts is not known. Possible explanations include the identified role of laminin type 1 in the regulation of several cell functions. Moreover, laminin has been reported to be directly involved in the regulation of angiogenic responses (28). The covalent immobilization of laminin type 1 onto porous ePTFE vascular grafts results in a stimulation of not only peri-implant angiogenesis, but subsequent stimulation and progression of this angiogenesis to include the pores within the polymer. Regardless of the exact mechanisms underlying this process, the results indicate laminin type 1 treatment of grafts will accelerate spontaneous endothelialization of the lumenal surface. The previously reported poor clinical performance of currently available grafts may be overcome by treatment of these grafts with laminin type 1 to support the development of a stable, anti-thrombogenic endothelial cell lining.
Acknowledgments
Funding for this study was provided by the National Institutes of Health grant numbers HL63873 and DK 078175 to SKW.
Footnotes
ePTFE grafts provided by Impra/Bard, Tempe, AZ.
Mouse Laminin type 1 was obtained from Collaborative Biomedical Products, Bedford, MA.
The benefits accruing to the author or authors from a commercial or industrial party will be applied to a research fund, nonprofit institution or other organization with which the authors are associated.
References
- 1.De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplant. 1997;63(6):824–30. doi: 10.1097/00007890-199703270-00006. [DOI] [PubMed] [Google Scholar]
- 2.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–24. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 3.Nagae T, Tsuchida H, Peng SK, Furukawa K, Wilson SE. Composite porosity of expanded polytetrafluoroethylene vascular prosthesis. Cardiovasc Surg. 1995;3(5):479–84. doi: 10.1016/0967-2109(95)94445-3. [DOI] [PubMed] [Google Scholar]
- 4.Kidd KR, Nagle RB, Williams SK. Angiogenesis and neovascularization associated with extracellular matrix modified porous implants. J Biomed Mater Res. 2002;59:366–377. doi: 10.1002/jbm.1253. [DOI] [PubMed] [Google Scholar]
- 5.Kohler TR, Stratton JR, Kirkman TR, Johansen KH, Zierler BK, Clowes AW. Conventional versus high porosity polytetrafluoroethylene grafts: Clinical evaluation. Surg. 1992;12:901–907. [PubMed] [Google Scholar]
- 6.Mickucki SA, Greisler HP. Understanding and manipulating the biological response to vascular implants. Semin Vasc Surg. 1999;12(1):18–26. [PubMed] [Google Scholar]
- 7.Madri JA, Williams SK. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983;97(1):153–65. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoying JB, Williams SK. Effects of basic fibroblast growth factor on human microvessel endothelial cell migration on collagen I correlates inversely with adhesion and is cell density dependent. J Cell Physiol. 1996;168(2):294–304. doi: 10.1002/(SICI)1097-4652(199608)168:2<294::AID-JCP8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Davies GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces and matrix guided pathways. Exper Cell Res. 1995;216(1):113–123. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- 10.Gamble J, Meyer G, Noack L, Furze J, Kovach N, Harlant J, Vadas M. β1 integrin activation inhibits in vitro tube formation: effects on cell migration, vacuole coalescence and lumen formation. Endothelium. 1999;7(1):23–34. doi: 10.3109/10623329909165309. [DOI] [PubMed] [Google Scholar]
- 11.Amos RA, Anderson AB, Clapper DL, Duquette PH, Duran LW, Hohle SG, Sogard DJ, Swanson MJ, Guire PE. Biomaterial surface modification using photochemical coupling technology. In: Wise DL, Trantolo DE, Altobelli MJ, Yaszemski JD, Gresser ER, Schwartz, editors. Encyclopedic Handbook of Biomaterials and Bioengineering, Part A: Materials. Vol. 1. New York: Marcell Dekker, Inc; 1995. pp. 895–926. [Google Scholar]
- 12.Clapper DL, Daws KM, Guire PE. Photoimmobilized ECM peptides promote cell attachment and growth on biomaterials. Trans Soc Biomat. 1994;17:345. [Google Scholar]
- 13.Ahlswede KM, Williams SK. Microvascular endothelial cell sodding of 1 mm expanded polytetrafluoroethylene vascular grafts. Arterioscler Thromb. 1994;14(1):25–31. doi: 10.1161/01.atv.14.1.25. [DOI] [PubMed] [Google Scholar]
- 14.Peters BP, Goldstein IJ. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the identification of α-D-galactopyranosyl groups. Exp Cell Res. 1979;120:321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- 15.Veith FJ, Gupta S, Daly V. Management of early and late thrombosis of expanded polytetrafluoroethylene (PTFE) femoropopliteal bypass grafts: Favorable prognosis with appropriate reoperation. Surg. 1980;87(5):581–587. [PubMed] [Google Scholar]
- 16.Kohler TR, Kaufman JL, Kacoyanis G, Clowes A, Donaldson MC, Kelley E, Skillman J, Couch NP, Whitamore AD, Mannick JA. Effect of aspirin and dipyridamole on the patency of lower extremity bypass grafts. Surg. 1984;96(3):462–466. [PubMed] [Google Scholar]
- 17.Schmidt SP, Hunter TJ, Hirko M. Small diameter vascular prostheses: Two designs of PTFE and endothelial cell seeded and non-seeded Dacron. J Vasc Surg. 1985;2:292–297. [PubMed] [Google Scholar]
- 18.Williams SK, Rose DG, Jarrell BE. Microvascular endothelial cell sodding of ePTFE vascular grafts: Improved patency and stability of the cellular lining. J Biomed Mater Res. 1994;28:203–212. doi: 10.1002/jbm.820280210. [DOI] [PubMed] [Google Scholar]
- 19.Meinhart JG, Deutsch M, Fischlein T, Howanietz N, Foschl A, Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg. 2001;7(5):S327–S331. doi: 10.1016/s0003-4975(01)02555-3. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchida H, Wilson SE, Ishimaru S. Healing mechanisms of high-porosity PTFE grafts: significance of transmural structure. J Surg Res. 1997;71(2):187–195. doi: 10.1006/jsre.1997.5158. [DOI] [PubMed] [Google Scholar]
- 21.Durante KR, Wu HD, Sauvage LR, Shi Q, Wechezak AR, Coan DE, Kaplan S, Patel MD, Walker MW. Implant site: a determinant of completeness of arterial prosthesis healing in the dog and possibly in humans. Ann Vasc Surg. 1990;4(2):171–178. doi: 10.1007/BF02001374. [DOI] [PubMed] [Google Scholar]
- 22.Gray JL, Kang SS, Zenni GC, Kim DU, Burgess WH, Drohan W, Winkles JA, Haudenschild CC, Greisler HP. FGF-1 affixation stimulates ePTFE endothelialization without intimal hyperplasia. J Surg Res. 1994;57(5):597–612. doi: 10.1006/jsre.1994.1189. [DOI] [PubMed] [Google Scholar]
- 23.Salzmann DL, Kleinert LB, Berman SS, Williams SK. The effects of porosity on endothelialization of ePTFE implanted in subcutaneous and adipose tissue. J Biomed Mater Res. 1997;34:463–476. doi: 10.1002/(sici)1097-4636(19970315)34:4<463::aid-jbm7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchida H, Kashyap A, Cameron BL, Peng SK, Wilson SE. In vivo study of a high-porosity polytetrafluoroethylene graft: endothelialization, fluid leakage, and the effect of fibrin glue sealing. J Invest Surg. 1993;6(6):509–18. doi: 10.3109/08941939309141641. [DOI] [PubMed] [Google Scholar]
- 25.Ponce ML, Nomizu M, Delgado MC, Kuratomi Y, Hoffman MP, Powell S, Yamada Y, Kleinman HK, Malinda KM. Identification of endothelial cell binding sites on the laminin gamma 1 chain. Circ Res. 1999;84(6):688–94. doi: 10.1161/01.res.84.6.688. [DOI] [PubMed] [Google Scholar]
- 26.Rose RW, Grant DS, O’Hara MD, Williamson SK. The role of laminin-1 in the modulation of radiation damage in endothelial cells and differentiation. Radiat Res. 1999;152(1):1428. [PubMed] [Google Scholar]
- 27.Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman HK, Ponce ML. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J. 1999;13(1):53–62. [PubMed] [Google Scholar]
- 28.Ponce ML, Nomizu M, Kleinman HK. An angiogenic laminin site and its antagonist bind through the alpha(v)beta3 and alpha5beta1 integrins. FASEB J. 2001;15(8):1389–97. doi: 10.1096/fj.00-0736com. [DOI] [PubMed] [Google Scholar]