Abstract
Previous studies revealed that curcumin is neuroprotective in diseases of the central nervous system such as cerebral ischemia and traumatic brain injury. However, the effect of curcumin on intracerebral hemorrhage remains unclear. We, therefore, investigated the pre-clinical effect of curcumin treatment on neurological outcomes following intracerebral hemorrhage, using a mouse model. Intracerebral hemorrhage was induced by autologous blood injection into the right basal ganglia. Curcumin (150 mg/kg) was administered 15 min after intracerebral hemorrhage. Grid walk and neurological scores were evaluated at 1, 3, 7, and 14 days post-injury. Mice were killed at 24 h or 28 days following injury, for histological examination. Evans Blue and water content in the ipsilateral and contralateral hemispheres were measured to evaluate the extent of blood–brain barrier disruption and brain edema. Zonula occludens-1 was detected by immunostaining. In situ zymography was used to measure the localization and focal enzymatic activity of matrix metalloproteinase. Our results demonstrated that curcumin reduced brain edema, measured by alleviated water content and Evans Blue leakage at 24 h (p<0.05). Lateral ventricle measurements indicated that curcumin reduced brain tissue loss in the ipsilateral hemisphere (p<0.05). The same dose of curcumin also significantly attenuated neurological deficits at 1 and 3 days of intracerebral hemorrhage (p<0.05). Immunostaining showed that tight junction continuity around the hematoma was better sustained in curcumin-treated mice than in vehicle-treated mice. At 24 h, the number of matrix metalloproteinase-positive cells was significantly reduced by curcumin (p<0.05). Our study suggests that curcumin ameliorates intracerebral hemorrhage damage by preventing matrix metalloproteinase-mediated blood–brain barrier damage and brain edema, which might provide therapeutic potential for intracerebral hemorrhage.
Introduction
Intracerebral hemorrhage (ICH) represents ∼15% of all strokes, and has much higher mortality than ischemic stroke. Although hemorrhagic volume is a key factor that affects ICH outcome, several studies have shown that the hematoma itself can lead to various degrees of brain edema around it, which is associated with neurological deficits in patients (Ropper, 1986; Zazulia et al., 1999). Increasing permeability of the blood–brain barrier (BBB), which remains intact to large molecules for the first several hours after ICH, contributes to the formation of brain edema 12 h later (Yang et al., 1994).
Curcumin is a yellow substance from the root of the plant Curcuma longa Linn. It was reported that curcumin alleviated neuronal injury in animal models of several neurological disorders, including ischemic stroke (Jiang et al., 2007; Thiyagarajan and Sharma, 2004), traumatic brain injury (TBI) (Wu et al., 2006) and Parkinson's disease (Wang et al., 2010). It functions through diverse neuroprotective mechanisms including antioxidation, anti-apoptosis, anti-inflammation, preventing BBB damage, and reducing edema (Dohare et al., 2008: Jiang et al., 2007; Rathore et al., 2008; Thiyagarajan and Sharma, 2004; Zhao et al., 2010). These studies led to clinical trials in progress, such as for Alzheimer's disease (Baum et al., 2008) over the next few years, and the results may provide a deeper understanding of the therapeutic potential of curcumin and other curcuminoids in neurological disorders.
These aforementioned studies reported improved neuroprotection and neurological outcomes when curcumin was administered. However, whether or not curcumin can reduce injury in animal model of ICH remains unclear. In the present study, we used mouse model of ICH to test the hypothesis that curcumin is neuroprotective after ICH, and can improve the neurological outcome of the animals.
Methods
ICH mouse model
All experimental procedures were approved by the Shanghai Jiao Tong University experimental ethics committee. Mice were anesthetized with a single intraperitoneal dose of ketamine (100 mg/kg) and xylazine (10 mg/kg). The mice were placed in a stereotactic frame (RWD Life Science co., Shenzhen, China) and subjected to ICH using autologous blood infusion (Belayev et al., 2003). A 1-mm burr hole was drilled 2.5 mm lateral to the midline and 0.5 mm anterior to bregma. Autologous whole blood (50 μL) was drawn from the femoral artery into a capillary tube. A 30-gauge needle was advanced 3.0 mm into the right striatum. A total of 50 μL autologous whole blood was injected via double injection technique using a microinfusion pump (WPI, Sarasota, FL). An initial amount of 5 μL was delivered at a rate of 1.5 μL/min. Following a 10-min interval without injection, the remaining 45 μL was delivered at a rate of 3 μL/min. The needle was then left in place for another 10 min to minimize backflow. After the withdrawal of the needle, the scalp was sutured. The animals was allowed to recover in a temperature-controlled incubator for 45 min post-procedure, with body temperature maintained at 37°C using a feedback-controlled heating pad, before being returned back into the cage.
Curcumin treatment
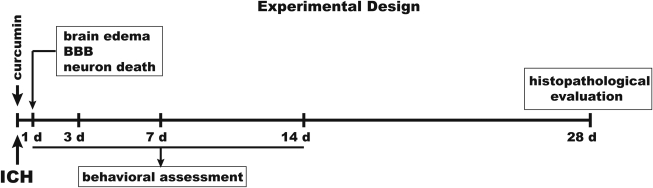
Curcumin (Purity ≥98%; LKT Laboratories Inc., St. Paul, MN) dissolved in corn oil was given intraperitoneally at a dose of 150 mg/kg 15 min after ICH (Fig. 1). This dose approximated those that have been used in experimental models of ischemic stroke or multiple sclerosis. Control mice received intraperitoneal injections of equal volumes of corn oil.
FIG. 1.
Experimental design. Autologous blood injection and curcumin treatment were performed on day 0. Animals received 150 mg/kg of curcumin injection intraperitoneally15 min after blood injection. BBB disruption, brain edema, and neuronal death were assessed after 24 h. Behavioral assessment was performed after injection at days 1, 3, 7, and 14. Histopathological measurements were conducted to examine brain tissue loss and ventricle enlargement at day 28.
Measurement of Evans Blue
The integrity of the BBB was investigated by measuring the extravasation of Evans Blue in curcumin-treated animals and controls (n=8 in each group). Evans Blue dye (2% in saline, 4 mL/kg) was given intravenously 22 h after ICH. Two h after Evans Blue injection, the chest wall was opened under lethal anesthesia and the animal was perfused with 0.01 M phosphate-buffered saline (PBS) through the left ventricle to remove the intravascular localized dye, until colorless perfusion fluid was obtained from the right atrium. After decapitation, the brain was removed and dissected into left and right hemispheres and each hemisphere was weighed. Brain samples were then placed in 1 mL 50% trichloroacetic acid solution and then homogenized and centrifuged at 21,000g for 20 min. The supernatant (containing Evans Blue) was diluted 1:3 with ethanol and its absorbance at 610 nm was measured using a spectrophotometer. The tissue content of Evans Blue was quantified from a linear standard curve and was expressed as micrograms per gram of brain tissue.
Measurement of brain water content
Mice were killed under deep pentobarbital anesthesia at 24 h. Brain tissue samples were weighed before and after dehydration in an oven at 100°C for >24 h. Tissue water content (%) was calculated as [(wet weight-dry weight)/wet weight]×100.
Fluoro-Jade® C (F-J C) staining
F-J C staining was used to assess dying neurons (Schmued et al., 1997) by incubating brain sections in potassium permanganate and F-J C (Millipore, Temecula, CA) working solution. At high magnification (objective magnification×40) aided by using an ocular reticule, the number of FJ-C positive neurons were counted in four fields immediately adjacent to hematoma as described (Xue et al., 2006). The sum of four fields averaged from three sections was used to depict the number of FJ-C positive cells per mouse. Analyses were evaluated following blind procedure.
Histopathological evaluation
Mice were killed by an overdose of ketamine/xylazine and were transcardiac perfused with 4% paraformaldehyde. The brains were removed, stored in 4% paraformaldehyde for 1 day, and then immersed in 25% sucrose for 3 days at 4°C. The brains were embedded and sectioned with a cryostat. Coronal sections from 1 mm anterior and 1 mm posterior to the blood injection site were used for hematoxylin and eosin staining. ImageJ software (National Institutes of Health) was used to trace and tabulate lateral ventricular areas of damaged brain.
Behavioral assessments
All animals underwent behavioral testing after surgery and were scored by experimenters who were blinded to both neurological and treatment conditions. Grid walking was used to evaluate motor coordination and balance by testing the ability of animals to traverse a grid. The apparatus consists of a bridge made of two tall walls connected by a floor of small, irregularly spaced bars forming a grid. Animals were videotaped and the number of drops made during a 1-m distance was recorded. Total neurological score was calculated as the sum of scores on forward visual placing (range: 0–2), lateral visual placing (0–2), dorsal (0–2) and lateral (0–2) tactile placing, proprioceptive placing (0–2), and postural reflex (0–2). Therefore, the maximal possible score is 12, as previously described by Belayev and associates (2003). The tests were performed at 1, 3, 7, and 14 days post-injury.
Immunohistological staining
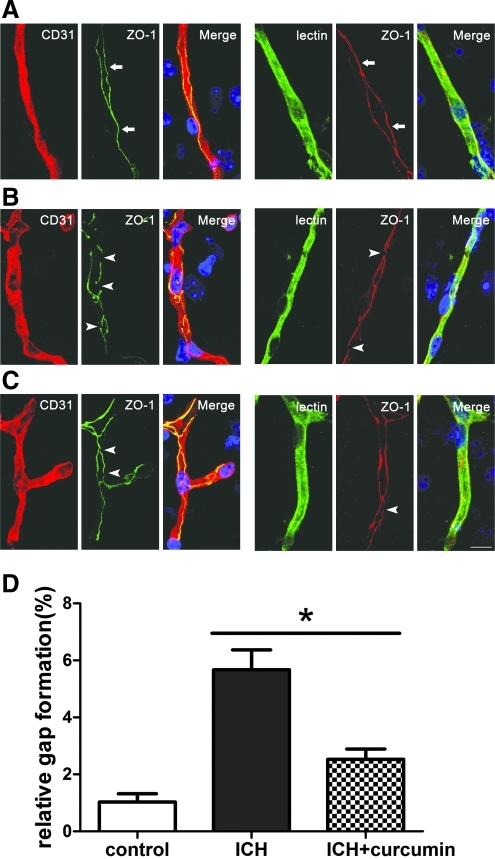
Brain cryosections (10 μm) were fixed in 4% paraformaldehyde for 5 min, washed, and blocked for 30 min in 10% (w/v) bovine serum albumin dissolved in 0.1 M PBS and incubated over night at 4°C with anti-zonula occludens-1(ZO1, 1:100 dilution, Invitrogen, Carlsbad, CA) and anti-CD31(1:100 dilution, BD Biosciences, Franklin Lakes, NJ) antibodies in PBS. After washing, sections were incubated for 1 h with secondary Cy3-conjugated donkey anti-goat antibody (1:500 dilution, Jackson ImmunoResearch, West Grove, PA), followed by Alexa-488-conjugated goat anti-rabbit antibody (1:500 dilution, Invitrogen, Carlsbad, CA). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (1:1000 dilution, Sigma-Aldrich, St Louis, MO) for 5 min. Fluorescein-lycopersicin esculentum lectin (Vector Laboratories, Burlingame, CA) staining was used to identify microvessels. Confocal microscopic images were acquired using a confocal laser-scanning microscope (Leica, Wetzlar, Hesse, Germany). Images were analyzed by Image Pro Plus (Media Cybernetics, Bethesda, MD). Gap length is presented as percentage (%) of whole tight junction staining of ZO-1 as described (Bauer et al., 2010). At least four vessels around the hematoma per brain were analyzed.
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from brain blocks of 2×2×2 mm3 size, including the hematoma and adjacent brain tissue, taken 12 h after blood injection. Reverse transcription was performed using a PrimeScript RT reagent kit (TaKaRa Bio Inc., Otsu, Shiga, Japan), and the reaction mixture was subjected to quantitative real-time PCR matrix metalloproteinase (MMP)-9 and GAPDH, which was used as an internal control. For a 10 μL of q-PCR reaction, 5 μL of SYBR Premix Ex Taq II (TaKaRa Bio Inc., Otsu, Shiga, Japan), 0.4 μL of 10 μM forward primer, 0.4 μL of 10 μM reverse primer, 0.1 μL of mixture of reverse transcription reaction, and 3 μL of water were included. The primers used to amplify target genes by q-PCR were as following: MMP-9: 5′ GGACCCGAAGCGGACATTG 3′, 5′ CGTCGTCGAAATGGGCATCT 3′; GAPDH: 5′ AGGTCGG TGTGAACGGATTTG 3′, 5′ TGTAGACCATGTAGTTGAG GTCA 3′ constructed by Invitrogen Corp. (Carlsbad, CA). After 40 cycles, the relative levels of gene expression were quantified with SDS software (Applied Biosystems, Carlsbad, CA). Data are expressed as copy number normalized by dividing the copy number for each sample by the copy number of the corresponding GAPDH housekeeping gene.
In situ zymography of MMP activity
In situ zymography was used to detect the enzymatic activity of the gelatinases, MMP-2 and MMP-9, as described (Xue et al., 2006). The substrate for proteolytic degradation is gelatin. Briefly, 10-μm unfixed cryostat brain sections were cut serially through the damaged brain. A reaction buffer containing 25μg/mL of fluorescein isothiocyanate-labeled DQ-gelatin (EnzChek, Invitrogen, Carlsbad, CA) was applied to these sections for 2 h at 37°C. Slides were washed, mounted, and photographed adjacent to the hematoma by a digital camera. Where gelatinolytic activity was present in brain tissue, the in situ degradation of fluorescein isothiocyanate-labeled DQ-gelatin resulted in emission of fluorescence at that location. Cells with gelatinolytic activity were counted in four photographic areas, and the average of the four fields per mouse was calculated.
Statistical analysis
Statistical analyses were performed using Instat (version 3.0, Graphpad Software) for both parametric and nonparametric comparisons. P values of <0.05 were considered significant. Data were analyzed to ensure normal distribution, and intergroup comparisons were made by analysis of variance followed by Bonferroni or Dunn post-hoc tests.
Results
Curcumin reduces ICH-induced neuronal damage in mice
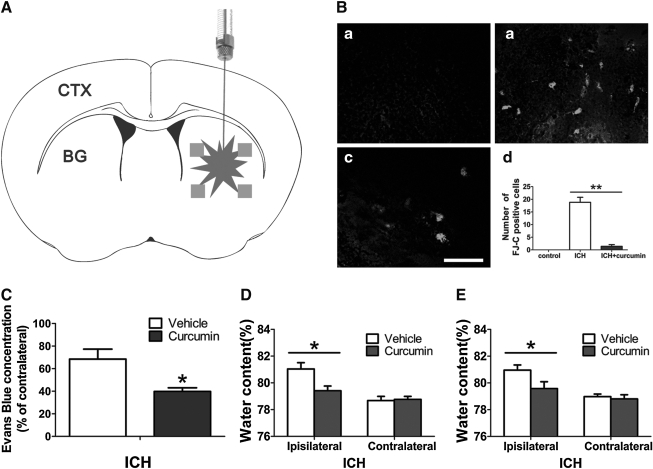
Quantitative data of FJ-C staining of dying neurons revealed that the ICH- induced injury was significantly reduced in curcumin-treated mice compared to the vehicle-treated ICH mice (p<0.05) (Fig. 2A), with curcumin-treated mice having less FJ-C positive staining (Fig. 2B). The sum number of FJ-C positive dying neurons in three sections surrounding the injection site was significantly increased after intracerebral injection of autologous blood, compared with sham-control. The results show that curcumin administered intraperitoneally 15 min after ICH attenuated neuronal death and reduced the number of dying neurons detected by FJ-C (Fig. 2B).
FIG. 2.
The number of degenerating neuron in intracerebral hemorrhage (ICH) is reduced by curcumin. (A) The schematic diagram shows the four areas (squares) around the hematoma that were counted for Fluoro-Jade-positive cells (FJ-C). CTX, cortex; BG, basal ganglia. (B) FJ-C staining shows the degenerating neuron at 24 h in the uninjured intact group (a), ICH group (b), and curcumin-treated group (c). These figures show brain areas adjacent to the hematoma. Scale bar=100μm; (d) To quantify neuronal injury, FJ-C positive cells was measured. Values are mean±SEM (**p<0.01; n=3 animals per group). There are three mice per group. Evans Blue contents (C) and Brain water contents (D,E) 24 h after ICH. The mice were treated with curcumin (vehicle) 15 min (D) or 2 h (E) after ICH. Values are mean±SEM. *p<0.05 vs. vehicle.
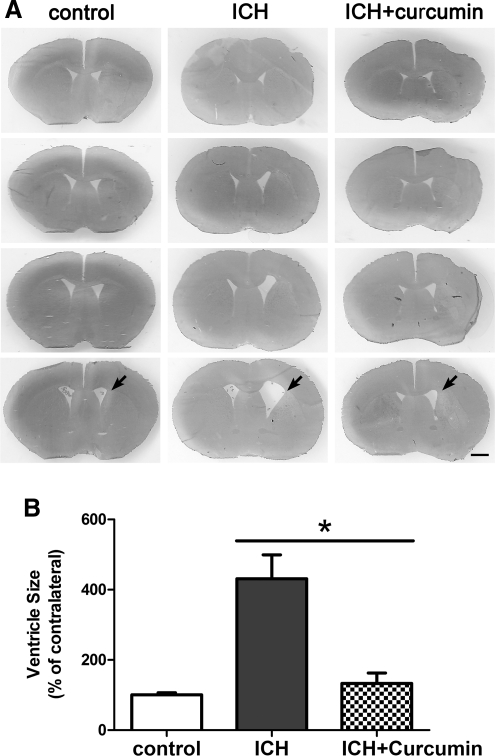
In addition, curcumin reduced brain tissue loss in the ipsilateral caudate and ameliorated ventricular enlargement (431±68% vs. 133±29%, p<0.05) (Fig. 3).
FIG. 3.
The changes of ventricle sizes after intracerebral hemorrhage (ICH). (A) Ventricle sizes 28 days after ICH in mice treated with curcumin. Scale bar=1 mm. (B) For ventricle measurement, the ipsilateral ventricle size was expressed as a percentage of that of the contralateral. Values are mean±SEM, *p<0.05 vs. vehicle.
Curcumin protects the BBB and alleviates brain edema
There was a marked increase in Evans Blue content in the ipsilateral hemisphere as compared with the contralateral hemisphere in control animals 24 h after ICH (12.5±0.9 vs. 7.3±0.2 μg/g, in the control group, n=8, p<0.05). Curcumin markedly reduced the extravasation of Evans Blue in the ipsilateral hemispheres of treated animals as compared with vehicle controls (68.4±8.8 % vs. 39.8±3.2 %, n=8, p<0.05; Fig. 2C).
Normal mouse brain water content is ∼78%; brain swelling occurred in the perihematomal zone of the ipsilateral hemisphere after ICH. Mean hemisphere weight in the ipsilateral side was larger than that in the contralateral side in both groups. Brain water content was significantly increased 24 h after ICH in the ipsilateral hemisphere (81.0±0.6% vs. 78.7±0.3% in the control group, p<0.05, Fig. 2D). Curcumin treatment reduced the water content of the ipsilateral hemisphere (79.4±0.4%, n=10, p<0.05, Fig. 2D). Curcumin reduced brain edema in the ipsilateral side when administered up to 2 h after injury (81.0±0.4% vs. 79.6±0.5%, n=10, p<0.05) (Fig. 2E).
Curcumin attenuated neurological deficits
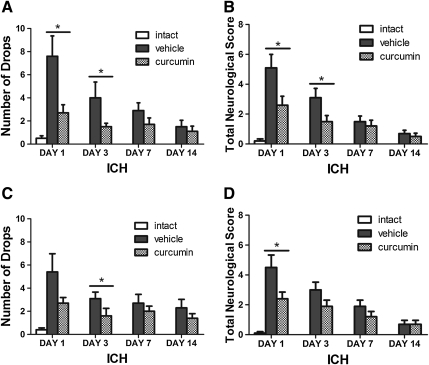
Neurological deficits were observed in all mice subjected to ICH (p<0.01) (Fig. 4). Significant improvement was observed on day 1 and day 3 after ICH, compared to vehicle (p<0.05). There was a trend toward an improvement in neurological function on day 7 and day 14, but this did not reach significance (p>0.05 compared to vehicle) (Fig. 4).
FIG. 4.
Curcumin confers behavioral recovery after intracerebral hemorrhage (ICH). Mice received intracerebral injections of 50 μL blood and were treated by curcumin I.P. at 150 mg/kg after 15 min (A,B) and 2 h (C,D), respectively. Bar graphs (mean±SEM) show that the number of drops in the grid walking test and the neurological score are significantly reduced at days 1 and 3 compared with vehicle treatment (n=10 animals per group).
Curcumin treatment reduced MMP activity and protected the continuity of tight junction
In the mice treated with vehicle, gap formation by ZO-1-positive staining was much greater than in the sham group, suggesting that ICH interrupted the integrity of the BBB. We demonstrated that a combination of increased gelatinolytic activity and tight junction gap formation was consistent with BBB disruption and should be investigated in further studies of barrier functionality. Curcumin treatment reduced tight junction gap formation compared with vehicle (Fig. 5).
FIG. 5.
Intracerebral hemorrhage (ICH) correlates with localization changes of ZO-1. (The blue staining represented 4,6-diamidino-2-phenylindole [DAPI].) (A) microvessels in the cortex of control mice showed a continuous, linear labeling of ZO-1 along the whole vessel (arrows). The tight junction proteins are located at the cell margins of CD31-positive endothelial cells or lectin-positive microvessles. (B) ICH significantly increased gap length and disruption of ZO-1. The microvessels showed an irregular and diffuse staining and gap formation (arrowheads). (C) Treatment with curcumin showed less redistribution and gap formation (arrowheads). Scale bar=10 μm. (D) Bar graph shows the gap length. Values are mean±SEM, *p<0.05, ICH vs. curcumin treatment, n=3 animals per group. Color image is available online at www.liebertonline.com/neu
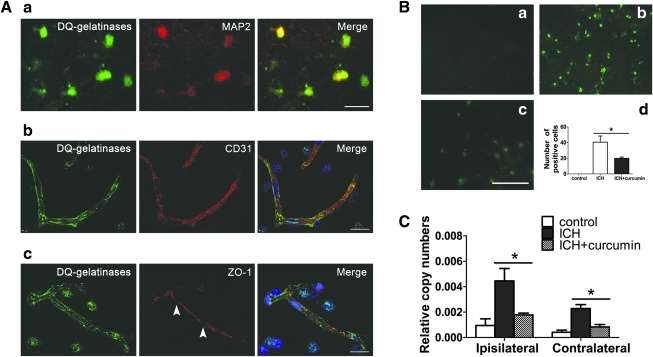
MMP activity was inversely related to tight junction integrity. Gelatinolytic activity was mostly associated with neurons (MAP2-positive) and vascular endothelial cells (CD31-positive) (Fig. 6A). Co-localization was not observed with GFAP, an astrocyte marker (data not shown). These data are in line with previous reports in an ICH model of collagenase injection, which indicated that neurons and endothelial cells are the main target of MMPs (Wang and Tsirka, 2005). Double staining showed that ZO-1 gaps match active MMPs. To assess whether curcumin suppress the gelatinolytic activity in ICH in mice, brain tissue around the hematoma was analyzed by using in situ zymography. ICH increased the number of gelatinolytic activity positive cells around the hematoma, which was significantly reduced by curcumin treatment. In the intact striatum; only background fluorescence was observed (Fig. 6B) (n=3, in each group).
FIG. 6.
(A) Increased gelatinolytic activity within perihematomal neurons after intracerebral hemorrhage (ICH). Double staining of gelatinolytic activity (green) and cell specific markers of neurons (MAP2) or endothelial cells (CD-31) and BBB integrity (ZO-1) were visualized. The blue staining represented 4,6-diamidino-2-phenylindole (DAPI). Scale bar=10 μm. (B) The increased gelatinolytic activity in ICH is reduced by curcumin. In situ zymography shows negligible gelatinase activity in the uninjured intact brain (a); b and c show the gelatinase activity in the ICH group (b) and the curcumin-treated group (c). Scale bar=100 μm. (d): Bar graph shows the number of positive cells in in situ zymography at 24 h of ICH. Values are mean±SEM, *p<0.05, ICH vs. curcumin treatment, n=3 animals per group. (C) Effect of curcumin on MMP-9 gene expression in both hemispheres. Expression of MMP-9 was assessed in the perihematoma region at 12 h post-ICH using qRT-PCR. Data are expressed as normalized relative copy number of each sample, dividing by the copy number of the corresponding GAPDH housekeeping gene. (mean±SEM; n=8 animals per group). Color image is available online at www.liebertonline.com/neu
MMP-9 mRNA level were measured at 12 h after blood injection. Levels of MMP-9 mRNA increased in both hemispheres; MMP-9 mRNA levels were significantly reduced by curcumin in the both hemispheres at 12 h (Fig. 6C).
Discussion
Curcumin has been shown to be neuroprotective in cerebral ischemia (Dohare et al., 2008). As ICH shares several pathological characteristics with cerebral ischemia, some effective therapy may also be beneficial to ICH. Our study shows that curcumin protects against acute ICH-induced brain injury in a mouse model. It reduces perihematomal brain edema, BBB disruption, and behavioral deficits. We chose a mouse model of ICH because significant neuropathology could be observed within 24 h, and phase I clinical trials had demonstrated that oral administration of up to 8 g/day of curcumin safely exhibits bioactivity, with serum levels peaking at 1–2 h (∼1.8 μM), followed by a gradual decline within 12 h (Cheng et al., 2001). Our results show that curcumin reduced brain edema when administered as a single dose up to 2 h after ICH, suggesting the therapeutic window of curcumin treatment is not restricted to the 15-min time point.
Curcuminoids contain numerous pharmacologically diverse polyphenolic components that have diverse mechanisms of action, including antioxidation, anti-inflammation, and neuroprotection in diseases of the central nervous system (CNS). Because there are parallel and simultaneous activations of pathways that are deleterious to brain tissues, it has been suggested that curcumin, being the most active multi-target component of curcuminoids, may be neuroprotective and promote functional recovery (Lapchak, 2011). It is reported that curcumin can be adequately absorbed from the gastrointestinal tract and undergo significant biotransformation (Pan et al., 1999). It is noteworthy that previous studies on the murine pharmacokinetics of orally taken curcumin in murine reported that the serum concentration of curcumin was low and that the urine concentration was negligible. Similarly, curcumin blood levels were undetectable between 2 and 12 h after oral dosing of 1 g/kg in rats (Cheng et al., 2001). Curcumin is most effective when administered peripherally at high doses (Ghoneim et al., 2002). Curcumin concentrations in the brain and in plasma were determined to be 1.1 μM and 1.6 μM, respectively, after intraperitoneal administration of curcumin at 100 mg/kg to mice (Pan et al., 1999). This dosage may yield adequate biological activity of curcumin in perihematomal brain tissue. In this study, we chose a dose similar to that used in previous reports.
Multiple forms of edema are present after ICH, but the main form is probably vasogenic. Disruption of the BBB occurs after ICH and contributes to brain edema formation. In previous experimental studies on edema formation after ICH, it was reported that the early phase was related to the coagulation cascade, thrombin production, and increased permeability of the barrier around the hematoma 8–2 h after onset, whereas the late phase was related to erythrocyte lysis and hemoglobin toxicity (Xi et al., 2006). Very recently, a study showed that a similar dose of curcumin reduced hematoma volume and vasogenic edema following collagenase injection ICH model (King et al., 2011). It also observed that curcumin treatment had significantly reduced the expression of pro-inflammatory mediators in ICH. In our study, the water content examination of ICH brain specimens clearly demonstrated the presence of brain tissue edema at 24 h after the injury; this edema was significantly reduced by the administration of curcumin, again supporting those findings.
Benefits of curcumin are evident not only in tissue morphology, but also in behavioral tests. Animals in the curcumin-treated group took much less time to recover from neurological deficit during both of the two tests comparing them to the vehicle-treated mice. In this study, we found that curcumin improved neurological outcome after ICH on the 1st and 3rd days. With regard to brain edema and factors affecting acute functional outcome, autologous blood model is known to cause far less lasting and less severe BBB disruption and neurological deficits than the collagenase injection (MacLellan et al., 2008). Nonetheless, the study of short-term MMP-mediated pathophysiological mechanisms may yet yield significant results in future studies using the collagenase model. Neurological function spontaneously recovered in the blood model, as the neurological scores of the ICH animals were very close to the baseline by the 7th day. Such relatively rapid and apparently complete behavioral recovery in untreated animals is similar to some experimental results available (MacLellan et al., 2008; Xue et al., 2010), which limits the use of this model in assessing long-term treatment efficacy. Nevertheless, the improvement in neurological outcome conferred by curcumin in the early phase may define the contribution of curcumin to early pathogenesis caused by MMP-induced acute secondary damage.
MMPs can directly interact with tight junction proteins, resulting in their degradation, and thus damage the BBB. These findings are in line with our results that ZO-1 is the target for ICH-induced tight junction integrity loss (gap formation). Furthermore, there is growing evidence showing direct action of MMPs on tight junction proteins: MMP-9 gene knock-out in mice reduced ZO-1 degradation after acute brain injury as compared with controls (Asahi et al., 2001); such tight junction protein degradation could also be reversed by MMP inhibition (Yang et al., 2007). Further evidence showed that tight junction protein contains a putative extracellular MMP cleavage site (Bojarski et al., 2004) and is a direct substrate for MMPs (Wachtel et al., 1999). Our findings that ICH results in BBB leakage through the activation of MMPs with subsequent tight junction degradation agrees with these previous findings. The distribution of tight junction inhibited by curcumin suggests that curcumin might protect the integrity of the BBB after ICH partly by inhibiting the MMP-mediated tight junction gap formation.
There is a close overlap between the brain water content and BBB permeability monitored by Evans Blue and MMP activation monitored by in situ zymography, which is in agreement with the reported activation of MMP-2 and MMP-9 in ICH. MMPs' upregulation and subsequent digestion of the components of the basement membrane significantly contributes to BBB damage and brain edema in ICH (Rosenberg and Navratil, 1997; Rosenberg et al., 1993; Wang and Tsirka, 2005). Our data indicate here, as reported previously (Abilleira et al., 2003), that MMPs' enzymatic activity, which plays a key role in ICH brain injury, is significantly upregulated. The mRNA of MMP-9 increased after blood injection differs from that observed in a collagenase injection model (Grossetete and Rosenberg, 2008). This difference could arise from the fact that these two models use different means of inducing ICH. Activation of MMP-9 and MMP-2 by hemorrhage was confirmed by direct measurement of in situ zymography perihematoma. Curcumin significantly decreased the transcriptional level of MMP-9 in both hemispheres, and inhibited hematoma-induced increase in MMP enzymatic activity, which may account for the reduction of brain edema and improvement of neurological function.
There is probably a mechanistic benefit beyond preventing the progression of neuronal degeneration and BBB disruption in perihematomal injury caused by MMP (Xue et al., 2006). To date, curcumin is well recognized as a broad-spectrum inhibitor, which effectively decreases MMPs' expression, and this is reflected by documented repression of the DNA binding and transcriptional activities of activator protein 1 (AP-1), a common upstream modulator of MMPs' gene expression (Kim et al., 2005; Wang et al., 2009). Curcumin was shown to have beneficial effect on inflammation responses by attenuated Il-1-induced pro-MMP-9 expression via c-Src-dependent PDGFR/PI3K/Akt/p300 cascade (Wu et al., 2008). These indicate that agents affecting MMPs may function through different mechanisms, including reduced expression of MMP genes, interference in the activation of pro-MMPs, and direct inhibition of the enzymatic activity of MMPs.
The results of this study suggest that curcumin ameliorates ICH damage by preventing MMP-mediated BBB damage and brain edema, and there is a pressing need for more mechanistic studies to further investigate these plasticity processes as a potential efficient and effective method to promote behavioral or clinical recovery after ICH.
Acknowledgments
This research was supported by Shanghai Committee of Science and Technology grants 09JC1409700, 10JC1410700, 10411954200 (L.G.B., Q.F.S.), 09140902400, 10JC1408100 (G.Y.Y., Y.T.W.). We thank the collaborative support of the Med-X research Institute (http//med-x.sjtu.edu.cn) and Ms. Karena Shen for assistance with manuscript preparation.
Author Disclosure Statement
No competing financial interests exist.
References
- Abilleira S. Montaner J. Molina C.A. Monasterio J. Castillo J. Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J. Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- Asahi M. Wang X. Mori T. Sumii T. Jung J.C. Moskowitz M.A. Fini M.E. Lo E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A.T. Burgers H.F. Rabie T. Marti H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow. Metab. 2010;30:837–848. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum L. Lam C.W. Cheung S.K. Kwok T. Lui V. Tsoh J. Lam L. Leung V. Hui E. Ng C. Woo J. Chiu H.F. Goggins W.B. Zee B.C. Cheng K.F. Fong C.Y. Wong A. Mok H. Chow M.S. Ho P.C. Ip S.P. Ho C.S. Yu X.W. Lai C.Y. Chan M.H. Szeto S. Chan I.H. Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Belayev L. Saul I. Curbelo K. Busto R. Belayev A. Zhang Y. Riyamongkol P. Zhao W. Ginsberg M.D. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke. 2003;34:2221–2227. doi: 10.1161/01.STR.0000088061.06656.1E. [DOI] [PubMed] [Google Scholar]
- Bojarski C. Weiske J. Schoneberg T. Schroder W. Mankertz J. Schulzke J.D. Florian P. Fromm M. Tauber R. Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J. Cell Sci. 2004;117:2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- Cheng A.L. Hsu C.H. Lin J.K. Hsu M.M. Ho Y.F. Shen T.S. Ko J.Y. Lin J.T. Lin B.R. Ming–Shiang W. Yu H.S. Jee S.H. Chen G.S. Chen T.M. Chen C.A. Lai M.K. Pu Y.S. Pan M.H. Wang Y.J. Tsai C.C. Hsieh C.Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Dohare P. Garg P. Jain V. Nath C. Ray M. Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav. Brain Res. 2008;193:289–297. doi: 10.1016/j.bbr.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Ghoneim A.I. Abdel–Naim A.B. Khalifa A.E. El-Denshary E.S. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol. Res. 2002;46:273–279. doi: 10.1016/s1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- Grossetete M. Rosenberg G.A. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J. Cereb. Blood Flow Metab. 2008;28:752–763. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- Jiang J. Wang W. Sun Y.J. Hu M. Li F. Zhu D.Y. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood–brain barrier damage. Eur. J. Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Kim S.Y. Jung S.H. Kim H.S. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005;337:510–516. doi: 10.1016/j.bbrc.2005.09.079. [DOI] [PubMed] [Google Scholar]
- King M.D. McCracken D.J. Wade F.M. Meiler S.E. Alleyne C.H. Dhandapani K.M. Attenuation of hematoma size and neurological injury with curcumin following intracerebral hemorrhage in mice. J. Neurosurg. 2011;115:116–123. doi: 10.3171/2011.2.JNS10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak P.A. Neuroprotective and neurotrophic curcuminoids to treat stroke: a translational perspective. Expert Opin. Investig. Drugs. 2011;20:13–22. doi: 10.1517/13543784.2011.542410. [DOI] [PubMed] [Google Scholar]
- MacLellan C.L. Silasi G. Poon C.C. Edmundson C.L. Buist R. Peeling J. Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Pan M.H. Huang T.M. Lin J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- Rathore P. Dohare P. Varma S. Ray A. Sharma U. Jagannathan N.R. Ray M. Curcuma oil: reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008;33:1672–1682. doi: 10.1007/s11064-007-9515-6. [DOI] [PubMed] [Google Scholar]
- Ropper A.H. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N. Engl. J. Med. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. Estrada E. Kelley R.O. Kornfeld M. Bacterial collagenase disrupts extracellular matrix and opens blood–brain barrier in rat. Neurosci. Lett. 1993;160:117–119. doi: 10.1016/0304-3940(93)90927-d. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–926. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- Schmued L.C. Albertson C. Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M. Sharma S.S. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- Wachtel M. Frei K. Ehler E. Fontana A. Winterhalter K. Gloor S.M. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J. Cell Sci. 1999;112:4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Wang H.H. Hsieh H.L. Wu C.Y. Sun C.C. Yang C.M. Oxidized low-density lipoprotein induces matrix metalloproteinase-9 expression via a p42/p44 and JNK-dependent AP-1 pathway in brain astrocytes. Glia. 2009;57:24–38. doi: 10.1002/glia.20732. [DOI] [PubMed] [Google Scholar]
- Wang J. Tsirka S.E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Wang M.S. Boddapati S. Emadi S. Sierks M.R. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson's disease cell model. BMC Neurosci. 2010;11:57. doi: 10.1186/1471-2202-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. Ying Z. Gomez–Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp. Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Wu C.Y. Hsieh H.L. Sun C.C. Tseng C.P. Yang C.M. IL-1 beta induces proMMP-9 expression via c-Src-dependent PDGFR/PI3K/Akt/p300 cascade in rat brain astrocytes. J. Neurochem. 2008;105:1499–1512. doi: 10.1111/j.1471-4159.2008.05318.x. [DOI] [PubMed] [Google Scholar]
- Xi G. Keep R.F. Hoff J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xue M. Hollenberg M.D. Yong V.W. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci. 2006;26:10,281–10,291. doi: 10.1523/JNEUROSCI.2806-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M. Mikliaeva E.I. Casha S. Zygun D. Demchuk A. Yong V.W. Improving outcomes of neuroprotection by minocycline, guides from cell culture and intracerebral hemorrhage in mice. Am. J. Pathol. 2010;176:1193–1202. doi: 10.2353/ajpath.2010.090361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.Y. Betz A.L. Chenevert T.L. Brunberg J.A. Hoff J.T. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood–brain barrier permeability in rats. J. Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Yang Y. Estrada E.Y. Thompson J.F. Liu W. Rosenberg G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Zazulia A.R. Diringer M.N. Derdeyn C.P. Powers W.J. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- Zhao J. Yu S. Zheng W. Feng G. Luo G. Wang L. Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem. Res. 2010;35:374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]