Abstract
Purpose
Multidrug resistance (MDR) represents a major obstacle to the success of antimicrobial fluoroquinolone (FQ) therapy. MDR-associated efflux protein pumps antimicrobial agents out of the corneal cells, leading to suboptimal eradication of microbes. This article examines whether long-term FQ (levofloxacin, ofloxacin, and gatifloxacin) therapy can modify the MDR phenotype (P-glycoprotein [P-gp]) on corneal epithelial cells (Statens Seruminstitut Rabbit Cornea [SIRC]).
Methods
To study the effect of FQ, SIRC cells without any exposure to FQ (control) were compared with the cells exposed to ofloxacin, levofloxacin, and gatifloxacin at a concentration of 10 μg/mL for 3 weeks. Efflux activity of P-gp was assessed by in vitro uptake studies (fluorescent and radioactive), flow cytometry, and quantitative real-time polymerase chain reaction (qRT-PCR).
Results
In the presence of FQ, elevated P-gp expression was noted with uptake, flow cytometry, and qRT-PCR analyses. This study confirms that long-term exposure to antibiotics, particularly FQ, can induce overexpression of P-gp efflux transporter present on the corneal cells. P-gp overexpression is commonly noticed in anticancer drug resistance cell lines; however, for the first time, this report describes overexpression of P-gp due to FQ exposure.
Conclusions
Based on this result, it is suggested that strategies should be developed and implemented not only to overcome resistance to ocular pathogen but also to FQ-induced cellular resistance.
Introduction
Ocular infections can cause severe necrosis and cell death to various internal structures in the eye, which can lead to vision loss and even blindness if left untreated. The effective use of antibiotics to treat ophthalmic infections requires an understanding of the disease and pharmacokinetics/pharmacodynamics of the antibiotics indicated for the treatment.1–3 The most common ocular infections seen by primary care physicians worldwide is bacterial conjunctivitis,4 bacterial keratitis,5 endophthalmitis,6 bacterial blepharitis,7 orbital cellulitis, and other periorbital infections.8 Broad-spectrum antibiotic therapy for bacterial infections is initially recommended to prevent any decline in vision or permanent vision loss that may require surgical intervention.1
Antibiotics can be administered systemically or topically to control ocular infection. Frequent or inappropriate, long-term use of antibiotics may result in the development of drug resistance in bacterial strains.1,2 Bacterial resistance has been continuously emerging worldwide, probably because of widespread and inappropriate dosing of broad-spectrum antibiotics for systemic infections, exacerbated by inadequate compliance to full treatment duration.9 There are many reports of clinical treatment failure due to drug-induced resistance in ocular pathogens. These reports highlight the need for reevaluation and further implementation of adequate treatment regimens for ocular anti-infective therapies.10,11
Fluoroquinolones (FQs) represent a class of antimicrobial agents that inhibit DNA synthesis by interaction with bacterial DNA gyrase and/or bacterial DNA topoisomerase.12
Despite the expanded activity, the use of FQs in ocular infections is limited by systemic toxicity upon administration. Besides ocular bacterial drug resistance, multidrug resistance (MDR) represents a major barrier to clinically successful FQ therapy. Subtherapeutic concentrations in the corneal epithelial cells and iris-ciliary body can result from MDR associated with efflux pumps. These proteins can pump antimicrobial agents out of cell, leading to suboptimal eradication of microbes.13–21
FQs are recognized by several eukaryotic multidrug transporters, most notably by 2 main members of the ABC superfamily, namely the MDR-related proteins (MRP) and the P-gp (MDR1).22 The most frequently described mechanism to explain MDR is the overexpression of P-gp, a 170-kDa transmembrane transport protein encoded by the MDR-1 gene.17
This work examines whether long-term use of FQs (levofloxacin, ofloxacin, and gatifloxacin) can modify MDR phenotype (P-gp expression) on a corneal cell line [Statens Seruminstitut Rabbit Cornea (SIRC) cells]. The SIRC cell line has been reported to moderately express P-gp.l9
In the present study, the SIRC cells were cultivated in medium containing 3 different FQs for several weeks. Therefore, this work proposes first the hypothesis that long-term use of FQs could influence P-gp expression in SIRC cells.
Every week, the expression of P-gp was evaluated by measuring accumulation of [14C]Erythromycin or Rhodamine-123 (Rho-123) inside SIRC cells.23,24 [14C]Erythromycin and Rho-123 have been widely applied as a radioactive and fluorescent P-gp substrate, respectively. These compounds do not appreciably bind to efflux pumps (hence does not act as an inhibitor) and can be detected in very low concentrations.19,23
Methods
Materials
Levofloxacin, ofloxacin, and gatifloxacin were obtained from Bosche Scientific (New Brunswick, NJ). SIRC cell line was purchased from ATCC (American Type Culture Collection, Manassas, VA). [14C]Erythromycin (specific activity: 51.3 mCi/mmol) was obtained from Moravek Biochemicals (Brea, CA). Rho-123 was procured from Sigma-Aldrich (St. Louis, MO). Trypsin–ethylenediaminetetraacetic acid solution and minimum essential medium (MEM) were obtained from Invitrogen (Carlsbad, CA). Calf serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Culture flasks (75 cm2 growth area), 12-well plates (3.8 cm2 growth area per well), and 96-well plates (0.32 cm2 growth area per well) were obtained from Corning Costar Corp. (Cambridge, MA). All other chemicals were of analytical reagent grade and were obtained from Sigma Chemicals (St. Louis, MO) and Fisher Scientific (Fair Lawn, NJ).
Cell culture
SIRC cell line is a commercially available rabbit corneal epithelial cell line. It is a well-known and established cell culture model that mimics corneal epithelium. This cell line has been selected as a model to perform in vitro studies, that is, to assess corneal physiology, transport, immunology, cytotoxicity, and pharmacology.19 SIRC cell line of passage number 423–430 was employed for these studies. To study the effects of FQs, 4 separate cultures of SIRC cells were simultaneously initiated in MEM with 10% calf serum non heat inactivated (NHI). The first culture (S-Control) was maintained without any exposure of FQs, second with ofloxacin (S-OFX), third with levofloxacin (S-LFX), and fourth with gatifloxacin (S-GFX) at a concentration of 10 μg/mL each. Cells were cultured in T-75 flasks at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every alternate day. Cells were allowed to reach 80%–90% confluence, which was confirmed with microscopic examination, and then passaged using TrypLE™ Express solution. The cells were seeded at a density of 3×106 cells/well in 12-well tissue culture–treated plastic plates, allowed to grow for 5–7 days, and then employed for further studies. Culture conditions were maintained for 3 weeks.
Preparation of drug solutions
[14C]Erythromycin (0.25 μci/mL) and Rho-123 (5 μM) were selected as model radioactive and fluorescent substrates, respectively, to study P-gp–mediated efflux.23,24 Uptake of these substrates was measured in SIRC cells (control and fluroquinolones treated) using radioactivity counter and fluorescence plate reader, respectively.
Uptake studies
Radioactive uptake of [14C]Erythromycin on SIRC cells
Uptake studies were performed in 12-well tissue culture–treated plates with confluent SIRC cells, usually 7–8 days postseeding. The medium was aspirated from wells and cells were washed (3×10 min) with 2 mL of Dulbecco's phosphate-buffered saline (DPBS) containing 130 mM NaCl, 2.5 mM KCl, 7.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgSO4, 5 mM glucose, and 20 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulforic acid] at pH 7.4. Then, 1 mL of drug solutions (each containing 0.25 μCi/mL of [14C]Erythromycin) were added to cells and were incubated at 37°C for 30 min. Then, solutions were removed and uptake process was terminated with ice-cold stop solution (200 mM KCl and 2 mM HEPES). Subsequently, cells were lysed overnight at room temperature with 1 mL of 0.1% (v/v) Triton-X in 0.3 N sodium hydroxide solution. Aliquots (500 μL) of the cell lysate were then added to scintillation vials containing 3 mL of scintillation cocktail (Fisher Scientific) and radioactivity was measured with a scintillation counter (Model LS-6500; Beckman Counter, Fullerton, CA), which can measure [14C] compounds with an efficiency of 95%. Uptake in each well was normalized to protein content, estimated by Bio-Rad protein estimation kit with bovine serum albumin as the standard. Each set of experiment was performed for control (S-Control) and FQ-treated (S-OFX, S-LFX, and S-GFX) SIRC cells for 3 weeks.
Fluorescent uptake of Rho-123 on SIRC cells
Fluorescent uptake studies were conducted with standard protocols, with minor modifications. Briefly, uptake studies were performed in 12-well tissue culture–treated plates with confluent cells, usually 7–8 days postseeding. The medium was aspirated from wells and cells were washed (3×10 min) with 2 mL of DPBS at pH 7.4. Rho-123 solution was prepared in DPBS at a concentration of 5 μM. Cells were then equilibrated in 1 mL Rho-123 solution in DPBS for 30 min at 37°C. At the end of each experiment, solution was removed and uptake was terminated with ice-cold stop solution (200 mM KCl and 2 mM HEPES). Subsequently, cells were lysed overnight at room temperature with 1 mL of 0.1% (v/v) Triton-X in 0.3 N sodium hydroxide solution. The lysate was transferred to a 96-well plate and assayed with a 96-well fluorescent microplate reader. Rho-123 fluorescence was measured at excitation-emission wavelengths of 485/535 nm, respectively, and quantified against a standard curve of Rho-123 (5 nM to 1 μM). The fluorescence of the cell lysates was corrected for autofluorescence of untreated cells. Uptake data in each well were normalized to the protein content, estimated by the Bradford reagent with bovine serum albumin as the standard. Each set of experiment was performed for control (S-Control) and FQ-treated (S-OFX, S-LFX, and S-GFX) SIRC cells for 3 weeks.
Flow cytometry analysis
P-gp function was also investigated by Rho-123 (fluorescent P-gp substrate) uptake. SIRC cell samples (S-Control, S-OFX, S-LFX, and S-GFX) were incubated with 5 μM of Rho-123 in DPBS (pH 7.4) for 1 h at 37°C, trypsinized, and washed (with cold DPBS) twice for any cell surface–attached Rho-123. After washing, cells were detached from the cultured plates with TrypLE express solution and then centrifuged at 1,000g at room temperature for 5 min to generate the pellet. The cell pellet was finally resuspended in DPBS for flow cytometry analysis. Fluorescence measurements of individual sample were performed with a Becton-Dickinson FACScalibur (San Jose, CA) equipped with an ultraviolet argon laser (excitation at 488 nm, emission at 530/30 and 570/30 nm band-pass filters). Analysis was gated to include single cells on the basis of forward and side light scatter and was based on acquisition of data from 10,000 total events. Log fluorescence was collected and displayed as single-parameter histograms.
Quantitative real-time polymerase chain reaction
To further confirm the FQ modulation of P-gp expression at molecular level, real-time polymerase chain reaction (RT-PCR) was conducted using Roche-LightCycler® 480. Primers were designed in PrimerQuestSM (Integrated DNA Technology, Coralville, Iowa). To enhence the simultaneous amplification efficiency of target and reference genes, 100–150-bp-long (Table 1) amplicons were designed. Ten microliters of PCR mix containing 100 ng template cDNA, 150 nmol forward and reverse primers for gene of interest, and 5 μL of Light Cycler 480 SYBR Green I Master mix (Roche Applied Sciences, Indianapolis, IN) were employed in this study. The specificity of the target amplicon was tested by melting curve analysis.25,26 Relative quantification was carried out by comparative Ct method. Samples for RT-PCR were prepared in triplicates. Quantitative values were obtained above the threshold PCR cycle number (Ct) at which an increase in signal associated with an exponential growth for PCR products was detected. Relative mRNA levels in each sample were normalized according to the expression levels of GAPDH and untreated cells. Initial standardization was carried out to ensure amplification of GAPDH and target genes with equal efficiencies. An induction ratio (treated/untreated) was determined from relative expression levels of the target gene using 2−ΔΔCt (Cttarget gene – CtGAPDH=ΔCt; ΔCt − Ctuntreated cells=ΔΔCt).27
Table 1.
Target and Reference Gene Sequences for Quantitative Real-Time Polymerase Chain Reaction Analysis
| Gene | Sequence |
|---|---|
| P-gp | GGTGCTGGTTGCTGCTTACATTCA (forward) CCCAACATCGTGCACATCAAACCA (reverse) |
| GAPDH | TCGACAGTCAGCCGCATCTTCTTT (forward) ACCAAATCCGTTGACTCCGACCTT (reverse) |
Results
Radioactive uptake of [14C]Erythromycin on SIRC cells
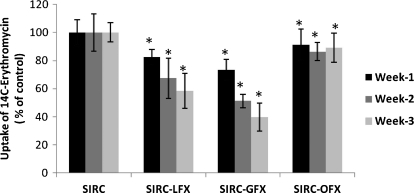
[14C]Erythromycin uptake was performed in the control (S-control) and FQ-treated (S-OFX, S-LFX, and S-GFX) SIRC cells. Uptake of [14C]Erythromycin was reduced by 17.5%, 32.6%, and 41.6% for S-LFX, 26.6%, 48.7%, and 60.2% for S-GFX, and 8.7%, 13.6%, and 10.6% for S-OFX for weeks 1, 2, and 3, respectively (Fig. 1). This result explains the induction of P-gp with antibiotic exposure for 3 weeks.
FIG. 1.
Radioactive uptake of [14C]Erythromycin by control and fluoroquinolone-treated SIRC cells. Each point represents mean SD of 3 determinations. *Statistically significant from control at a P value of <0.05. SIRC, Statens Seruminstitut Rabbit Cornea; SD, standard deviation; S-OFX, ofloxacin-treated SIRC cells; S-GFX, gatifloxacin-treated SIRC cells; S-LFX, levofloxacin-treated SIRC cell.
Fluorescent uptake of Rho-123 on SIRC cells
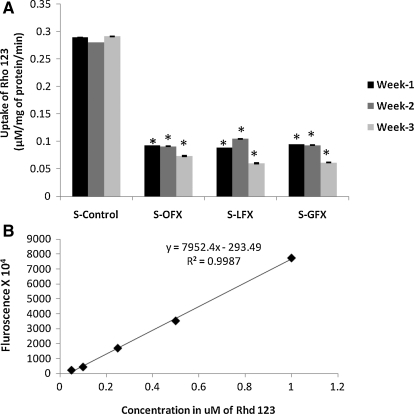
Uptake of Rho-123 (5 μM) was examined at 30 min in the control (S-control) and FQ-treated (S-OFX, S-LFX, and S-GFX) SIRC cells (Fig. 2). Rho-123 fluorescence was quantified against a standard curve of Rho-123. Uptake of Rho-123 was found to continuously decline from week 1 to 3. This result further supports the induction of P-gp expression with antibiotic exposure.
FIG. 2.
(A) Overall comparison of Rho-123 uptake with and without exposure of fluoroquinolones in SIRC cells. Each point represents mean SD of 4 determinations. *Statistically significant from control at a P value of <0.05. (B) Standard curve of Rho-123. Rho-123, Rhodamine-123; S-LFX, levofloxacin-treated SIRC cells.
Flow cytometry analysis
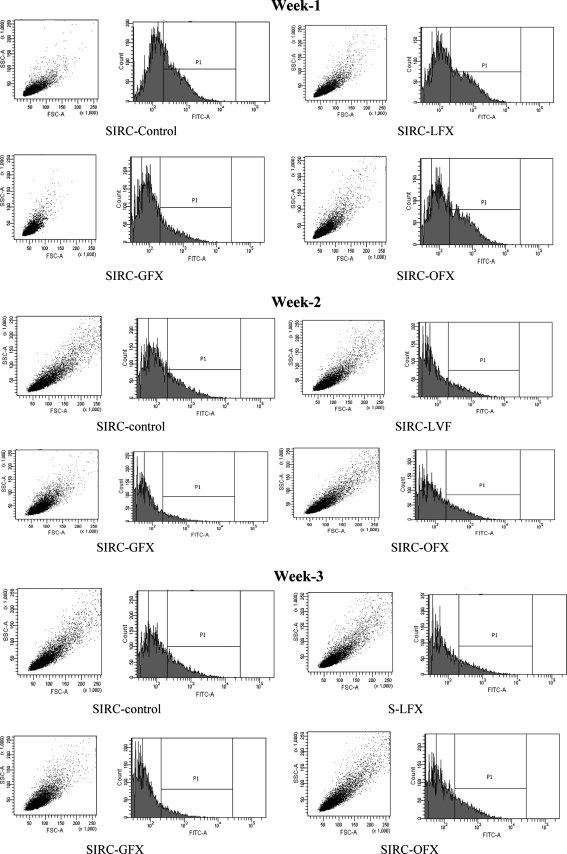
In case of S-LFX, S-OFX, and S-GFX cells, lower accumulation of Rho-123 relative to control indicates induction of P-gp expression due to antibiotic exposure for 3 weeks (Fig. 3 and Table 2).
FIG. 3.
Functional assay for the P-gp efflux pump on SIRC cells by flow cytometry analysis. P-gp, P-glycoprotein.
Table 2.
Comparison of % Event in Control and Fluoroquinolone-Treated Statens Seruminstitut Rabbit Cornea Cells by Flow Cytometry Analysis
| Uptake of Rho-123 | SIRC-control (% of event) | SIRC-LFX (% of event) | SIRC-GFX (% of event) | SIRC-OFX (% of event) |
|---|---|---|---|---|
| Week 1 | 100 | 86.6 | 49.3 | 86.4 |
| Week 2 | 100 | 59.37 | 34.7 | 85.1 |
| Week 3 | 100 | 52.7 | 29.89 | 82.4 |
SIRC, Statens Seruminstitut Rabbit Cornea; Rho-123, Rhodamine-123; S-OFX, ofloxacin-treated SIRC cells; S-GFX, gatifloxacin-treated SIRC cells; S-LFX, levofloxacin-treated SIRC cells.
Quantitative RT-PCR
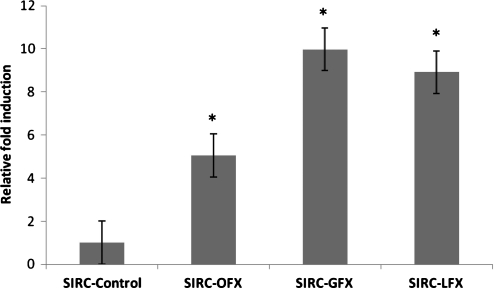
qRT-PCR was performed to compare the effect of long-term FQ exposure on P-gp gene expression by measuring mRNA levels in the treated versus nontreated corneal cells (Fig. 4). Elevation in P-gp mRNA expression level in FQ-treated cells was observed relative to untreated cells.
FIG. 4.
Relative fold induction of P-gp in SIRC cells treated with 3 fluoroquinolones. Data represent relative fold induction (n=3) of 3 different experiments. *Statistically significant from control at a P value of <0.05.
Discussion
FQ-induced bacterial resistance is emerging worldwide because of widespread and inappropriate dosing of broad-spectrum antibiotics in ocular infections. Such etiology may lead to well-recognized ocular pathogen resistance–based treatment failure. However, besides bacterial resistance, MDR represents a major obstacle to the success of FQs therapy, which has not been adequately examined. Development of MDR in ocular therapy can examined continuous dose escalation, which in turn may lead to systemic toxicity. The aim of the present study was to delineate whether long-term use of FQs can modify P-gp expression on corneal epithelial cells. In this study, we demonstrated that higher levels of P-gp expression at mRNA level was detected in SICR corneal epithelial cells exposed to FQs for 3 weeks. The SIRC cell line was cultivated in media containing 3 different FQs (levofloxacin, ofloxacin, and gatifloxacin) for 3 weeks, with P-gp functional activity being regularly determined.
From week 1, FQ-treated SIRC cells showed concomitant enhancement in P-gp expression, and diminished uptake of [14C]Erythromycin (Fig. 1) and Rho-123 (Fig. 2) were observed relative to control (nontreated) cells by both radioactive and nonradioactive uptake studies. [14C]Erythromycin and Rho-123 are well-known substrates of P-gp and are readily effluxed out by P-gp transporter expressed on the corneal cell membrane. Because of long-term FQ exposure of SIRC corneal cells, P-gp expression is gradually enhanced simultaneously lowering uptake of [14C]Erythromycin and Rho-123. Therefore, continuous overexpression of P-gp efflux transporter on FQ-treated SIRC cells may cause efflux of higher amount of P-gp substrates out of cells.
Moreover, significantly lower intracellular accumulation of Rho-123 was further confirmed by flow cytometry analysis in FQ-exposed cells when compared with control cells (Fig. 3). Table 2 clearly shows only 52.7%, 29.8%, and 82.4% Rho-123 accumulation in FQ-treated SIRC cells (S-LFX, S-GFX, and S-OFX, respectively) compared with control SIRC cells after 3 weeks. This result further indicates induction of P-gp after FQ exposure for 3 weeks.
From this discussion it can be suggested that long-term FQ exposure to corneal cells results in overexpression of P-gp efflux transporter. P-gp overexpression is a common phenomenon in anticancer resistance cell lines.18,28,29 This report may be the first work detailing the overexpression of P-gp due to FQ resistance.
Conclusion
In addition to FQ-based bacterial resistance, this study suggests that continuous exposure of FQs to SIRC cells can cause a significant increase in P-gp expression. P-gp expression modulators can be developed to reverse this process. However, for investigating new FQs and while using existing FQs for the treatment of various diseases (such as tuberculosis, cancer, and viral and fungal infections), particular attention must be given to the eventual consequences of their clinical use. Therefore, it is suggested that strategies should be developed and implemented not only to reduce ocular pathogen resistance but also FQ-based cellular resistance.
Acknowledgment
This work was supported by NIH (grants RO1 EY 09171-16 and RO1 EY 10659-14).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bertino J.S. Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin. Ophthalmol. 2009;3:507–521. doi: 10.2147/opth.s5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder R. Glasser D. Antibiotic therapy for ocular infection. West J. Med. 1994;161:579–584. [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes L. Maurice D. A fresh look at iontophoresis. Arch. Ophthalmol. 1984;102:1825–1829. doi: 10.1001/archopht.1984.01040031483028. [DOI] [PubMed] [Google Scholar]
- 4.Hovding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86:5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 5.Bourcier T. Thomas F. Borderie V. Chaumeil C. Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callegan M.C. Gilmore M.S. Gregory M., et al. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog. Retin. Eye Res. 2007;26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowalski R.P. Dhaliwal D.K. Ocular bacterial infections: current and future treatment options. Expert Rev. Anti. Infect. Ther. 2005;3:131–139. [PubMed] [Google Scholar]
- 8.Tovilla-Canales J.L. Nava A. Tovilla Y. Pomar J.L. Orbital and periorbital infections. Curr. Opin. Ophthalmol. 2001;12:335–341. doi: 10.1097/00055735-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Stratton C.W. Dead bugs don't mutate: susceptibility issues in the emergence of bacterial resistance. Emerg. Infect. Dis. 2003;9:10–16. doi: 10.3201/eid0901.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asbell P.A. Colby K.A. Deng S., et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am. J. Ophthalmol. 2008;145:951–958. doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Asbell P.A. Sahm D.F. Shaw M. Draghi D.C. Brown N.P. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J. Cataract Refract. Surg. 2008;34:814–818. doi: 10.1016/j.jcrs.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Shen L.L. Mitscher L.A. Sharma P.N. O'Donne T.J. Chu C.S. Rosen A.G. Pernet A.C. Mechanism of inhibition of DNA gyrase by quinolone antibacterials. A cooperative drugDNA- binding model. Biochemistry. 1989;28:2886–2894. doi: 10.1021/bi00435a039. [DOI] [PubMed] [Google Scholar]
- 13.Kwatra D. Vadlapatla R.K. Vadlapudi A.D. Pal D. Mitra A.K. Interaction of gatifloxacin with efflux transporters: a possible mechanism for drug resistance. Int. J. Pharm. 2010;395:114–121. doi: 10.1016/j.ijpharm.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper D.C. Mode of action of fluoroquinolones. Drugs. 1999;58(Suppl 2):6–10. doi: 10.2165/00003495-199958002-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hooper D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. Suppl. 2000;2:S24–S28. doi: 10.1086/314056. [DOI] [PubMed] [Google Scholar]
- 16.Baker R.S. Flowers C.W., Jr. Casey R. Fong D.S. Wilson M.R. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Arch. Ophthalmol. 1996;114:632–633. doi: 10.1001/archopht.1996.01100130624031. [DOI] [PubMed] [Google Scholar]
- 17.Marchal S. Merlin J.L. Colosetti P. Finance C. Influence of the fluoroquinolone ofloxacin on the intrinsic expression of multidrug resistance phenotype in HCT-8 human colon carcinoma cells. Oncol. Res. 1999;11:375–381. [PubMed] [Google Scholar]
- 18.Gottesman M.M. Fojo T. Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 19.Dey S. Patel J. Anand B.S. Jain-Vakkalagadda B. Kaliki P. Pal D. Ganapathy V. Mitra A.K. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 20.Karla P.K. Earla R. Boddu S.H. Johnston T.P. Pal D. Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr. Eye Res. 2009;34:1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karla P.K. Pal D. Quinn T. Mitra A.K. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int. J. Pharm. 2007;336:12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Bambeke F. Michot J.M. Tulkens P.M. Antibiotic efflux pumps in eukaryotic cells: occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J. Antimicrob. Chemother. 2003;51:1067–1077. doi: 10.1093/jac/dkg225. [DOI] [PubMed] [Google Scholar]
- 23.Dey S. Gunda S. Mitra A.K. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J. Pharmacol. Exp. Ther. 2004;311:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- 24.Robey R.W. To K.K. Polgar O. Dohse M. Fetsch P. Dean M. Bates S.E. ABCG2: a perspective. Adv. Drug Deliv. Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L. Zhang Y.D. Strong J.M. Reynolds K.S. Huang S.M. A regulatory viewpoint on transporter-based drug interactions. Xenobiotica. 2008;38:709–724. doi: 10.1080/00498250802017715. [DOI] [PubMed] [Google Scholar]
- 26.Wittwer C.T. Herrmann M.G. Gundry C.N. Elenitoba-Johnson K.S. Real-time multiplex PCR assays. Methods. 2001;25:430–442. doi: 10.1006/meth.2001.1265. [DOI] [PubMed] [Google Scholar]
- 27.Lage J.M. Leamon J.H. Pejovic T. Hamann S. Lacey M. Dillon D. Segraves R. Vossbrinck B. Gonzalez A. Pinkel D. Albertson D.G. Costa J. Lizardi P.M. Whole genome analysis of genetic alterations in small DNA sampls using hyperbranched strand displacement amplification and array-CGH. Genome Res. 2003;13:294–307. doi: 10.1101/gr.377203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehnert M. Chemotherapy resistance in breast cancer. Anticancer Res. 1998;18:2225–2226. [PubMed] [Google Scholar]
- 29.Gottesman M.M. Pastan I. Ambudkar S.V. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 1996;6:610–617. doi: 10.1016/s0959-437x(96)80091-8. [DOI] [PubMed] [Google Scholar]