Abstract
Subarachnoid hemorrhage (SAH) results in significant long-lasting cognitive dysfunction. Therefore, evaluating acute and long-term outcomes after therapeutic intervention is important for clinical translation. The aim of this study was to use minocycline, a known neuroprotectant agent, to evaluate the long-term benefits in terms of neurobehavior and neuropathology after experimental SAH in rats, and to determine which neurobehavioral test would be effective for long-term evaluation. SAH was induced by endovascular perforation in adult male Sprague-Dawley rats (n=118). The animals were treated with intraperitoneal injection of minocycline (45 mg/kg or 135 mg/kg) or vehicle 1 h after SAH induction. In the short-term, animals were euthanized at 24 and 72 h for evaluation of neurobehavior, brain water content, and matrix metalloproteinase (MMP) activity. In the long-term, neurobehavior was evaluated at days 21–28 post-SAH, and histopathological analysis was done at day 28. High-dose but not low-dose minocycline reduced brain water content at 24 h, and therefore only the high-dose regimen was used for further evaluation, which reduced MMP-9 activity at 24 h. Further, high-dose minocycline improved spatial memory and attenuated neuronal loss in the hippocampus and cortex. The rotarod, T-maze, and water maze tests, but not the inclined plane test, detected neurobehavioral deficits in SAH rats at days 21–28. This study demonstrates that minocycline attenuates long-term functional and morphological outcomes after endovascular perforation-induced SAH. Long-term neurobehavioral assessments using the rotarod, T-maze, and water maze tests could be useful to evaluate the efficacy of therapeutic intervention after experimental SAH.
Key words: endovascular perforation, histopathology, long-term neurobehavior, minocycline, subarachnoid hemorrhage
Introduction
Outcome of aneurysmal subarachnoid hemorrhage (SAH) remains poor, and existing evidence suggests that many factors including early brain injury and cerebral vasospasm cause poor outcome (Cahill et al., 2006; Hansen-Schwartz et al., 2007; Macdonald et al., 2007). Animal studies usually focus on only one or a few factors among them, and the difference in endpoints may cause lack of translation of treatments effective in animals to humans. Moreover, long-term neurobehavioral assessment after therapeutic intervention is imperative, since SAH patients have long-lasting neurological deficits. Survivors of SAH have problems in memory, mood, learning, or neuropsychological function in the medium to long term (Hackett et al., 2000; Jeon et al., 2010; Kreiter et al., 2002; Ogden et al., 1993). Further, long-term cognitive deficits affect up to 50–60% survivors, and only fewer than half of previously employed SAH survivors return to work in the long term (Anderson et al., 2006; Mayer et al., 2002; Takata et al., 2008). Long-term neurobehavioral assessment as a measure of whole brain function is recommended to evaluate the efficacy of therapeutic interventions for experimental stroke (Stroke Therapy Academic Industry Roundtable, 1999). Further, long-term evaluation of potential neuroprotective agents is important for clinical translation. However, studies on long-term functional and morphological changes following therapeutic intervention after endovascular perforation-induced SAH are few (Silasi and Colbourne, 2009; Thal et al., 2008).
Minocycline is a second-generation tetracycline antibiotic that has been shown to be neuroprotective in various models of stroke (Homsi et al., 2009; Lee et al., 2007; Murata et al., 2008; Xu et al., 2004; Yrjanheikki et al., 1998) and in acute ischemic stroke in humans (Lampl et al., 2007). It has biological effects that are separate and distinct from its antimicrobial effects (Domercq and Matute, 2004; Yrjanheikki et al., 1998). Further, minocycline inhibits the activity and expression of matrix metalloproteinase (MMP)-9 (Stirling et al., 2005), which has also been implicated in the pathogenesis of early brain injury after SAH (Sehba et al., 2004; Suzuki et al., 2010a; Zongduo et al., 2010). A recently published study showed that minocycline improved outcomes and protected against early brain injury in an autologous blood injection SAH rat model (Guo et al., 2011). However, the long-term effect of minocycline on neurobehavior, neurocognition, and histopathology was not evaluated.
Furthermore, the endovascular perforation SAH model that we have used in this study mimics clinical mechanisms of artery rupture, and shows a high mortality and acute metabolic changes similar to clinical findings, and the model is considered to be the most suitable for investigating pathophysiological changes after SAH (Titova et al., 2009). Given limited studies in this model (Silasi and Colbourne, 2009; Thal et al., 2008), it would be worthwhile to examine which neurobehavioral tests would be useful for evaluating the long-term neurobehavior of SAH rats. Moreover, to our knowledge no study has investigated if the prevention of early brain injury eventually leads to an improvement in long-term neurological outcomes and memory impairments after experimental SAH.
The aim of this study was thus to examine (1) the potential long-term beneficial effects of minocycline after endovascular perforation-induced SAH in rats, and (2) to determine which neurobehavioral test would detect the long-term effects of SAH among a large number of neurobehavioral tests in the endovascular perforation rat SAH model.
Methods
Experimental animals
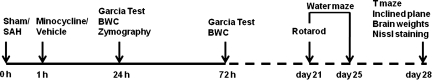
All the protocols used in this study were approved by the Institutional Animal Care and Use Committee at Loma Linda University. One hundred and eighteen adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing between 280 and 350 g were randomly assigned to the following 4 groups: sham, SAH+vehicle, SAH+minocycline 45 mg/kg, and SAH+minocycline 135 mg/kg. Minocycline or vehicle was administered 1 h after surgery, and outcomes were assessed in the short and long term (Fig. 1).
FIG. 1.
Schematic illustration of the timeline of the experiments (SAH, subarachnoid hemorrhage; BWC, brain water content).
Subarachnoid hemorrhage modeling
The endovascular perforation SAH rat model was produced as previously described with modifications (Benderson et al., 1995; Schwartz et al., 2000; Suzuki et al., 2010b). Anesthesia was induced with 3% isoflurane and maintained with 2–3% isoflurane. The animals were transorally intubated and respiration was maintained with a small rodent respirator (Harvard Apparatus, Holliston, MA). The left common carotid artery was exposed and the external carotid artery (ECA) was fashioned into a 3-mm stump. A 4-0 monofilament nylon suture was advanced into the internal carotid artery (ICA) from the ECA stump until resistance was felt. The suture was advanced further 3 mm to perforate the bifurcation of the anterior and middle cerebral arteries. The suture was immediately withdrawn to reperfuse the ICA. In sham animals the suture was advanced until resistance was felt, after which it was withdrawn without perforating the artery. The animals were returned to their cages after recovery from anesthesia and had free access to food and water.
Minocycline administration
Minocycline was purchased from Sigma-Aldrich, St. Louis, MO. The treatment groups received 45 mg/kg or 135 mg/kg of minocycline intraperitoneally 1 h after SAH induction. The drug was dissolved in phosphate-buffered saline (PBS; 0.1 mol/L, pH 7.4) as previously described (Homsi et al., 2009; Morimoto et al., 2005). The vehicle group received the same volume of PBS intraperitoneally 1 h after SAH induction.
Mortality
Mortality was assessed at 1–3 h, 3–6 h, 6–24 h, and weekly thereafter.
Experiment 1: Early brain injury after subarachnoid hemorrhage
SAH grading
The SAH grading of the animals at 24 and 72 h was determined by assessing high-resolution photographs of the brains at the time of sacrifice, as previously described (Sugawara et al., 2008). The animals received a total score ranging from 0 to 18.
Garcia test
We used the Garcia test with modifications (Garcia et al., 1995; Ostrowski et al., 2005) to evaluate the neurobehavioral deficits at 24 and 72 h post-SAH. Briefly, six parameters were tested, which allowed a total score of 18. Higher scores indicated greater function. These six tests included: spontaneous activity, symmetry in the movement of all four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch.
Brain water content (BWC)
Animals were sacrificed at 24 and 72 h post-SAH (n=6, respectively). The brains were quickly removed and separated into left and right hemispheres, brainstem, and cerebellum, and the specimens were weighed (wet weight). The samples were then placed in an oven at 105°C for 72 h (Suzuki et al., 2010a) and weighed again (dry weight). The percentage of BWC was calculated using the following formula: [(wet weight – dry weight)÷wet weight]×100.
Zymography
MMP-9 activity was measured by gelatin zymography as previously described (Chen et al., 2009; Tsubokawa et al., 2006; Wu et al., 2010). The rats were euthanized at 24 h post-SAH (n=5, respectively), and transcardially perfused with chilled (4°C) PBS, pH 7.4. The left hemisphere (perforation side) was homogenized in Ripa buffer (Santa Cruz Biotechnology, Santa Cruz, CA) for protein extraction. The samples were loaded and separated by 10% zymogram gelatin gel (Invitrogen, Carlsbad, CA). Following electrophoresis, the gel was renatured and then incubated with development buffer at 37°C for 48 h. Staining was performed with 0.5% Coomassie blue R-250 for 1 h and then destained with three changes of solution. Optical densities were normalized to positive controls and calculated as percentages above sham.
Experiment 2: Long-term neurobehavior assessment
Rotarod test
The rotarod test was performed at day 21 post-SAH to assess sensorimotor coordination and balance. We used the rotarod (Columbus Instruments, Columbus, OH) consisting of a rotating horizontal cylinder (7 cm diameter) divided into 9.5-cm-wide lanes. The animal was placed on the cylinder and was allowed to walk forward to avoid falling off the cylinder. The cylinder was started at 4 RPM and accelerated by 2 RPM every 5 sec (Hartman et al., 2009). A photobeam circuit recorded the latency to fall off the cylinder.
T-maze test
The T-maze test was performed at day 28 post-SAH to assess short-term or working memory (Fathali et al., 2010). The animal was placed in the stem of the T-maze (40×10 cm) and allowed to explore until an arm (46×10 cm) of the maze was chosen. Each animal performed 10 trials, and the spontaneous alternation rate was expressed as a ratio of alternating choices to the total number of choices (Zhou et al., 2010).
Water maze test
This test for learning and memory was performed at days 21–25 post-SAH (Lekic et al., 2010a,2010b). Briefly this test required that the animal find a submerged platform (11 cm diameter) in a metal pool (110 cm diameter) filled with water up to 15 cm of the upper edge and made opaque with non-toxic white tempera paint. An overhead camera recorded the swim path, allowing for quantification of swim distance and time spent in the probe quadrant by a computerized tracking system (Noldus Ethovision; Noldus, Tacoma, WA). The cued water maze test was performed on day 21. This test was used as a control to assess any sensorimotor and/or motivational deficits that could affect performance during the spatial water maze test. In this test the platform was made visible (5 mm above the surface of the water) with a 20-cm-tall pole placed over the platform. Each animal performed 10 trials per day in 5 blocks of 2 consecutive trials with a 10-min interval between successive blocks. The animal was released into the water opposite the platform, and the location of the platform was changed for each block of trials. The animal was allowed to remain on the platform for 5 sec after finding it or being guided to it. The spatial water maze test was performed on days 22 to 25 post-SAH. In this test the platform was submerged 1 cm below the surface of the water. Each animal performed 10 trials per day in 5 blocks of 2 consecutive trials with a 10-min interval between successive blocks. The location of the platform was changed every day and was kept in the same place throughout the day. The animal was allowed to remain on the platform for 10 sec after finding it or being guided to it. At the beginning of each day, the animal was tested with a 60-sec probe trial in which the platform was removed from the water. In this trial we recorded the amount of time spent in the target quadrant from which the platform was removed.
Inclined plane test
The inclined plane test for hindlimb function was performed at day 28 post-SAH. The rats were placed in a box (70 cm long, 20 cm wide, 10 cm tall) with an analog protractor and hinged base that was elevated at 5° intervals until the animal slipped backwards. The relative angle at which the animal could not maintain its position and started to slip backwards was measured (Lekic et al., 2010b; Wagner et al., 2010).
Experiment 3: Neuropathological analysis at day 28
Gross evaluation of brain and brain weights
At day 28 post-SAH the brains of the animals were harvested and grossly examined for signs of bleeding. The brain samples were divided into supra- and infratentorial regions and weighed on a high-precision balance (sensitivity±0.001 g). In order to determine changes in brain weight, we evaluated the ratio of brain weight to the body weight. Hemispheric weight loss has been used as the primary variable to estimate brain damage in neonatal hypoxia ischemia rats (Fathali et al., 2010; Zhou et al., 2010).
Histopathological analysis
At 28 days after SAH, the animals (n=8, respectively) were sacrificed under deep anesthesia and transcardially perfused with 300 mL of ice-cold PBS (0.1 mol/L, pH 7.4), followed by 300 mL of 4% paraformaldehyde solution. The brains were quickly removed and post-fixed in 4% formaldehyde solution at 4°C overnight, followed by 30% sucrose (weight/volume) for 3 days. Coronal sections 10 μm thick were taken every 500 μm at −3 mm to −4.3 mm from the bregma according to the coordinates in Paxinos and Watson (Paxinos and Watson, 1998) using a cryostat (CM3050S; Leica Microsystems, Buffalo Grove, IL), and mounted on poly-L-lysine-coated slides (Lekic et al., 2010b). Nissl staining was performed using 0.5% cresyl violet as previously described (Ostrowski et al., 2005). The sections were observed under a light microscope for neuronal morphology and cell count. The loss of neuronal density was evaluated according to established methods (Lekic et al., 2010a; MacLellan et al., 2008). Cells were counted at 400×magnification in five areas (250×250-μm grids) per hemisphere in the cortex, hippocampus, and diencephalon. Morphometric analysis involved computer-assisted (Image J 4.0 software; Media Cybernetics Inc., Silver Spring, MD) hand delineation of the cortex, hippocampus, and diencephalon, as previously described for other regions of the brain (Lekic et al., 2010a; MacLellan et al., 2008) based on Paxinos and Watson (Paxinos and Watson, 1998). The area of tissue in each of the three regions was calculated using the following equation as previously described with modifications (Lekic et al., 2010a; MacLellan et al., 2008): area of tissue loss=(area of uninjured side – area of injured side)/area of uninjured side.
Statistical analysis
Data were analyzed using the commercially available software (Sigma Plot 10.0; Systat Software, Inc., San Jose, CA). Statistical significance was verified by one-way analysis of variance (ANOVA), followed by Tukey's test for multiple comparisons as appropriate. Differences in BWC and MMP activity among groups were compared using Fisher's test. Water maze cued learning swim distance data were averaged into five blocks of two trials each, and analyzed with a group (sham, vehicle, and minocycline 135 mg/kg)×cued blocks (first, second, third, fourth, and fifth) repeated-measures ANOVA. Water maze spatial learning swim distance data were averaged into five blocks of two trials each, and analyzed with a group (sham, vehicle, and minocycline 135 mg/kg)×location (east, west, northeast, and southwest)×spatial blocks (first, second, third, fourth, and fifth) repeated-measures ANOVA. Water maze probe trial data (percentage of the trial spent searching the probe quadrant) were analyzed using group (sham, vehicle, and minocycline 135 mg/kg)×spatial location (east, west, northeast, and southwest) repeated-measures ANOVA. Significant ANOVA interactions were further explored using the conservative Scheffe post-hoc test. Difference in mortality was tested using Fisher's exact test, with p<0.05 considered statistically significant.
Results
Mortality
There was no mortality in the sham animals. In the short term, 9 out of 39 (23%) rats died in the vehicle group, 2 out of 10 (20%) rats died in the low-dose treatment group, and 2 out of 20 (10%) rats died in the high-dose treatment group. In the long term, 3 out of 13 animals (23%) died in the vehicle group, and 2 out of 11 (18%) animals died in the high-dose treatment group. Mortality was not significantly different between groups (p>0.05), and 72.2% of the total mortality occurred within 1–3 h post-SAH induction, and 27.8% of the mortality occurred at 6–24 h post-SAH induction. There was no mortality after 24 h in our study.
Experiment 1: Early brain injury after subarachnoid hemorrhage
SAH grade
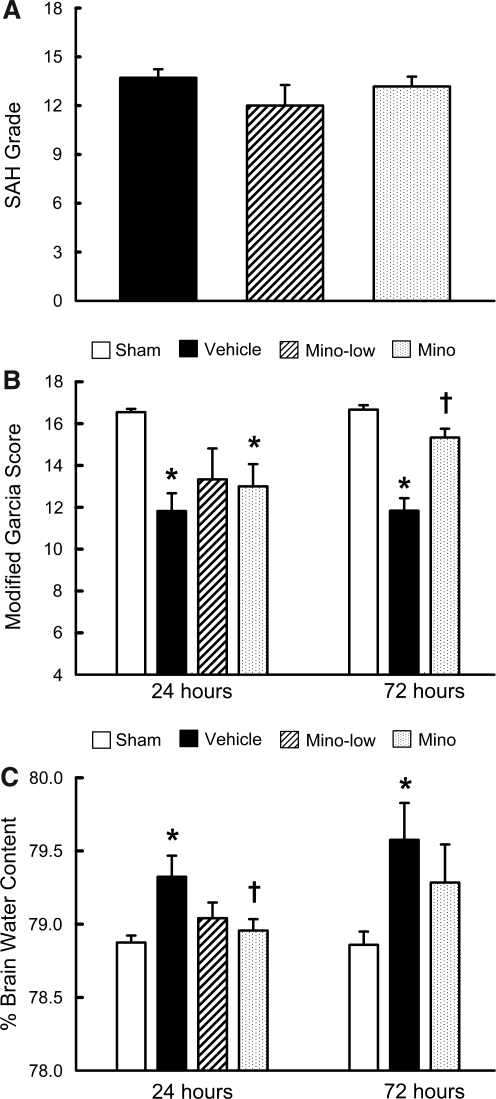
The average SAH grade was 13.7±0.5 in the vehicle group (n=17), 12±1.3 in the low-dose treatment group (n=6), and 13.2±0.6 in the high-dose treatment group (n=17). There was no significant difference (p>0.05, Fig. 2A) in the SAH grades among the groups.
FIG. 2.
Subarachnoid hemorrhage (SAH) grading score (A), neurological score (B), and brain water content in the left hemisphere (C), in SAH groups treated with phosphate-buffered saline (Vehicle), 45 mg/kg of minocycline (Mino-low), 135 mg/kg of minocycline (Mino), and sham-operated rats (Sham), at 24 and 72 h post-SAH (*p<0.05 versus sham; †p<0.05 versus vehicle).
Garcia test
The Garcia score was significantly lower in the vehicle group compared to the sham group at 24 h (11.8±0.9 versus 16.6±0.2) and 72 h (11.8±0.6 versus 16.7±0.2) post-SAH (p<0.05, Fig. 2B). High-dose minocycline significantly improved Garcia scores at 72 h (15.3±0.4 versus 11.8±0.6; p<0.05, Fig. 2B).
Brain water content
BWC in the left hemisphere (perforation side) was significantly reduced in the high-dose minocycline group compared to the vehicle group at 24 h (78.9±0.1% versus 79.3±0.1%) post-SAH (p<0.05, Fig. 2C). However, minocycline failed to reduce BWC at 72 h, although there was a trend toward reduction (79.2±0.3% versus 79.6±0.3%; p=0.09, Fig. 2C). There was no significant difference in BWC in the right hemisphere, cerebellum, and brainstem among the groups (data not shown). Since low-dose minocycline showed no significant improvements in neurological scores and BWC compared with the vehicle group, only the high-dose group was used for further analysis.
Matrix metalloproteinase activity
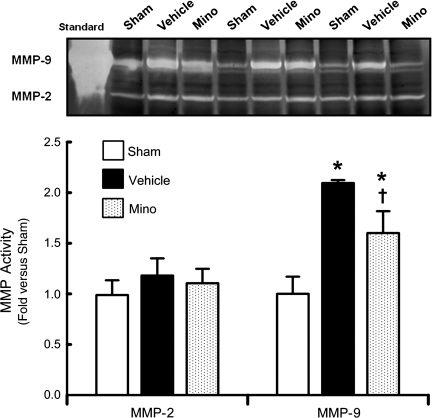
MMP-9 activity in the left hemisphere (perforation side) was significantly increased in the vehicle group compared to the sham animals (2.1±0.03 versus 1±0.17-fold) at 24 h (p<0.05, Fig. 3), and high-dose minocycline significantly reduced MMP-9 activity (1.6±0.22-fold) (p<0.05, Fig. 3). There was no difference in MMP-2 activity among the groups (p>0.05).
FIG. 3.
Changes in matrix metalloproteinase (MMP)-2 and MMP-9 activity at 24 h post-SAH (n=5 each). Gelatin zymography and quantitative analysis of MMP-2 and MMP-9 activity in sham-operated rats (Sham) and the SAH groups treated with phosphate-buffered saline (Vehicle), or 135 mg/kg of minocycline (Mino). MMP-9 activity is expressed as percentage of mean sham levels. MMP-9 standard was loaded as positive control (Standard; *p<0.05 versus sham; †p<0.05 versus vehicle; SAH, subarachnoid hemorrhage).
Experiment 2: Long-term neurobehavior assessment
Rotarod test
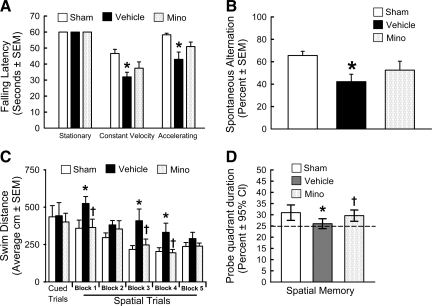
The vehicle group had a significantly shorter latency to fall compared to the sham group, in both the constant and accelerating velocity rotarod tests at day 21 post-SAH (p<0.05, Fig. 4A). High-dose minocycline improved the performance in both the tests, but the difference was not significant between the minocycline and vehicle groups (p>0.05, Fig. 4A).
FIG. 4.
Long-term neurobehavior assessment. Rotarod (A), T-maze (B), and water maze test spatial trials (C) and probe trials (D) in the sham-operated rats (Sham), and SAH groups treated with vehicle (Vehicle) or 135 mg/kg minocycline (Mino; *p<0.05 versus sham, †p<0.05 versus vehicle; SAH, subarachnoid hemorrhage; SEM, standard error of the mean; CI, confidence interval).
T-maze test
The vehicle group had significantly worse working memory than the sham group in the T-maze test at day 28 post-SAH (p<0.05, Fig. 4B). High-dose minocycline improved the performance, but was not significant compared with the vehicle group (p>0.05, Fig. 4B).
Inclined plane test
All the groups performed equally in the inclined plane test (data not shown).
Water maze test
All the groups performed equally in the cued trials (p>0.05, Fig. 4C). In the spatial maze test, the vehicle group had significantly worse spatial learning than the sham animals, and traveled a longer distance to find the platform (p<0.05, Fig. 4C). Animals in the high-dose minocycline group traveled significantly a shorter distance to find the platform (p<0.05, Fig. 4C), and performed better with subsequent blocks. In the probe trials, the vehicle group spent significantly less time in the target quadrants from which the probe had been removed compared to the sham group, and minocycline treatment improved the duration spent in the probe quadrant (p<0.05, Fig. 4D). Further, repeated-measures ANOVA showed no group differences for the cued water maze test. For spatial memory, there was a significant group×block interaction in vehicle group (p=0.002), which performed worse than the minocycline and sham groups, which did not differ. There was a main effect of group for the probe trial (p=0.02), and the vehicle group performed worse than the minocycline and sham groups, which did not differ.
Experiment 3: Brain examination at day 28
Gross examination of the brain and brain weights
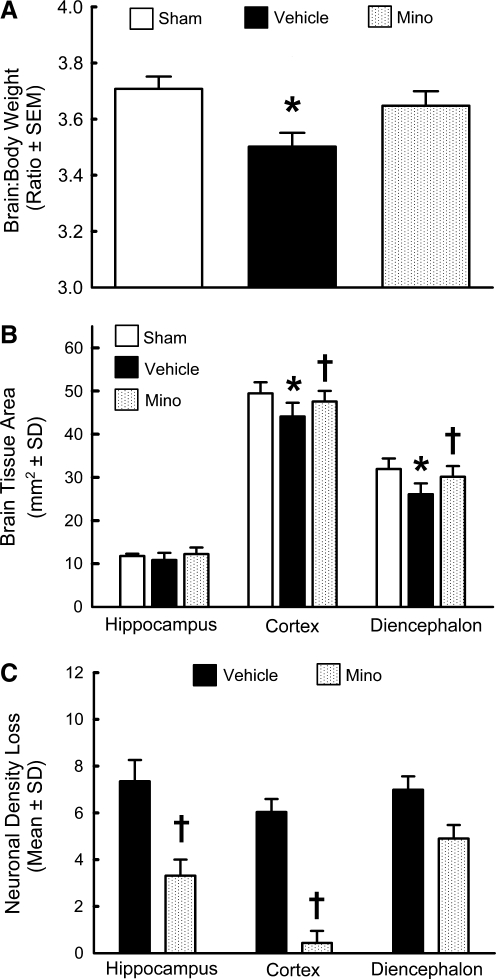
Gross examination of the brains of the animals sacrificed at 28 days post-SAH demonstrated discoloration on the ventral side, which showed evidence of bleeding in both the vehicle and the treatment groups. We also observed cavitation in the diencephalon in one of the vehicle animals, where blood was localized after SAH. The ratio of supratentorial brain weight to the body weight was significantly reduced in the vehicle group (p<0.05), but not in the minocycline group, compared to the sham group (p>0.05, Fig. 5A), although there was no significant difference between the vehicle and minocycline groups. Further, body weights were not significantly different among the groups (data not shown).
FIG. 5.
Neuropathological analysis at day 28. Brain weights (A), brain tissue area (B), and neuronal density loss (C), in the hippocampus, cortex, and diencephalon at day 28 post-SAH in the sham-operated rats (Sham), and SAH animals treated with phosphate-buffered saline (Vehicle), or 135 mg/kg minocycline (Mino; n=8 each; *p<0.05 versus sham; †p<0.05 versus vehicle; SAH, subarachnoid hemorrhage).
Histopathological analysis
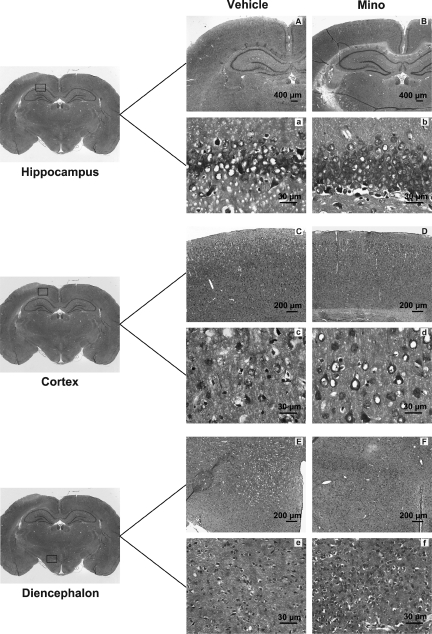
Morphometric analysis revealed significant tissue loss in the cortex and the diencephalon ipsilateral to the perforation site (p<0.05, Fig. 5B). Compared to the vehicle group the tissue loss was significantly less in the treatment group, both in the cortex and the diencephalon (p<0.05, Fig. 5B). Neuronal counts revealed a significant decrease in neuronal density loss in the treatment group compared to the vehicle group in the hippocampus CA1 region and in the cortex (p<0.05, Fig. 5C). Further, the damaged neurons exhibited a dark and shrunken appearance (Fig. 6), as has been previously reported (Cheng et al., 2011; Ostrowski et al., 2005). Relatively fewer damaged neurons were detected in the treatment group in the hippocampus (Fig. 6b), cortex (Fig. 6d), and diencephalon (Fig. 6f), compared to the vehicle group (Fig. 6a, 6c, and 6e), respectively.
FIG. 6.
Nissl staining of brain sections at day 28 post-SAH in the SAH animals treated with phosphate-buffered saline (Vehicle), or 135 mg/kg minocycline (Mino). Damaged neurons exhibited shrunken and dark appearance. Less neuronal loss is seen in the minocycline group in the hippocampus (B, b), cortex (D, d), and diencephalon (F, f ), compared to the vehicle group hippocampus (A, a), cortex (C, c), and diencephalon (E, e). Scale bars represent 400 μm in the hippocampus, and 200 μm in the cortex and diencephalon under low magnification in all panels. Under high magnification scale bars represent 30 μm in all panels (SAH, subarachnoid hemorrhage).
Discussion
Long-term evaluation of a therapeutic intervention after SAH is clinically relevant since functional outcomes are a major endpoint for clinical translation. This study demonstrates the long-term efficacy of minocycline in terms of improved functional outcome, neurocognition, and histopathology, after endovascular perforation-induced SAH in rats. Among the long-term neurobehavioral tests performed, the rotarod, T-maze, and water maze tests could detect long-term neurobehavioral impairments in this model. Other important findings in this study were the first to show neuronal and tissue loss in the diencephalon, cortex, and hippocampus, as well as a significant loss in brain weight in a clinically relevant SAH rat model at day 28 post-SAH.
Minocycline has been shown to be neuroprotective in various non-SAH animal models. It is highly lipophilic and can penetrate the brain tissue effectively and is well tolerated (Yrjanheikki et al., 1998). Moreover, minocycline is known to have pleiotropic effects, including anti-apoptotic (Domercq and Matute, 2004), anti-inflammatory (Yrjanheikki et al., 1998), antioxidant (Morimoto et al., 2005), and MMP inhibitory actions (Domercq and Matute, 2004). A recently published study showed that minocycline reduced MMP-9 expression and protected against early brain injury after autologous blood injection-induced SAH in rats (Guo et al., 2011). We showed that neuroprotection conferred by minocyline in the acute phase rendered long-term improvements in functional outcomes, memory abilities, and histopathological changes after SAH. The endovascular perforation SAH rat model that we used in our study mimics a clinical scenario (Titova et al., 2009) more closely than a blood injection model, which makes this study clinically relevant.
In this study different time points were chosen for the short-term and long-term neurobehavior analysis, based on observations from previous studies that focused on neurobehavioral outcomes after SAH. Following SAH, several pathological mechanisms are activated after the initial bleed, which can lead to early brain injury (Sehba et al., 2011). Early brain injury, which occurs during the first 72 h following SAH, is associated with acute sensorimotor deficits likely resulting from various insults like increased intracranial pressure, decreased cerebral blood flow, blood–brain barrier disruption, and global cerebral edema (Sehba et al., 2011; Ostrowski et al., 2006). Sensorimotor deficits were present at days 1–3 after SAH by endovascular perforation in rats and mice (Ostrowski et al., 2005; Sozen et al., 2009). We therefore chose 24 and 72 h for acute neurobehavioral assessment using the Garcia test, which showed significantly worse performance by the SAH group on this sensorimotor test. However, a previous study by Thal and associates showed that there was no difference in neurobehavioral performance up to 7 days, between treated and untreated rats evaluated by a beam balance task, a prehensile traction task, the rotarod, and a 6-point motor function score (Thal et al., 2008). Further, significant long-lasting motor deficits were not detected over days 3–21 in a intraluminal perforation SAH rat model, which was assessed using the tapered beam test, the forelimb asymmetry test, and the horizontal ladder task (Silasi and Colbourne, 2009). We therefore attempted to determine whether the inclined plane test would be sensitive to detect any deficits, if present, in the long-term. Since there is a possibility of improved motor function and rehabilitation that can occur as a result of repeated motor learning (Bar-Haim et al., 2010; Niemeier et al., 2011; Piron et al., 2010), we used the inclined plane test at day 28 only to determine if any motor deficit was present after SAH in the long-term. The inclined plane test in this study failed to detect motor deficits at day 28. However, it would be interesting to see whether this test is sensitive to detect deficits if used for evaluation in the acute phase. Also, vestibulomotor dysfunction was shown over the 4-week period after SAH by two injections of blood into the cisterna magna in rats (Takata et al., 2008), so we therefore used the rotarod test to detect if dysfunctions in sensorimotor coordination and balance would be present at day 21.
Clinically, a substantial portion of survivors of SAH have long-term cognitive impairments, rather than focal neurological deficits, causing disability (Hackett and Anderson, 2000; Jeon et al., 2010; Kreiter et al., 2002; Mayer et al., 2002). Patients with good neurological outcomes demonstrated impaired memory and cognitive function at 12 months after SAH (Ogden et al., 1993). However, few studies have assessed long-term neurobehavioral function after treatment in an endovascular perforation SAH model (Silasi and Colbourne, 2009; Thal et al., 2008). To assess cognitive function, the Morris water maze has been used in experimental SAH; cognitive deficits were not observed at days 2–4, but were present at day 5 in a pre-chiasmatic injection rat model (Jeon et al., 2010), at day 21 in an endovascular perforation rat model (Silasi and Colbourne, 2009), and at days 29–35 in a double-hemorrhage rat model (Takata et al., 2008). We therefore performed water maze testing at days 21–25, and T-maze testing at day 28 post-SAH to determine cognitive dysfunction, which revealed significant deficits in spatial learning and memory, as well as short-term or working memory. Based on these observations, we propose to use the modified Garcia test for acute study, and the rotarod, water maze, and T-maze tests for the long-term study in the endovascular perforation SAH model in rats. These tests could be beneficial to assess the acute and long-term outcomes to determine therapeutic efficacy, which is important for clinical translation (Stroke Therapy Academic Industry Roundtable, 1999).
Although the mechanisms underlying cognitive deficits have not been fully elucidated, various factors have been attributed. It seems that acute physiological changes induced by early brain injury, and delayed complications such as cerebral vasospasm, could lead to acute sensorimotor, long-term functional, and morphological consequences following SAH. The acute increase in intracranial pressure, transient global ischemia, microthromboemboli, and vasospasm all cause cerebral hypoperfusion, leading to neuronal cell death (Jeon et al., 2010; Kreiter et al., 2002; Ostrowski et al., 2006; Prunell et al., 2005; Takata et al., 2008). Also, blood components collecting in the subarachnoid space could cause neuronal cell death (Ostrowski et al., 2006). In fact, neuronal loss was shown in the cortex and hippocampus at day 35 in a rat double-hemorrhage model (Takata et al., 2008), which correlated with cognitive deficits. Similarly, we observed neuronal loss in the cortex and CA1 region of the hippocampus at day 28 in the endovascular perforation rat model. In addition, we first demonstrated that tissue injury and neuronal loss also occurred in the diencephalon, where blood is localized after SAH, and has a key role in memory processing (Vann and Aggleton, 2003; Vann, 2009). Minocycline attenuated neuronal loss in the cortex and CA1 region, which is consistent with previous studies that have shown reduced neuronal death with minocycline treatment. Various mechanisms including reduced oxidative stress (Morimoto et al., 2005), inhibition of microglia proliferation and activation (Tikka et al., 2001; Yrjanheikki et al., 1998), and inhibition of glutamate-induced toxicity (Tikka et al., 2001), have been proposed to reduce neuronal loss in vivo and in vitro with minocycline.
Our study had limitations. Given the pleiotropic effects of minocycline, further evaluation is required to determine the mechanism of neuroprotection provided by minocycline after SAH. Also, the mechanisms and pathophysiological processes involved in cognitive deficits were not evaluated, and this needs to be explored for effective intervention.
In conclusion, this study showed that early treatment with minocycline after SAH provided long-term benefits in terms of cognitive function and improved histopathology. Thus, long-term neurobehavioral assessments would be beneficial to evaluate the efficacy of therapeutic interventions for experimental SAH, using the rotarod, T-maze, and water maze tests.
Acknowledgments
This study is partially supported by National Institutes of Health grants NS053407 to J.H.Z., and NS060936 to J.T.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson S.W. Todd M.M. Hindman B.J. Clarke W.R. Torner J.C. Tranel D. Yoo B. Weeks J. Manzel K.W. Samra S. Effects of intraoperative hypothermia on neuropsychological outcomes after intracranial aneurysm surgery. Ann. Neurol. 2006;60:518–527. doi: 10.1002/ana.21018. [DOI] [PubMed] [Google Scholar]
- Bar-Haim S. Harries N. Nammaourah I. Oraibi S. Malhees W. Loeppky J. Perkins N.J. Belokopytov M. Kaplanski J. Lahat E. MERC project. Effectiveness of motor learning coaching in children with cerebral palsy: a randomized controlled trial. Clin. Rehabil. 2010;24:1009–1020. doi: 10.1177/0269215510371428. [DOI] [PubMed] [Google Scholar]
- Bederson J.B. Germano I.M. Guarino L. Cortical blood flow and cerebral perfusion pressure in a new non-craniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- Cahill J. Calvert J.W. Zhang J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- Cheng O. Ostrowski R.P. Wu B. Liu W. Chen C. Zhang J.H. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Hartman R. Ayer R. Marcantonio S. Kamper J. Tang J. Zhang J.H. Matrixmetalloproteinases inhibition provides neuroprotection against hypoxia-ischemia in developing brain. J. Neurochem. 2009;111:726–736. doi: 10.1111/j.1471-4159.2009.06362.x. [DOI] [PubMed] [Google Scholar]
- Domercq M. Matute C. Neuroprotection by tetracyclines. Trends Pharmacol. Sci. 2004;25:609–612. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Fathali N. Ostrowski R.P. Lekic T. Jadhav V. Tong W. Tang J. Zhang J.H. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit. Care. Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J.H. Wagner S. Liu K.F. Hu X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- Guo Z.D. Wu H.T. Sun X.C. Zhang X.D. Zhang J.H. Protection of minocycline on early brain injury after subarachnoid hemorrhage in rats. Acta Neurochir. Suppl. 2011;110:71–74. doi: 10.1007/978-3-7091-0353-1_13. [DOI] [PubMed] [Google Scholar]
- Hackett M.L. Anderson C.S. Health outcomes 1 year after subarachnoid hemorrhage: An international population-based study. The Australian Cooperative Research on subarachnoid hemorrhage study group. Neurology. 2000;55:658–662. doi: 10.1212/wnl.55.5.658. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J. Vajkoczy P. Macdonald R.L. Pluta R.M. Zhang J.H. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol. Sci. 2007;28:252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Hartman R. Lekic T. Rojas H. Tang J. Zhang J.H. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi S. Federico F. Croci N. Palmier B. Plotkine M. Leroux C.M. Tehrani M.J. Minocycline effects on cerebral edema: Relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 2009;1291:122–132. doi: 10.1016/j.brainres.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Jeon H. Ai J. Sabri M. Tariq A. Macdonald R.L. Learning deficits after experimental subarachnoid hemorrhage in rats. Neuroscience. 2010;169:1805–1814. doi: 10.1016/j.neuroscience.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Kreiter K.T. Copeland D. Bernardini G.L. Bates J.E. Peery S. Claassen J. Du Y.E. Stern Y. Connolly E.S. Mayer S.A. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002;33:200–209. doi: 10.1161/hs0102.101080. [DOI] [PubMed] [Google Scholar]
- Lampl Y. Boaz M. Gilad R. Lorberboym M. Dabby R. Rapoport A. Anca-Hershkowitz M. Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Lee C.Z. Xue Z. Zhu Y. Yang G.Y. Young W.L. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- Lekic T. Hartman H. Rojas H. Manaenko A. Chen W. Ayer R. Tang J. Zhang J.H. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J. Neurotrauma. 2010a;27:627–637. doi: 10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic T. Rolland W. Hartman R. Kamper J. Suzuki H. Tang J. Zhang J.H. Characterization of brain injury, neurobehavioral profiles, and histopathology in a rat model of cerebellar hemorrhage. Exp. Neurol. 2010b;227:96–103. doi: 10.1016/j.expneurol.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R.L. Pluta R.M. Zhang J.H. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat. Clin. Pract. Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- MacLellan C.L. Silasi G. Poon C.C. Edmundson C.L. Buist R. Peeling J. Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Mayer S.A. Kreiter K.T. Copeland D. Bernardini G.L. Bates J.E. Peery S. Classen J. Du Y.E. Connolly E.S., Jr Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59:1750–1758. doi: 10.1212/01.wnl.0000035748.91128.c2. [DOI] [PubMed] [Google Scholar]
- Morimoto N. Shimazawa M. Yamashima T. Nagai H. Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res. 2005;1044:8–15. doi: 10.1016/j.brainres.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Murata Y. Rosell A. Scannevin R.H. Rhodes K.J. Wang X. Lo E.H. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeier J.P. Kreutzer J.S. Marwitz J.H. Gary K.W. Ketchum J.M. Efficacy of a brief acute neurobehavioural intervention following traumatic brain injury: a preliminary investigation. Brain Inj. 2011;25:680–690. doi: 10.3109/02699052.2011.573520. [DOI] [PubMed] [Google Scholar]
- Ogden J.A. Mee E.W. Henning M. A prospective study of impairment of cognition and memory and recovery after subarachnoid hemorrhage. Neurosurgery. 1993;33:572–587. doi: 10.1227/00006123-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Ostrowski R.P. Colohan A.R. Zhang J.H. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol. Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- Ostrowski R.P. Colohan A.R.T. Zhang J.H. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Sterotaxic Coordinates. Academic Press; Sydney: 1998. [Google Scholar]
- Piron L. Turolla A. Agostini M. Zucconi C.S. Ventura L. Tonin P. Dam M. Motor learning principles for rehabilitation: a pilot randomized controlled study in poststroke patients. Neurorehabil. Neural Repair. 2010;24:501–508. doi: 10.1177/1545968310362672. [DOI] [PubMed] [Google Scholar]
- Prunell G.F. Svendgaard N.A. Alkass K. Mathiesen T. Delayed cell death related to acute cerebral blood flow changes following subarachnoid hemorrhage in the rat brain. J. Neurosurg. 2005;102:1046–1054. doi: 10.3171/jns.2005.102.6.1046. [DOI] [PubMed] [Google Scholar]
- Schwartz A.Y. Masago A. Sehba F.A. Bederson J.B. Experimental models of subarachnoid hemorrhage in the rat: a refinement of the endovascular filament model. J. Neurosci. Methods. 2000;96:161–167. doi: 10.1016/s0165-0270(00)00156-4. [DOI] [PubMed] [Google Scholar]
- Sehba F.A. Mostafa G. Knopman J. Friedrich V., Jr. Benderson J.B. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J. Neurosurg. 2004;101:633–640. doi: 10.3171/jns.2004.101.4.0633. [DOI] [PubMed] [Google Scholar]
- Sehba F.A. Pluta R.M. Zhang J.H. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol. Neurobiol. 2011;43:27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silasi G. Colbourne F. Long-term assessment of motor and cognitive behaviours in the intraluminal perforation model of subarachnoid hemorrhage in rats. Behav. Brain Res. 2009;198:380–387. doi: 10.1016/j.bbr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Sozen T. Tsuchiyama R. Hasegawa Y. Suzuki H. Jadhav V. Nishizawa S. Zhang J.H. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D.P. Koochesfahani K.M. Steeves J.D. Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Sugawara T. Ayer R. Jadhav V. Zhang J.H. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J. Neurosci. Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Ayer R. Sugawara T. Chen W. Sozen T. Hasegawa Y. Kanamaru K. Zhang J.H. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit. Care Med. 2010a;38:612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Hasegawa Y. Kanamaru K. Zhang J.H. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010b;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K. Sheng H. Borel C.O. Laskowitz D.T. Warner D.S. Lombard F.W. Long-term cognitive dysfunction following experimental subarachnoid hemorrhage: new perspectives. Exp. Neurol. 2008;213:336–344. doi: 10.1016/j.expneurol.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Thal S.C. Mebmer K. Elsaesser R.S. Zausinger S. Neurological impairment in rats after subarachnoid hemorrhage—A comparison of functional tests. J. Neurol. Sci. 2008;268:150–159. doi: 10.1016/j.jns.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Tikka T. Fiebich B.L. Goldsteins G. Keinanen R. Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titova E. Ostrowski R.P. Zhang J.H. Tang J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol. Res. 2009;31:568–581. doi: 10.1179/174313209X382412. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T. Solaroglu I. Yatsushige H. Cahill J. Yata K. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- Vann S.D. Aggleton H.P. Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic tract. J. Neurosci. 2003;23:3506–3514. doi: 10.1523/JNEUROSCI.23-08-03506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann S.D. Gudden's ventral tegmental nucleus is vital for memory: re-evaluating diencephalic inputs for amnesia. Brain. 2009;132:2372–2384. doi: 10.1093/brain/awp175. [DOI] [PubMed] [Google Scholar]
- Wagner A.P. Stöcker K. Balseanu A.T. Rogalewski A. Diederich K. Minnerup J. Margaritescu C. Schäbitz W.F. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010;41:1027–1031. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- Wu B. Ma Q. Khatibi N. Chen W. Sozen T. Cheng O. Tang J. Ac-YVAD-CMK decreases blood brain barrier degradation by inhibiting caspase-1 activation of interleukin-1β in intracerebral hemorrhage mouse model. Trans. Stroke Res. 2010;1:57–64. doi: 10.1007/s12975-009-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. Fagan S.C. Waller J.L. Edwards D. Borlongan C.V. Zheng J. Hill W.D. Feuerstein G. Hess D.C. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rat. BMC Neurol. 2004;26:1–7. doi: 10.1186/1471-2377-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J. Keinanen R. Pellikka M. Hokfelt T. Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Lekic T. Fathali N. Ostrowski R.P. Martin R.D. Tang J. Zhang J.H. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;4:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zongduo G. Xiaochuan S. Zhaohui H. Yong J. Xiaodong Z. Zhang J.H. Matrix metalloproteinase-9 potentiates early brain injury after subarachnoid hemorrhage. Neurol. Res. 2010;32:715–720. doi: 10.1179/016164109X12478302362491. [DOI] [PubMed] [Google Scholar]