Abstract
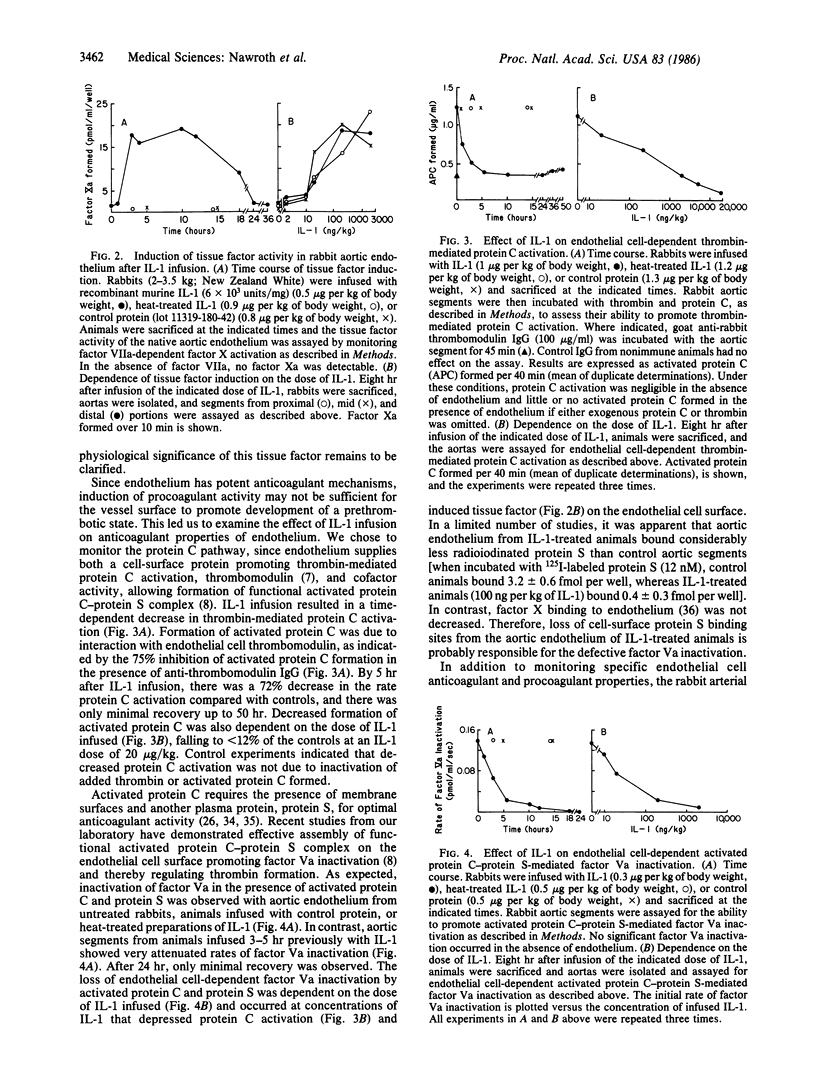
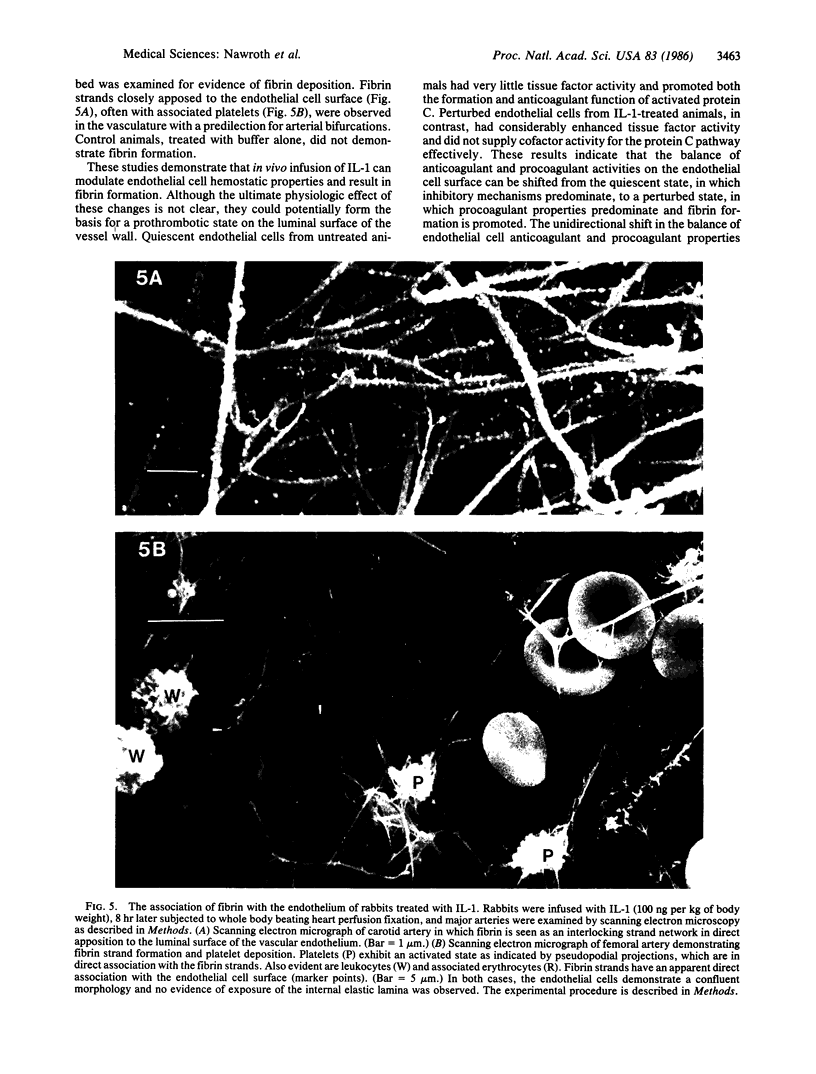
Previous studies demonstrated that endothelial cells participate actively in both anticoagulant and procoagulant reactions. Although anticoagulant mechanisms predominate on the surface of quiescent endothelial cells, perturbed endothelial cells can promote coagulation through the coordinated induction of procoagulant activity and suppression of anticoagulant mechanisms. Purified recombinant interleukin 1 was infused intravenously into rabbits and coagulant properties of the native aortic endothelium were subsequently studied. Interleukin 1 infusion resulted in a time- and dose-dependent induction of the procoagulant cofactor tissue factor, while concomitantly blocking the protein C anticoagulant pathway. Tissue factor activity increased greater than 10-fold by 3-5 hr after the infusion, while endothelial cell-dependent thrombin-mediated protein C activation decreased by 72% and assembly of functional activated protein C-protein S complex on the vessel surface was decreased by greater than 90%. Scanning electron microscopy of major arteries demonstrated fibrin strands closely associated with the luminal endothelial cell surface with a predilection for bifurcations. Interleukin 1, a mediator of the inflammatory response, can shift the balance of procoagulant and anticoagulant reactions on the endothelium unidirectionally favoring clot formation. The surface of perturbed endothelium can thus provide a template, facilitating the development of a prethrombotic state, and provides a model for the early stages of thrombosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertina R. M., Broekmans A. W., van der Linden I. K., Mertens K. Protein C deficiency in a Dutch family with thrombotic disease. Thromb Haemost. 1982 Aug 24;48(1):1–5. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans A. W., Veltkamp J. J., Bertina R. M. Congenital protein C deficiency and venous thromboembolism. A study of three Dutch families. N Engl J Med. 1983 Aug 11;309(6):340–344. doi: 10.1056/NEJM198308113090604. [DOI] [PubMed] [Google Scholar]
- Colucci M., Balconi G., Lorenzet R., Pietra A., Locati D., Donati M. B., Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983 Jun;71(6):1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Recurrent venous thromboembolism in patients with a partial deficiency of protein S. N Engl J Med. 1984 Dec 13;311(24):1525–1528. doi: 10.1056/NEJM198412133112401. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Nixon R. R., Esmon C. T. Determination of functional levels of protein C, an antithrombotic protein, using thrombin-thrombomodulin complex. Blood. 1984 Jan;63(1):15–21. [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–973. [PubMed] [Google Scholar]
- Esmon N. L., DeBault L. E., Esmon C. T. Proteolytic formation and properties of gamma-carboxyglutamic acid-domainless protein C. J Biol Chem. 1983 May 10;258(9):5548–5553. [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Griffin J. H., Evatt B., Zimmerman T. S., Kleiss A. J., Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981 Nov;68(5):1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPPELER R. Das Verhalten von Faktor V im Serum unter normalen und pathologischen Bedingungen. Z Klin Med. 1955;153(2):103–113. [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Kisiel W., McMullen B. A. Isolation and characterization of human factor VIIa. Thromb Res. 1981 May 1;22(3):375–380. doi: 10.1016/0049-3848(81)90130-4. [DOI] [PubMed] [Google Scholar]
- Lyberg T., Galdal K. S., Evensen S. A., Prydz H. Cellular cooperation in endothelial cell thromboplastin synthesis. Br J Haematol. 1983 Jan;53(1):85–95. doi: 10.1111/j.1365-2141.1983.tb01989.x. [DOI] [PubMed] [Google Scholar]
- Maynard J. R., Dreyer B. E., Stemerman M. B., Pitlick F. A. Tissue-factor coagulant activity of cultured human endothelial and smooth muscle cells and fibroblasts. Blood. 1977 Sep;50(3):387–396. [PubMed] [Google Scholar]
- Meltzer M. S., Oppenheim J. J. Bidirectional amplification of macrophage-lymphocyte interactions: enhanced lymphocyte activation factor production by activated adherent mouse peritoneal cells. J Immunol. 1977 Jan;118(1):77–82. [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y., Bach R. Tissue factor revisited. Prog Hemost Thromb. 1982;6:237–261. [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. I. Characterization of the reaction products formed during the activation of bovine prothrombin. J Biol Chem. 1974 Jan 25;249(2):594–605. [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Rodgers G. M., Shuman M. A. Prothrombin is activated on vascular endothelial cells by factor Xa and calcium. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7001–7005. doi: 10.1073/pnas.80.22.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]
- Seligsohn U., Berger A., Abend M., Rubin L., Attias D., Zivelin A., Rapaport S. I. Homozygous protein C deficiency manifested by massive venous thrombosis in the newborn. N Engl J Med. 1984 Mar 1;310(9):559–562. doi: 10.1056/NEJM198403013100904. [DOI] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Drillings M., Kisiel W., Nawroth P., Nossel H. L., LaGamma K. S. Activation of factor IX bound to cultured bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):913–917. doi: 10.1073/pnas.81.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Nawroth P. P., Harris K., Esmon C. T. Cultured bovine aortic endothelial cells promote activated protein C-protein S-mediated inactivation of factor Va. J Biol Chem. 1986 Jan 15;261(2):713–718. [PubMed] [Google Scholar]
- Stern D. M., Nawroth P. P., Kisiel W., Handley D., Drillings M., Bartos J. A coagulation pathway on bovine aortic segments leading to generation of Factor Xa and thrombin. J Clin Invest. 1984 Dec;74(6):1910–1921. doi: 10.1172/JCI111611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D., Nawroth P., Handley D., Kisiel W. An endothelial cell-dependent pathway of coagulation. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2523–2527. doi: 10.1073/pnas.82.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by a new protein. A possible function for bovine protein S. J Biol Chem. 1980 Jun 25;255(12):5521–5524. [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., Tans G., Rosing J., Hemker H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981 Apr 10;256(7):3433–3442. [PubMed] [Google Scholar]