Abstract
Objective:
This study explored the association between long-term epilepsy surgery outcome and changes in depressive symptoms.
Methods:
Adults were enrolled between 1996 and 2001 in a multicenter prospective study to evaluate outcomes of resective epilepsy surgery. The extent of depressive symptoms and depression case status (none, mild, or moderate/severe) were assessed using the Beck Depression Inventory (BDI) preoperatively and 3, 12, 24, 48, and 60 months postoperatively. A mixed-model repeated-measures analysis was performed, adjusting for covariates of seizure location, gender, age, race, education, and seizure control.
Results:
Of the total 373 subjects, 256 were evaluated at baseline and 5 years after surgery. At baseline, 164 (64.1%) were not depressed, 34 (13.3%) were mildly depressed, and 58 (22.7%) had moderate to severe depression. After 5 years, 198 (77.3%) were not depressed, 20 (7.8%) were mildly depressed, and 38 (14.8%) were moderately to severely depressed. Five years after surgery, the reduction in mean change from baseline in BDI score was greater in subjects with excellent seizure control than in the fair and poor seizure control groups (p = 0.0006 and p = 0.02 respectively). Those with good seizure control had a greater reduction in BDI score than the poor seizure control group (p = 0.02) and borderline significant reduction compared with the fair seizure control group (p = 0.055).
Conclusion:
Although study participants had initial improvement in depressive symptoms, on average, after resective surgery, only patients with good or excellent seizure control had sustained long-term improvement in mood.
Approximately 20%–50% of people with epilepsy have significant mood problems.1 In cross-sectional studies, severity of depressive symptoms is more strongly associated with poor quality of life than seizure frequency.2 However, depressive symptoms may occur peri-ictally,3 suggesting that seizures may cause mood changes.
A few short-term clinical trials showed that antidepressants and psychotherapy modestly improve depression in people with epilepsy.4 In contrast, we previously found that resective epilepsy surgery significantly improves mood 2 years after resection.5 In patients without epilepsy, the 5-year risk of depression relapse is up to 80%.6 This study explores the association between seizure control and depression symptom 5 years after surgery.
METHODS
The study sample, surgical protocol, data collection, and psychiatric evaluation were described in detail previously.7 Adult subjects were enrolled between 1996 and 2001 in a multicenter, prospective study to evaluate outcomes of resective epilepsy surgery.
A standardized protocol to record history, neurologic evaluation, MRI, video-EEG monitoring, and neuropsychiatric assessments was administered. The Beck Depression Inventory (BDI) was validated for depression assessments in people with epilepsy,8 including subjects from this cohort.5 BDI scores between 0 and 11 were classified as not depressed, scores between 12 and 15 were classified as mild depression, and scores >15 were classified as moderate/severe depression. Assessments were conducted at baseline preoperatively and at 3, 12, 24, 48, and 60 months postoperatively. Epilepsy control was rated as excellent for subjects who were seizure-free (and no auras) for all 5 years, good if subjects were seizure-free for 2 consecutive years but not all 5, fair if subjects were seizure-free for 1 year but never 2 consecutive years, and poor if subjects never had a 1-year period of seizure freedom.
Standard protocol approvals, registrations, and patient consents.
An institutional review board approved all procedures in the study.
Statistical methods.
The change from baseline BDI score was calculated at each follow-up time point, and the distribution was examined. A mixed-model repeated-measures analysis (implemented with SAS PROC MIXED) was performed to compare change from baseline BDI score among different seizure control groups. The model included resection location, gender, age, race, education, time, and seizure control as fixed effects as well as random intercept and slope. Baseline depression and anxiety diagnosis, as established by the World Mental Health Composite International Diagnostic Interview (CIDI), were also further controlled in the model in the separate analysis. The significance level was set at 0.05.
RESULTS
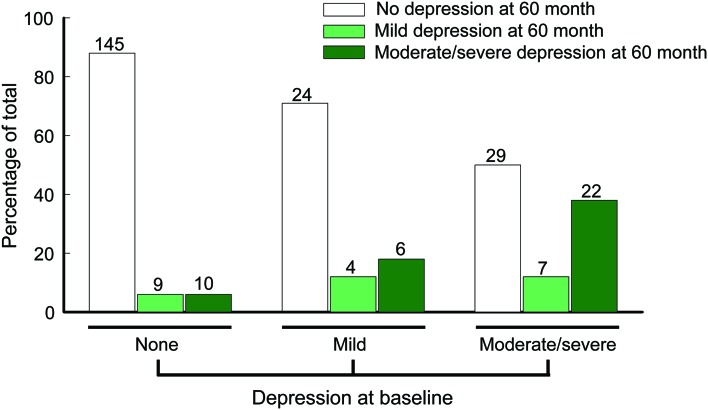
Of the 373 adult subjects who were recruited and completed at least one BDI (table 1), 164 (64.1%) were not depressed, 34 (13.3%) were mildly depressed, and 58 (22.7%) had moderate to severe depression at baseline, as classified by BDI score cutoffs. After 5 years, among 256 subjects who were reevaluated, 198 (77.3%) were not depressed, 20 (7.8%) were mildly depressed, and 38 (14.8%) were moderately to severely depressed (figure 1). Of the subjects with missing data at 5 years, 13 had moderate to severe depression, and 94 were not depressed at their last visit. There was no significant difference in baseline BDI score between completers and those with missing BDI data at 5 years (p = 0.39). Among the 164 subjects who were not depressed at baseline, 10 subjects had BDI scores at the moderate to severe depression range at 5 years (figure 1). Of the 10 subjects who developed moderate to severe depressive symptoms at the 5 year follow-up, 4 met the criteria for an anxiety disorder within 1 year before their surgery and 2 had depressive disorders, as determined by the CIDI, within 1 year before their surgery; furthermore, 2 had excellent, 3 had good, 1 had fair, and 4 had poor seizure control after surgery.
Table 1.
Demographic and clinical characteristics of study sample
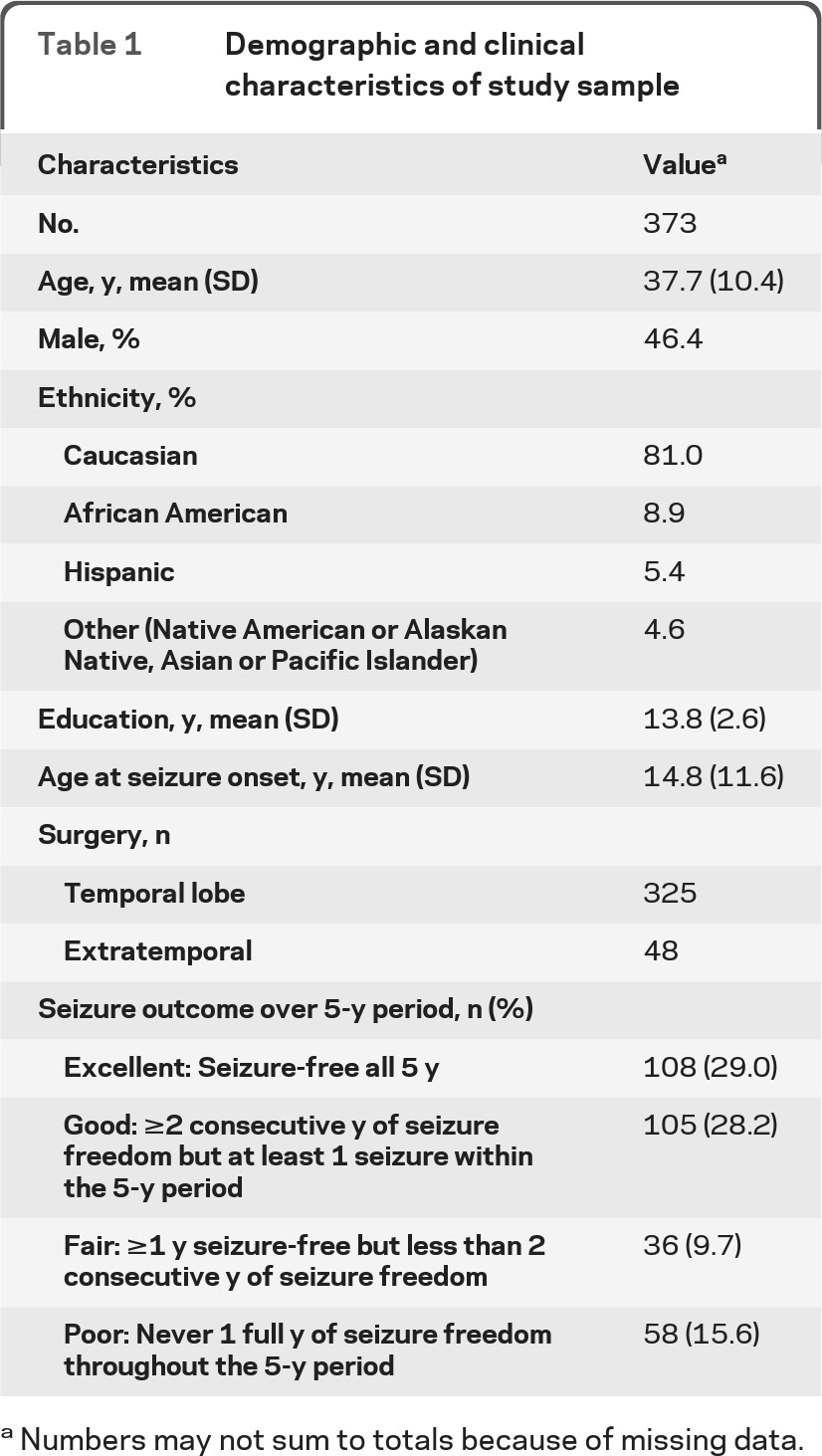
Numbers may not sum to totals because of missing data.
Figure 1. Baseline and 5-year postsurgery depression frequency.
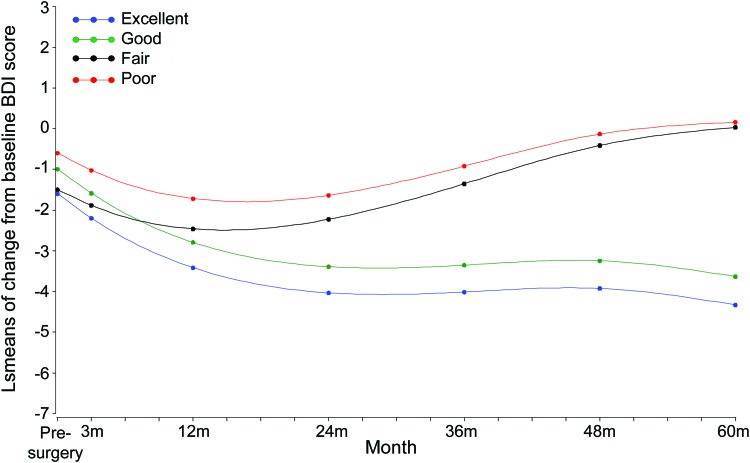
Five years after surgery, differences in mean change from baseline BDI scores were demonstrated between excellent and poor seizure control (p = 0.006) as well as between excellent and fair seizure control (p = 0.02), respectively (figure 2; table e-1 on the Neurology® Web site at www.neurology.org). Study participants with good seizure control had greater reductions in BDI scores (less depressive symptoms) than those with poor seizure control (p = 0.02) (figure 2). Seizure location (including laterality), age, race, and education were not associated with depression outcomes. Subjects with depressive disorder and anxiety disorders at baseline, as determined by the CIDI, had greater reductions in the change in BDI score during follow-up than people without depressive and anxiety disorders at baseline (figure e-1, table e-2). The association between seizure control and BDI was similar with or without controlling for baseline diagnosis.
Figure 2. Change in depression over time.
BDI = Beck Depression Inventory; LS = least-squares.
DISCUSSION
Whereas most study participants in this cohort had initial improvement in depressive symptoms after surgery, only subjects with good or excellent seizure control had sustained long-term improvement 5 years after surgery. In contrast to cross-sectional studies showing no relationship between seizure frequency and depression score,2 we found that good seizure control was associated with more improved depressive symptoms. Further, we previously found that subjects with excellent seizure control have an improvement in quality of life (including the mental health component of the quality-of-life measure) compared with subjects with persistent seizures after surgery.9 In contrast to previous studies, this study demonstrated that patients with good seizure control had depressive scores comparable to those of subjects who were seizure-free throughout the 5 years. Interestingly, even subjects with poorly controlled seizures demonstrated an initial drop in BDI score. The initial drop may be due to a placebo response postoperatively, a transient effect of surgery, a result of the additional care provided by being enrolled in a study, or partial improvement with seizure frequency. Conversely, 10 subjects who reported no depressive symptoms at baseline, reported moderate to severe depressive symptoms after 5 years. However, at least 2 of those patients had a history of depression, and 4 had comorbid anxiety disorders. Some of these patients may continue to have emotional lability due to limbic dysregulation.
Although the linear mixed-effects model we used demonstrates how BDI scores changed per subpopulation over time, it does not link the timing of seizure breakthrough to depressive symptoms. Our data cannot determine whether depressive symptoms are a part of a peri-ictal phenomenon or a psychological response to experiencing breakthrough seizures. Another limitation of the study is that access to and quality of mental health care including antidepressant medication use, cognitive behavioral therapy, or other therapeutic strategies shown to be efficacious for epilepsy and depression were not recorded systematically. In depression clinical trials as well as the clinical setting, as many as 70% of patients discontinue antidepressant medications within 1 year of treatment,10 but we do not know the patterns of mental health care in this study cohort. Another limitation is that depression assessments were done by BDI score, which reflects state rather than trait characteristics, is based on self-reporting of symptoms, and may be systematically biased on persons with epilepsy because of overlap of the disorder with somatic symptoms and side effects of antiepileptic drug use. Whereas the CIDI was administered at baseline and at 2 years after surgery, it was not administered at 3, 4, and 5 years after surgery.
Because psychotherapeutic and psychotropic management were not adequately monitored in this study, we may not draw conclusions on whether epilepsy surgery directly improves mood symptoms. However, subjects who achieved good seizure control after surgery also experienced long-term mood improvement. Those with only fair or poor outcomes after surgery did not, in the long term, appear to experience similar benefits. Further research is needed to explore the long-term temporal relationship between seizures and mood symptoms. Measurement of access to and quality of mental health care should be incorporated into epilepsy surgical outcomes studies.
Supplementary Material
GLOSSARY
- BDI
Beck Depression Inventory
- CIDI
Composite International Diagnostic Interview.
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Hamid contributed to the design and conceptualization of the study, analysis and interpretation of the data, statistical analysis, and drafting and revising the manuscript. Dr. Liu contributed to the analysis and interpretation of the data, statistical analysis, and drafting and revising the manuscript. Dr. Cong contributed to the analysis and interpretation of the data, statistical analysis, and drafting and revising the manuscript. Dr. Devinsky contributed to the analysis and interpretation of the data and drafting and revising the manuscript. Dr. Berg contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Vickrey contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Sperling contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Shinnar contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Langfitt contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Walczak contributed to the design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Barr contributed to the analysis and interpretation of the data, and drafting and revising the manuscript. Dr. Dziura contributed to the design of the study, analysis and interpretation of the data, and statistical analysis. Dr. Bazil contributed to the interpretation of the data and drafting and revising the manuscript. Dr. Spencer contributed to the design and conceptualization of the study.
DISCLOSURE
Dr. Hamid serves as Managing Editor of the Journal of Muslim Mental Health and receives research support from the National EpiFellows Foundation and the Epilepsy Foundation. Dr. Liu and Dr. Cong report no disclosures. Dr. Devinsky served on a speaker's bureau for and has received speaker honoraria from UCB; has served as a consultant for Medivation, Inc.; serves as an Associate Editor of Epilepsy and Behavior and Co-Editor of Reviews in Neurological Diseases; receives royalties from the publication of Cognitive and Behavioral Neurology (Oxford University Press, 2002); and receives research support from the NIH/NINDS. Dr. Berg receives research support from the NIH/NINDS; has received funding for travel and speaker honoraria from Eisai Inc., the British Pediatric Neurological Association1, and the Epilepsy Research Center (Melbourne); has received funding for travel from UCB, the American Epilepsy Society, and the International League Against Epilepsy; has received awards from the American Epilepsy Society and British Pediatric Neurological Association; has served as a consultant for Dow Agro Science; serves on the editorial boards of Epileptic Disorders and Epilepsy & Behavior; and is past Chair of the ILAE's Commission on Classification and Terminology, current Chair of the ILAE's Task Force on Classification-Diagnostic Manual, member of the ILAE's Pediatric Commission's Task Force on Autism, member of the AES's Commission on Nonepileptic Seizures, member ad hoc Task Force of the ILAE Commission on Therapeutic Strategies, member of the AES Suicidality Task Force, and steward for the NINDS Benchmarks in Epilepsy Research. Dr. Vickrey serves on scientific advisory boards for the Sports Concussion Institute, National Multiple Sclerosis Society, and the NIH; has received funding for travel from the French National Foundation on Alzheimer's Disease and Related Disorders and the NIH; serves on the editorial boards of Neurorehabilitation and Neural Repair, Journal of Medical Economics, and Circulation: Cardiovascular Quality and Outcomes; serves as a consultant for EMD Serono, Inc. and the Research Triangle Institute; serves as a National Advisory Neurological Disorders and Stroke council member; and receives research support from Genentech, Inc., the NIH (NIA, NINDS), the US Veterans Administration Health Services Research and Development Service, the California Office of Statewide Health Planning and Development, the California Department of Public Health, the State of California Department of Health Services, University of Pennsylvania Udall Center, the American Heart Association, the National Multiple Sclerosis Society, and SCAN Foundation. Dr. Sperling serves on scientific advisory boards for UCB and Vertex Pharmaceuticals; has received speaker honoraria from UCB; serves as an Associate Editor for Epilepsia; serves as a consultant for Sunovion Pharmaceuticals Inc.; served on speakers' bureaus for UCB and Pfizer Inc; and receives research support from UCB, Marinus Pharmaceuticals, Inc., Sunovion Pharmaceuticals Inc., Eisai Inc., Novartis, Lundbeck Inc., Vertex Pharmaceuticals, NeuroPace, Inc., Medtronics, Inc., Sepracor Inc., and the NIH. Dr. Shinnar has served on scientific advisory boards for Questcor, King Pharmaceuticals, and Sunovion Pharmaceuticals Inc.; serves on the editorial board of Pediatric Neurology; receives royalties from publication of Febrile Seizures (Academic Press, 2002); serves as a consultant for Questcor and Eisai Inc.; serves on speakers' bureaus for Questcor, UCB, and Eisai Inc.; and receives research support from the NIH/NINDS. Dr. Langfitt serves as an Associate Editor for Epilepsia and for the Journal of the International Neuropsychological Society and has received research support from the NIH/NINDS. Dr. Walczak reports no disclosures. Dr. Barr serves as an Associate Editor of The Clinical Neuropsychologist and Neuropsychology Review and on the editorial boards of Neuropsychology, Archives of Clinical Neuropsychology, and Assessment; serves as a consultant for the Brainscope Company; is President Elect of Division 40 (Clinical Neuropsychology) of the American Psychological Association; and receives research support from Finding a Cure for Epilepsy and Seizures (FACES). Dr. Dziura serves on data safety monitoring boards for MannKind Corporation and Chelsea Therapeutics. Dr. Bazil serves on the editorial boards of Current Neurology and Neuroscience Reviews and The Medical Letter; has received speaker honoraria from Pfizer Inc; serves as a consultant for Pfizer Inc and UCB; and receives research support from Sepracor Inc. and Pfizer Inc. Dr. Spencer is deceased; disclosures are not included for this author.
REFERENCES
- 1. Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry 2003; 54: 388–398 [DOI] [PubMed] [Google Scholar]
- 2. Boylan LS, Flint LA, Labovitz DL, et al. Depression but not seizure frequency predicts quality of life in treatment resistant epilepsy. Neurology 2004; 62: 258–261 [DOI] [PubMed] [Google Scholar]
- 3. Mula M, Jauch R, Cavanna A, et al. Interictal dysphoric disorder and periictal dysphoric symptoms in patients with epilepsy. Epilepsia 2010; 51: 1139–1145 [DOI] [PubMed] [Google Scholar]
- 4. Gilliam F, Kanner AM. The treatment of depression in epilepsy. Epilepsy Behav 2002; 3(5S): 2–9 [DOI] [PubMed] [Google Scholar]
- 5. Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology 2005; 65: 1744–1749 [DOI] [PubMed] [Google Scholar]
- 6. Boland RJ, Keller MB. The course of depression. In: Davis KL, Charney D, Coyle JT, Nemeroff C. eds. Neuropsychopharmacology: The Fifth Generation of Progress. Brentwood, TN: American College of Neuropsychopharmacology; 2002: 1009–1015 [Google Scholar]
- 7. Berg AT, Vickrey BG, Langfitt J, et al. The Multicenter Study of Epilepsy Surgery: recruitment and selection for surgery. Epilepsia 2003; 44: 1425–1433 [DOI] [PubMed] [Google Scholar]
- 8. Jones JE, Hermann BP, Woodard JL, et al. Screening for major depression in epilepsy with common self-report depression inventories. Epilepsia 2005; 46: 731–735 [DOI] [PubMed] [Google Scholar]
- 9. Spencer SS, Berg AT, Vickrey BG, et al. Health-related quality of life over time since resective epilepsy surgery. Ann Neurol 2007; 62: 327–334 [DOI] [PubMed] [Google Scholar]
- 10. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int J Clin Psychopharmacol 2002; 17: 265–271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.