Abstract
Amyloid β-protein (Aβ) self-assembly into toxic oligomers and fibrillar polymers is believed to cause Alzheimer’s disease (AD). In the AD brain, a high percentage of Aβ contains Met-sulfoxide at position 35, though the role this modification plays in AD is not clear. Oxidation of Met35 to sulfoxide has been reported to decrease Aβ assembly and neurotoxicity, whereas surprisingly, Met35 oxidation to sulfone yields similar toxicity to unoxidized Aβ. We hypothesized that the lower toxicity of Aβ-sulfoxide might result not only from structural alteration of the C-terminal region, but also from activation of methionine-sulfoxide reductase (Msr), an important component of the cellular antioxidant system. Supporting this hypothesis, we found that the low toxicity of Aβ-sulfoxide correlated with induction of Msr activity. In agreement with these observations, in MsrA−/− mice the difference in toxicity between native Aβ and Aβ-sulfoxide was essentially eliminated. Subsequently, we found that treatment with N-acetyl-Met-sulfoxide could induce Msr activity and protect neuronal cells from Aβ toxicity. In addition, we measured Msr activity in a double-transgenic mouse model of AD and found that it was increased significantly relative to non-transgenic mice. Immunization with a novel methionine sulfoxide-rich antigen for six months led to antibody production, decreased Msr activity, and lowered hippocampal plaque burden. The data suggest an important neuroprotective role for the Msr system in the AD brain, which may lead to development of new therapeutic approaches for AD.
Oxidative stress occurs in biological systems when generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as hydroxyl radicals and peroxynitrite ions, exceeds the system’s capacity to eliminate these species (1). This situation may result from a disturbance in production and/or distribution of antioxidants or from environment-induced elevation of ROS/RNS. Oxidative stress is a major deleterious mechanism in Alzheimer’s disease (AD) (2), other neurodegenerative diseases (3), and normal aging (4). In AD, oxidative damage markers, including lipid peroxidation and nitration, nucleic acid oxidation, and protein carbonylation are increased in vulnerable brain areas relative to age-matched healthy individuals (5).
AD is characterized pathologically by extracellular amyloid plaques comprising predominantly fibrillar amyloid β-protein (Aβ) and intracellular neurofibrillary tangles made of hyperphosphorylated tau (6). Amyloid plaques are surrounded by inflammation, including activated microglia and astrocytes, which contribute to creation and maintenance of oxidative stress (6). Though historically amyloid plaques were thought to cause AD (7), current evidence indicates that the pathological process leading to AD begins with synaptic injury by neurotoxic Aβ oligomers, whereas formation of plaques and tangles are downstream events (8). Oxidative stress is one of the earliest consequences of toxic insults mediated by soluble Aβ oligomers (9). Mitochondria are particularly sensitive to oxidative stress and reduced metabolic activity resulting from oxidative damage to vital mitochondrial components has been demonstrated in AD (10). Consequently, antioxidant therapy has been associated with reduced risk for AD (11, 12).
Aβ exists predominantly in two major forms comprising 40 (Aβ40) or 42 (Aβ42) amino acid residues. Genetic, physiologic, and biochemical evidence indicates that Aβ42 plays a predominant role in the pathogenesis of AD (13). A single Met residue in Aβ, Met35, is located in the middle of the hydrophobic C-terminal region (Aβ(29–42)). Therefore, the dramatic increase in polarity of the Met side chain that occurs upon oxidation has a profound effect on the hydropathy of the entire region (14). Met is highly susceptible to oxidation in vivo, particularly under conditions of oxidative stress. The sulfoxide form has been found to comprise 10–50% of Aβ in amyloid plaques of AD brain (15–18), though it is not clear whether its existence contributes to AD etiology or results from the highly oxidative environment around amyloid plaques where fibrillar Aβ may be trapped for long periods.
In addition to oxidation of Met to Met-sulfoxide (Met(O)), Met can undergo a second oxidation reaction yielding Met-sulfone (Met(O2)). Met(O2) has been found in the antioxidant protein DJ-1 in brains from patients with AD or Parkinson’s disease (PD) (19) and may exist in Aβ (20), though its formation requires high activation energy and consequently Met(O2) is not commonly found in vivo. When it does happen, the in vivo oxidation of Met to Met(O2) is considered irreversible (21). In contrast, oxidation of Met to Met(O) is reversible and the reverse reaction is catalyzed in vivo by the methionine-sulfoxide reductase (Msr) system, comprising peptide-methionine (S)-S-oxide reductase (EC 1.8.4.11, MsrA) and peptide-methionine (R)-S-oxide reductase (EC 1.8.4.12, MsrB), which reduce the S and R enantiomers of the sulfoxide group, respectively, providing protection against oxidative stress (22). Mammalian MsrA is encoded by a single gene (23) and is found in both the cytosol and mitochondria due to alternative splicing of an N-terminal mitochondrial signal sequence and myristoylation of the cytosolic form (24). MsrA levels decrease with aging (25) and in AD (26). Studies in MsrA−/− mice have shown increased vulnerability to oxidative stress (27) and oxidative pathology associated with AD (28) and PD (29). Conversely, overexpression of MsrA in various organisms has been shown to provide enhanced protection against oxidative stress and extend survival rate (30–32).
Several laboratories have reported lower toxicity of Aβ-Met(O) relative to WT Aβ (33). This lower toxicity largely has been attributed to the tendency of Aβ-Met(O) to aggregate with slower kinetics (34) and/or form smaller oligomers relative to WT Aβ (14), which correlate with structural differences between native and oxidized Aβ in the C-terminal region (35, 36). However, recent examination of the sulfoxide and sulfone forms of Aβ alongside the WT form found that although Aβ-Met(O) showed reduced toxicity, as expected, the toxicity of Aβ-Met(O2), which was used as a control, was surprisingly similar to that of WT Aβ in assays of neuronal apoptosis, dendritic spine morphology, and Ca2+ homeostasis (37). These data suggested that the lower activity of Aβ-Met(O) might result not only from an altered structure in the C-terminal region of Aβ or alteration of Aβ oligomerization, but also from other mechanisms, possibly Msr activation, which might be unique to the sulfoxide form, despite the similarity in the structure and calculated dipole moment between Met(O) and Met(O2) (14, 38). Consistent with this hypothesis, a recent study has reported elevated MsrA activity and mRNA levels in human neuroblastoma (IMR-32) cells in response to treatment with Aβ42-Met(O) suggesting that the cells sensed the presence of Met(O) in Aβ and upregulated MsrA to provide enhanced cellular protection (39).
To test the hypothesis that Msr activation contributes to the lower toxicity observed for Aβ-Met(O) relative to Aβ-Met(O2) and WT Aβ, here, we compared the effect of the WT, sulfoxide, and sulfone forms of Aβ40 and Aβ42 on the viability and Msr activity of rat primary cortical neurons. The findings led us to explore the role of the different Msr isoforms in the cellular response to Aβ by using the same experimental paradigm in primary neurons from WT and MsrA−/− mice. In addition, we hypothesized that the Msr system could be used as a target for development of therapeutic agents against Aβ-induced oxidative stress and to test this hypothesis, we studied the possibility of inducing a neuroprotective response by activating the Msr system, both in cell culture, using a Met(O) derivative, and in vivo by immunization with a Met(O)-rich antigen.
Materials and Methods
Peptides synthesis
Aβ40, [Met(O)35]Aβ40, [Met(O2)35]Aβ40, Aβ42, [Met(O)35]Aβ42, and [Met(O2)35]Aβ42 were synthesized by incorporating FMOC-Met(O) or FMOC-Met(O2) (EMD Biosciences, San Diego, CA) in position 35 where appropriate, purified, and characterized in the UCLA Biopolymers Laboratory. Quantitative amino acid analysis and mass spectrometry were used to characterize the expected compositions and molecular weights, respectively, for each peptide. N-acetyl-D,L-Met(O) (Ac-Met(O)) was purchased from Sigma (St. Louis, MO).
Preparation of peptide solutions
Purified peptides were stored as lyophilized powders at −20°C. Before use, peptides were treated with 1,1,1,3,3,3-hexafluoroisopropanol (HFIP, TCI America, Portland, OR) to disassemble pre-formed aggregates and stored as dry films at −20°C as described previously (40). Immediately before use, peptide films were dissolved in 60 mM NaOH at 10% of the desired volume, diluted with cell-culture media followed by 1 min sonication and added to the cells at a final concentration of 10 μM unless otherwise stated. The final NaOH concentration was ≤6 mM and the pH change of the media was negligible.
Animals
All experiments were compliant with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the respective Animal Research Councils and the Ethics Committees of UCLA or KU. Pregnant (E18) Sprague-Dawley rats and C57Bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Double-transgenic (2×Tg) mice overexpressing familial-AD linked mutant forms of amyloid β-protein precursor (APP) and presenilin 1 (B6C3 Tg(APPswe, PSEN1dE9)85Dbo/J) and control, non-Tg mice on the same genetic background were purchased from Jackson Laboratories (Bar Harbor, ME). MsrA−/− mice were bred and maintained in house.
Cell culture
Primary cortical or hippocampal neurons were prepared as described previously (37). Briefly, E18 pregnant rats or mice were euthanized with CO2 and the pups were collected immediately. The brains were dissected in chilled Leibovitz’s L-15 medium (ATCC, Manassas, VA) in the presence of 1 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA) and the cells were suspended in Dulbecco’s Modified Eagle’s Medium (DMEM, obtained from ATCC) containing 10% heat-inactivated fetal bovine serum (ATCC) and penicillin/streptomycin (1 μg/ml), and plated in poly D-lysine (0.1 mg/ml, Sigma)-coated 96-well COSTAR plates (Corning, Lowell, MA) at a density of 3×105 cells/ml. The cultures were maintained for 6 d before treatment with peptides. Twenty-four hours after plating, the medium was replaced with fresh medium supplemented with 5 μM cytosine β-D-arabinofuranoside (Sigma) to inhibit the proliferation of glial cells. PC-12 cells were cultured and differentiated with 50 ng/ml nerve growth factor (NGF) 24 h prior to treatment with peptides as described previously (41).
MTT reduction assay
Cells were treated with freshly prepared Aβ analogues for 48 h. Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell-metabolism assay, as described previously (41). Briefly, following treatment, 15 μl of MTT were added to each well and incubated for 4 h at 37°C. Then, stop solution was added and kept overnight at 25°C. The optical density was measured using a Synergy plate reader (Bio-TEK instruments, Winooski, VT). The cell viability results of three independent experiments (6 wells per data point) were normalized to the medium control group and expressed as mean ± SEM. Neuroprotection experiments were performed in a similar manner using 10 μM Aβ42 in the absence or presence of Ac-Met(O).
Lactate dehydrogenase (LDH) release assay
Neurons were incubated with Aβ analogues for 48 h and cell death was assayed by measuring the release of LDH as described previously (42). Data from 6 independent experiments (6 wells per data point) were normalized to medium control and expressed as mean ± SEM.
Measurement of Msr activity
Total Msr activity was measured in rat or mouse primary cortical neurons, or differentiated PC-12 cells, as described previously (32). Briefly, the cells were treated with 10 μM of each peptide for 24 h. Similarly, in protection experiments, differentiated PC-12 cells were treated for 24 h with Ac-Met(O) in the absence or presence of 10 μM Aβ42. Following the incubation, the culture medium was removed and the cells were washed with PBS, pH 7.4. Then, the cells were lysed in PBS by 1-min sonication in an ice-water bath in the presence of a protease-inhibitor cocktail (Sigma). Insoluble material was removed by centrifugation at 14,000 g for 10 min at 4°C and supernates were stored at −80°C until use. Protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA) and used to normalize the volume used for determination of Msr activity. Supernates (100 μg protein) were incubated with 100 μl of 50 mM Tris-HCl, pH 7.5, containing 20 mM DTT and 200 μM dabsyl-Met(O) for 1 h at 37°C. Then, the reaction was stopped by adding an equal volume of acetonitrile and the mixture was analyzed by an HPLC system equipped with a C18 column using a gradient starting at 100% 0.14 M sodium acetate, pH 6.0, and increasing the percentage of acetonitrile to 70% over 30 min. The dabsyl-Met(O) and dabsyl-Met peaks were detected at 436 nm. The basal specific activity measured in control cells treated with medium alone was the following (in pmoles dabsyl-Met formed/min/mg protein): primary WT rat neurons − 240, primary WT mouse neurons − 150, primary MsrA−/− neurons − 80, PC-12 cells − 200. The specific activity in Aβ-treated cells was normalized to the medium-treated cells and expressed as % change of Msr activity (mean ± SEM).
Immunization of 2×Tg mice
2×Tg mice (43) were immunized with oxidized zea mays Met-rich protein (DZS18) (44) with complete Freund’s adjuvant for the first injection, followed by oxidized DZS18 with incomplete Freund’s adjuvant every 2 weeks for 6 months beginning at 3 months of age. 2×Tg mice injected with adjuvant only, unimmunized mice, and non-Tg mice of the same genetic background served as control groups (5 mice per group). At the end of the immunization period, the mice were euthanized, their brains were collected, frozen, and sectioned, and brain sections were analyzed for aggregated Aβ deposition using thioflavin S (ThS) staining. Additionally, Msr specific activity in brain were measured using the HPLC assay described above for cultures cells.
Detection of serum immunoglobulins by Western blot
Sera were collected from 2×Tg mice immunized with oxidized DZS18 or adjuvant alone, or unimmunized mice (n = 5 per group). Oxidized, recombinant DZS18 was loaded onto 4–20% gradient gels (Pierce, Rockford, IL, 5 μg per lane) and subjected to SDS-PAGE. The protein was transferred to nitrocellulose membranes and each lane was probed with serum from one mouse (1:500 dilution), followed by HRP-conjugated goat-anti-mouse IgG (1:5000 dilution, Invitrogen, Carlsbad, CA). The bands were visualized using ECL.
ThS staining
Coronal, 45-μm brain sections were prepared using a Microm HM 360 microtome (Harlow Scientific, Arlington, VA), immersed for 3 min each in 95% and 70% ethanol followed by 5-min incubation in 1% ThS in deionized water (Sigma) and quick rinses in 80% ethanol and deionized water. The sections then were dehydrated by consecutive 1 min incubations in 70%, 95%, and 100% ethanol and immersed in xylene for 3 min prior to aqueous mounting in glycerin jelly. ThS fluorescence was imaged and quantified using a fluorescence microscope (GE Healthcare, Waukesha, WI) with a high-content imaging system (GE Healthcare). The number of ThS-positive plaques per equivalent hippocampal area (plaque burden) was measured and quantified using ImageJ.
Data analysis
Data are expressed as mean ± SEM. They were analyzed by one-way analysis of variance (ANOVA) with post hoc Tukey’s test.
Results
Effect of WT and oxidized Aβ on neuronal viability
To investigate the neurotoxic effects of native and oxidized Aβ variants on cellular viability and survival, we used two different toxicity assays, MTT reduction and LDH release, in rat primary cortical or hippocampal neurons (Fig. 1). We used both assays because methods for determining Aβ toxicity are not consistent across the AD field. Each of these assays addresses a different aspect of cell toxicity: The MTT assay measures mitochondrial activity of viable cells, whereas the LDH assay detects membrane integrity as a direct measurement of cell death.
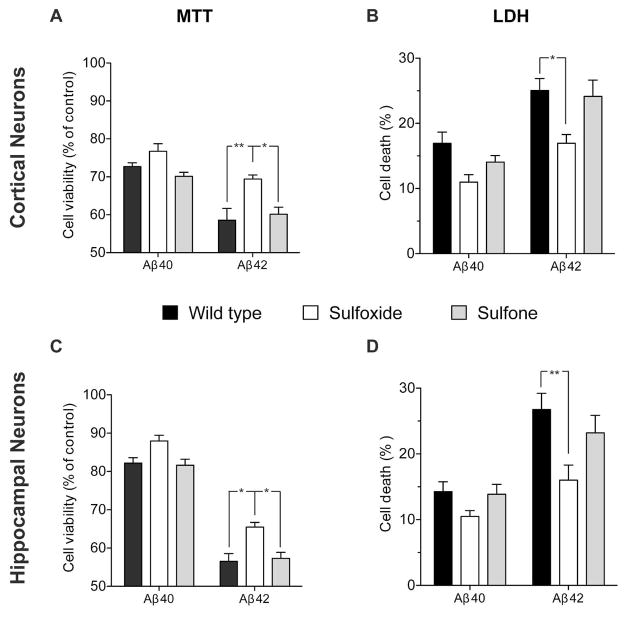
Figure 1. Comparison of neurotoxic effects of native and oxidized Aβ analogues.
Rat primary cortical (A, B) or hippocampal (C, D) neurons were cultured for 6 d and then treated with Aβ analogues. Cell viability was measured using the MTT reduction (A, C) or LDH release (B, D) assays following treatment with 10 μM of each Aβ analogue for 48 h as described previously (37). *p < 0.05; **p < 0.01.
Compared to cells treated with medium containing the same concentration of NaOH (used for initial peptide solubilization) but no Aβ, Aβ40 caused a decrease of 27±1% and 17±2% in cortical neuron viability, and 18±1% and 14±2% in hippocampal neuron viability in the MTT (Fig. 1A, C) and LDH (Fig. 1B, D) assays, respectively. Aβ40-Met(O) was 5–10% less toxic than WT Aβ40 whereas Aβ40-Met(O2) had similar toxicity to WT Aβ40. Overall, the differences observed among the Aβ40 analogues were not statistically significant. We also did not find significant differences between the response of cortical (Fig. 1A, B) and hippocampal (Fig. 1C, D) neurons to Aβ40 analogues.
Under the same conditions, Aβ42 showed 41±3% and 25±2% decrease in cortical neuron viability and 43±2% and 27±3% decrease in hippocampal neuron viability in the MTT (Fig. 1A, C) and LDH (Fig. 1B, D) assays, respectively. Similar to previously described data (37), Aβ42-Met(O) was significantly less toxic, causing 31±1% and 17±1% decrease in cortical neuron viability and 34±1% and 16±2% decrease in hippocampal neuron viability, respectively (Fig. 1). These levels of toxicity were similar to those of Aβ40 analogues in the LDH assay (Fig. 1A, B), whereas in the MTT assay, Aβ42-Met(O) showed toxicity that was intermediate between those induced by Aβ40 and Aβ42 (Fig. 1C, D). Aβ42-Met(O2) showed similar toxicity to WT Aβ42 in all cases, causing 40±2% and 24±3% decrease in cortical neuron viability and 43±2% and 23±3% decrease in hippocampal viability in the MTT and LDH assays, respectively (Fig. 1). Thus, as reported previously (37), both assays showed that despite the similar increase in dipole moment upon oxidation of Met35 to Met(O) or Met(O2) (14, 38), and despite the change in the oligomer size distribution of the sulfoxide and sulfone forms of Aβ42 relative to WT Aβ42 (14), only Aβ42-Met(O) was less toxic to cells than WT Aβ42.
Msr response to WT and oxidized Aβ
The toxicity of the six Aβ alloforms correlated with aggregation kinetics (37) but not with oligomer size distribution or polarity of the C-terminus (14). To test whether the Msr system might be involved, we measured cellular levels of Msr activity following treatment with each Aβ analogue. Because relatively high toxicity levels were observed following a 48-h incubation with Aβ42 or Aβ42-Met(O2) (Fig. 1), we used a 24-h incubation in these experiments.
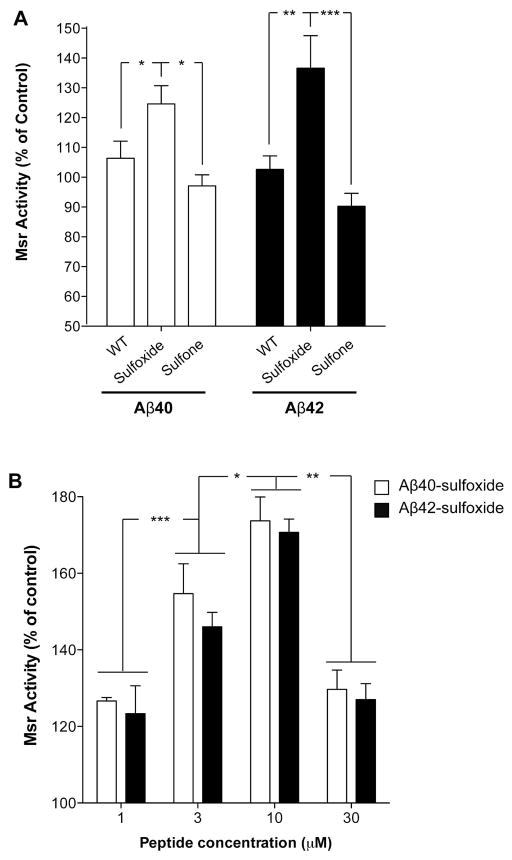
We found that WT Aβ40 and Aβ42 caused a small (6±6% and 3±5%, respectively), insignificant increase in Msr activity relative to untreated cells (Fig. 2A), whereas Aβ40-Met(O) and Aβ42-Met(O) increased total Msr activity significantly (25±6% and 27±11%, respectively) relative to untreated cells (Fig. 2A). In contrast, following treatment with Aβ40-Met(O2) or Aβ42-Met(O2), we observed a moderate, non-significant decrease of 3±4% and 10±4%, respectively, in Msr activity compared to control cells (Fig. 2A). Dose–response analysis of the effect of Aβ40-Met(O) or Aβ42-Met(O) on Msr activity showed that in both cases, the measured total Msr activity increased between 1–10 μM of Aβ and decreased at 30 μM Aβ, likely due to high levels of apoptosis at the highest concentration (Fig. 2B). Differences in Msr activity levels by Aβ40-Met(O) or Aβ42-Met(O) between Figs. 2A and B reflect experimental variability. These findings support the hypothesis that neurons sense the presence of the sulfoxide group in Aβ and respond by activating the Msr system as self-protection against oxidative stress.
Figure 2. Effect of native and oxidized Aβ on Msr activity.
Rat primary cortical neurons were grown for 6 d on poly-D-lysine-coated, 60-mm Petri plates. A) Cells were incubated in the presence or absence of 10 μM of each Aβ analogue for 24 h at 37°C. The cells were lysed in PBS, pH 7.4, centrifuged, and supernates were used to determine total specific Msr activity by HPLC using dabsyl-Met(O) as described previously (60). The results were normalized to untreated cells (240 pmol dabsyl-Met/min/mg protein defined as 100% specific Msr activity). The data are an average of 10–15 independent experiments. B) Cells were treated with Aβ40-sulfoxide or Aβ42-sulfoxide at the indicated concentrations and total specific Msr activity was determined as described above. The data are an average of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Examination of Aβ analogues in MsrA−/− neurons
To gain insight into the relative contribution of Msr isozymes to the response to Aβ-Met(O), we measured neurotoxicity and Msr activation in primary cortical neurons from MsrA−/− mice and compared the data to neurons from WT mice. In these experiments, we used only Aβ42 analogues because the differences observed among the different Aβ forms were similar in trend, yet greater in magnitude for Aβ42 than for Aβ40. We predicted that if MsrA were the main isozyme responsible for the lower observed toxicity of Aβ42-Met(O) relative to WT Aβ42 and Aβ42-Met(O2), the difference among the three Aβ analogues would disappear when tested in MsrA−/− neurons, i.e., Aβ42-Met(O) would show the same level of toxicity as the other two analogues. In contrast, if MsrB compensated for the absence of MsrA, we expected that each Aβ42 alloform would behave similarly, regardless of whether the neurons were WT or MsrA−/−.
In WT neurons, Aβ42-Met(O) was significantly less toxic than WT Aβ42 or Aβ42-Met(O2) in the MTT assay (Fig. 3A). In contrast, in neurons from MsrA−/− mice, Aβ42, Aβ42-Met(O), and Aβ42-Met(O2) caused a similar decrease in viability (Fig. 3A) and the differences among the three alloforms were insignificant. Thus, the absence of MsrA appeared to render the neurons more susceptible to the toxic effect of all three alloforms suggesting that MsrA was the predominant isozyme protecting the neurons from Aβ42-Met(O) toxicity. Similar results were obtained in the LDH assay. In neurons from WT mice, Aβ42-Met(O) caused significantly less cell death relative to WT Aβ42 or Aβ42-Met(O2). In contrast, in MsrA−/− neurons, Aβ42, Aβ42-Met(O), or Met(O2) induced similar levels of cell death, respectively and the differences were insignificant.
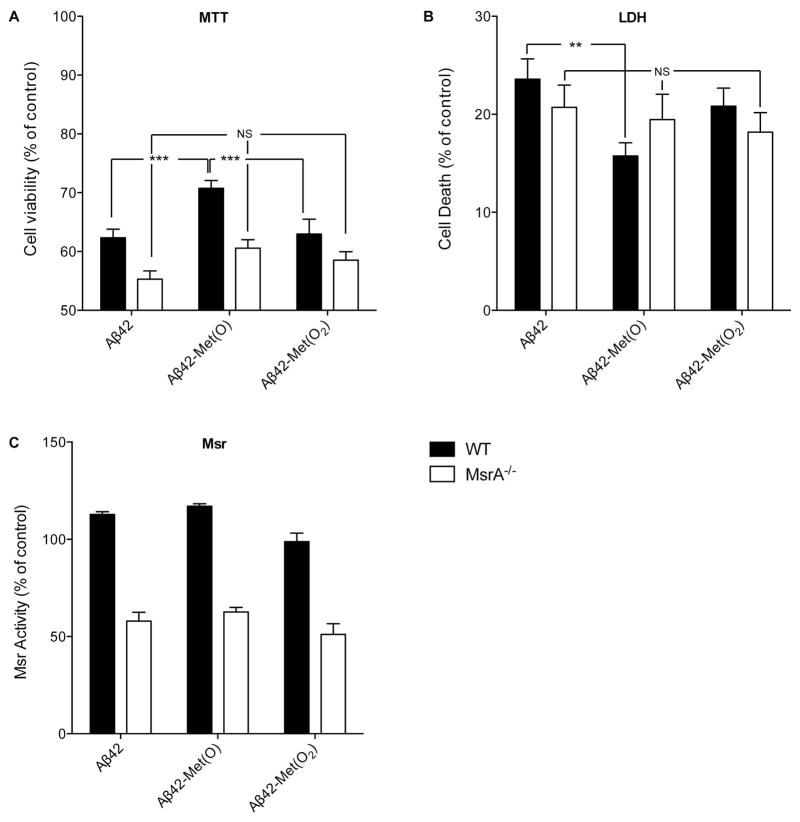
Figure 3. Response of MSRA−/− and WT mouse primary cortical neurons to native and oxidized Aβ42.
Primary MsrA−/− or WT mouse cortical neurons were treated with 10 μM of each Aβ analogues for 48h. A) Assessment of cell viability using the MTT assay. B) Assessment of cell death using the LDH assay. C) Measurement of specific Msr activity by HPLC using dabsyl-Met(O) as substrate. The results were normalized to untreated WT cells (150 pmol dabsyl-Met/min/mg protein defined as 100% specific Msr activity). The data are an average of 5–10 independent experiments. **p < 0.01, ***p < 0.001, NS – non-significant.
Measurement of Msr activity showed that the specific Msr activity in MsrA−/− neurons (80 pmol dabsyl-Met/min/mg protein) was approximately half that of WT neurons (150 pmol dabsyl-Met/min/mg protein), consistent with the absence of MsrA. The pattern of response of the MsrA−/− neurons to the three Aβ42 analogues was similar to that of WT neurons though the differences, which reflect MsrB only, did not reach statistical significance. These results suggest that in the absence of MsrA, neurons still sense the presence of the sulfoxide group in Aβ42-Met(O) and respond by elevation of MsrB activity, but this response provides little neuroprotection compared to WT neurons.
Induction of a protective Msr response by a Met(O) derivative in cell culture
Based on the findings described above, we asked next whether the Msr system could be induced to protect neurons against Aβ42 toxicity. Our first approach was an attempt to protect cultured cells by application of Ac-Met(O). Because of the exploratory nature of these experiments, we used here differentiated PC-12 cells rather than primary neurons.
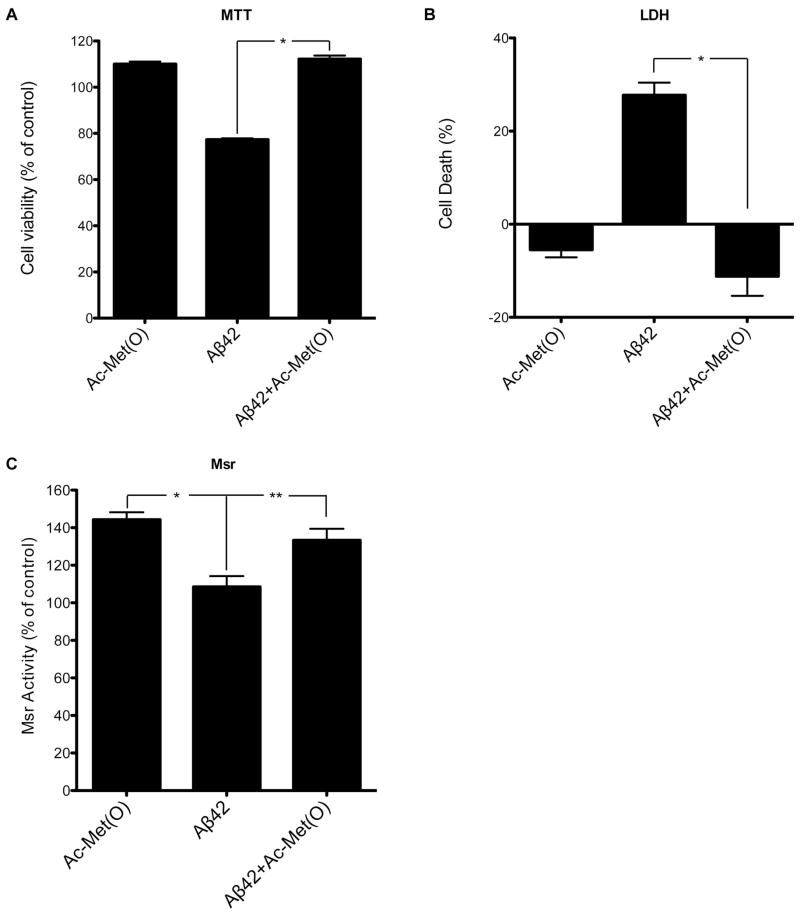
Evaluation of cell viability using the MTT (Fig. 4A) and LDH (Fig. 4B) assays showed that 1 mM Ac-Met(O) rescued Aβ42-induced toxicity to the levels of untreated cells. Lower concentrations of Ac-Met(O) showed partial rescue (data not shown). The rescue by 1 mM Ac-Met(O) correlated with a significant, 44±4% increase in Msr activity (Fig. 4C). The data suggest that induction of an Msr response by exposure of cells to Ac-Met(O) or derivatives thereof is a viable neuroprotective strategy against Aβ42-induced toxicity.
Figure 4. Ac-Met(O) increases Msr activity in differentiated PC-12 cells and protects the cells from Aβ42-induced toxicity.
Differentiated PC-12 cells were treated for 24 h with 1 mM Ac-Met(O), 10 μM Aβ42, or 10 μM Aβ42 + 1 mM Ac-Met(O). A) Assessment of cell viability using the MTT assay. B) Assessment of cell death using the LDH assay. C) Measurement of specific Msr activity by HPLC. The results were normalized to untreated cells (200 pmol dabsyl-Met/min/mg protein defined as 100% specific Msr activity). The data are an average of 10 independent experiments. *p < 0.05, **p < 0.01.
Induction of a protective Msr response by immunization with a Met(O)-rich antigen in vivo
To test whether induction of the Msr system could be beneficial in vivo, we immunized 2×Tg mice bearing FAD-linked mutant app and psen1 genes (45) with a Met(O)-rich antigen, which recently has been used to create a unique anti-Met(O) antibody (46). This antigen is an oxidized form of recombinant zea mays methionine-rich protein (DZS18). The anti-Met(O) antibody was developed to recognize Met(O) in any protein. It was shown to detect increased Met(O) levels in plasma from aged WT mice or MsrA−/− mice compared with young WT mice, from patients with AD compared with healthy age-matched individuals (46), and in symptomatic and pre-symptomatic persons carrying familial AD-linked mutations in app or psen1 compared to non-carriers from the same kindreds (47).
Here, we used oxidized DZS18 to immunize 2×Tg AD mice. These mice produce high levels of Aβ, particularly Aβ42, in their brain and display Aβ deposition in amyloid plaques as early as 4 months of age with progression up to 12 months of age (48). We hypothesized that immunization with the Met(O)-rich antigen initially would induce higher Msr activity, but over time would produce an immune response that might lead to decreased levels of Met(O) in proteins and subsequent decrease in Msr activity. Because a large portion of Aβ Met35 in amyloid plaques is oxidized to sulfoxide (15), we hypothesized also that immunization with oxidized DZS18 might help clear the amyloid burden in the brains of the mice.
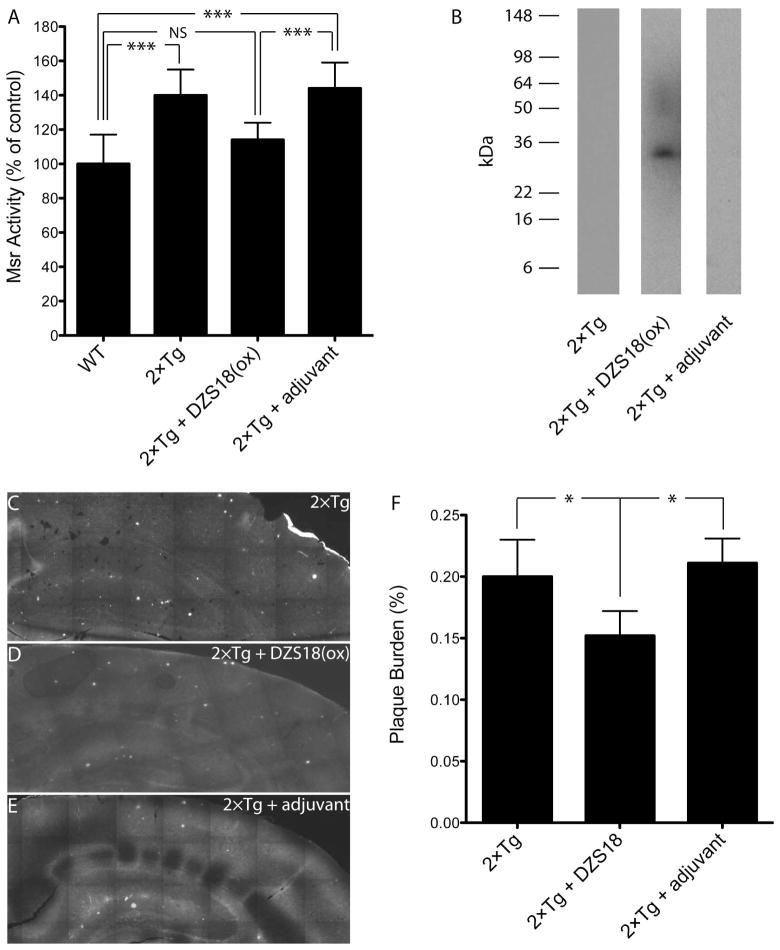
Mice were immunized every 2 weeks for 6 months beginning at 3 months of age. At the end of the immunization period, their serum was analyzed for Msr activity and production of anti-Met(O) antibodies, and brain sections were stained with ThS for visualization of amyloid plaques. As shown in Fig. 5, Msr activity in the brain of unimmunized 2×Tg mice was 40±15% higher than in WT mice. Immunization of the 2×Tg mice with oxidized DZS18, but not with adjuvant alone, caused a significant decrease in brain Msr activity by 26±10% (Fig. 5A), consistent with our prediction. Analysis of mouse serum showed immunoglobulins reactive towards oxidized DZS18 in immunized mice, but not in mice receiving adjuvant alone or unimmunized mice (Fig. 5B). ThS staining showed abundant plaques in unimmunized mice (Fig. 5C). Immunization with oxidized DZS18 caused a significant, 28±8% reduction in plaque burden in the hippocampus of the immunized mice relative to the control groups (Fig. 5D–F). The data suggest that immunization with a Met(O)-rich, non-Aβ antigen can produce amyloid plaque clearance in the brain of 2×Tg AD mice, presumably by removing the oxidized form of Aβ or other plaque-associated proteins, and that production of an immune response against Met(O) may alleviate in part the oxidative stress that causes increased Msr activity in these mice.
Figure 5. Immunization of 2×Tg mice with oxidized Met-rich protein reduces Msr activity and brain Aβ burden.
Mice were immunized for 6-months with oxidized Met-rich protein (DSZ18(ox)) or adjuvant alone. Non-Tg mice served as a negative control and unimmunized mice as a positive control. N = 5 mice per group. A) Measurement of specific Msr activity in mouse brain. ***p < 0.001. NS – non-significant. B) Westren blot analysis for detection of anti-Met(O) antibodies in sera of 2×Tg mice immunized with DZS18(ox), mice immunized with adjuvant alone, or unimmunized mice. The smear between 45–60 kDa in the 2×Tg + DZS18(ox) lane is an artifact that was not observed in other blots. C–E) Fluorescence microscopy images of hippocampal brain slices stained with Thioflavin S: C) Unimmunized mice. D) Mice immunized with DZS18(ox). E) Mice immunized with adjuvant alone. F) % Plaque burden was quantified by calculating the total ThS-stained area divided by the total hippocampal area measured and normalized to unimmunized mice using ImageJ (n = 5 mice per condition, *p < 0.05).
Discussion
Met35 is the primary target site for oxidants in Aβ (33). Formation of methionyl radicals and participation of Met in Fenton chemistry in the presence of transition metal ions leading to production of ROS have been hypothesized to play an important role in Aβ-induced toxicity. Once Met gets oxidized to sulfoxide or sulfone, its tendency to participate in further oxidation reactions or form radicals decreases substantially. Thus, if participation of Met35 in these reactions were important for Aβ-induced toxicity, Aβ would be predicted to become less toxic upon oxidation. This hypothesis has been supported by a number of studies comparing the toxicity of WT Aβ42 to Aβ42-Met(O) (33). Surprisingly, however, even though Aβ-Met(O2) is less likely than Aβ-Met(O) to form radicals or participate in Fenton chemistry, it induces the same levels of neurotoxicity and synaptotoxicity as WT Aβ (Fig. 1, (37, 49)). These results have led us to hypothesize that factors other than the effect of oxidation on Aβ conformation and assembly, for example, activation of the Msr system, contributed to the observed differences between the toxicity levels of WT Aβ and Aβ-Met(O).
The observation of a significant increase in Msr activity in response to Aβ-Met(O), but not WT Aβ or Aβ-Met(O2), in WT rat (Fig. 2) and mouse (Fig. 3) primary neurons suggests that Msr protects neurons from Aβ-Met(O) toxicity. Supporting our findings, similar observations have been made recently by Misiti et al. in IMR-32 cells, who reported also an increase in MsrA transcription upon treatment with Aβ-Met(O) (39). Interestingly, although Msr reduces the less toxic Aβ-Met(O) to the more toxic WT Aβ, the overall result is significantly lower toxicity (Fig. 1). This suggests that Msr-mediated reduction of Met(O) to Met in cellular proteins other than Aβ overrides the direct toxicity caused by Aβ itself through other mechanisms, emphasizing the role of oxidative damage in the array of toxic mechanisms induced by Aβ.
In both the MTT and LDH assays (Fig. 3A,B) the absence of MsrA resulted in elimination of the significant decrease in toxicity induced by Aβ42-Met(O) relative to WT Aβ42 or Aβ42-Met(O2). These results suggested that the main neuroprotective activity was provided by MsrA rather than MsrB. Indeed, the overall specific Msr activity found in MsrA−/− cells, which must be provided by MsrB, was about half that in WT neurons. Previously, ablation of the mouse MsrA gene was shown to lower the expression level of MsrB1 (50). Our data suggest that in response to treatment with Aβ42-Met(O), the neurons still upregulate MsrB (Fig. 3C), but this provides only partial protection and the observed toxicity is higher in the MsrA−/− mice than in WT mice. The putative partial protection provided by MsrB in MsrA−/− cells appeared to be higher in the LDH assay (Fig. 3B) than in the MTT assay (Fig. 3A). This observation suggests that in mitochondria, where MsrA is the main isoform (51), the capability of MsrB to mitigate oxidative stress is lower than in the cytosol. The mitochondrial MsrB isoforms, MsrB2 and MsrB3B are minor isoforms (52) and presumably have a limited capability to compensate for the absence of MsrA, whereas the cytosolic MsrB1 may offers somewhat higher levels of compensation as reflected in the LDH assay.
Overexpression of MsrA has been shown to be protective against oxidative stress in multiple systems (22), whereas MsrA ablation enhanced oxidative posttranslational modifications and resulted in accumulation of damaged proteins, similar to findings in neurodegenerative diseases (53). These studies and the data presented here suggest that activating the Msr system using sulfoxide-containing compounds may serve as a novel route for development of therapeutic agents against AD and other neurodegenerative diseases, and for general reduction of aging-related oxidative stress. Here, we used Ac-Met(O) to test this hypothesis and found that this simple amino-acid derivative induced elevated Msr activity and protected differentiated PC-12 cells against Aβ42-induced toxicity (Fig. 4). These results provide proof of principle for activation of Msr using non-toxic Met(O) derivatives and suggest that exploration of derivatives with higher activity and desirable pharmacokinetic characteristics may yield novel drug candidates for conditions in which oxidative stress is a major deleterious mechanism.
The experiments using Ac-Met(O) did not distinguish between MsrA and MsrB because the compound we used comprised all four diastereomers. MTT experiments using Ac-L-Met(O) or Ac-D-Met(O) showed that both isomers significantly protected differentiated PC-12 cells against Aβ-induced toxicity to a similar extent (data not shown). Experiments using the R- or S- enantiomers of the sulfoxide group will be pursued in the future.
Immunization has been explored widely as a therapeutic approach for AD, with mixed results (54). Immunization with Aβ-derived antigens or passive immunization with anti-Aβ antibodies has been shown to reduce Aβ burden in patients with AD and in animal models (55). However, neuroinflammation, induction of vasogenic edema and/or microhemorrhages, and other adverse effects have raised concerns regarding the safety of this approach and multiple attempts have been made to develop safer immunization strategies (56). One such strategy is to use surrogate antigens based on Aβ-unrelated protein sequences that may promote Aβ clearance without causing the problems mentioned above (57). Here we used a similar approach with a unique antigen – oxidized maize Met-rich protein, which has no sequence similarity with Aβ, and observed a significant reduction of plaque burden in the 2×Tg mice (Fig. 5C–F). These data suggest that anti-Met(O) antibodies, similar to the one reported previously (58) were produced in the mice (Fig. 5B) and contributed to the observed clearance of deposited Aβ via binding to Aβ-Met(O). This offers an advantage relative to antibodies that recognize Aβ itself because only an aberrant form of Aβ, the one containing Met(O), is targeted.
The immunization likely reduced the overall brain oxidative stress using several mechanisms: First, by reducing the number of amyloid plaques, the inflammatory processes surrounding the plaques were relieved. A cellular immune response (e.g., activation of microglia) also might have participated in plaque clearance, though exploration this aspect of the immune response was beyond the scope of the current work. Second, the antibodies might have promoted clearance of other oxidized (Met(O)-containing) proteins resulting in lower overall cellular oxidative damage. Third, because oxidative damage is known to upregulate Aβ production (59), the decrease in oxidative stress signals might have lowered Aβ production and further facilitated reduction in Aβ burden.
Msr activity has been shown to decline in post mortem AD brain (26), yet to increase in cell culture in response to Aβ. Our study presents for the first time evidence showing that Msr levels are elevated in a mouse model of AD at an age in which abundant plaque deposition is observed (Fig. 5). These findings could reflect simply a difference between mice and humans. However, if mouse models of AD represent relatively early stages of the disease, our findings suggest that in early AD, Msr activation is one way by which the brain attempts to mitigate oxidative stress, yet this attempt fails in late stages. Testing this hypothesis in human studies will validate the Msr system as a new therapeutic target and may lead to development of novel treatments for AD that would utilize natural brain defense mechanisms. Such treatments may have a broad impact because oxidative stress is a common deleterious mechanism in many degenerative diseases and in normal aging.
Acknowledgments
Funding Statement
The work was supported by NIH/NIA grants AG027818 to GB and AG027363 to JM and by Alzheimer’s Association Grant IIRG-07-5833 to GB.
We thank Dr. David Teplow for the use of his microplate reader.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid β-protein
- APP
amyloid β-protein precursor
- ANOVA
analysis of variance
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DTT
dithiothreitol
- FAD
familial AD
- HFIP
1,1,1,3,3,3-hexafluoroisopropanol
- LDH
lactate dehydrogenase
- Msr
methionine-sulfoxide reductase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ThS
thioflavin S
References
- 1.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 2.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Role of free radicals in the neurodegenerative diseases - Therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 4.Terman A, Brunk UT. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 5.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 7.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 8.Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. Amyloid β-protein assembly and Alzheimer disease. J Biol Chem. 2009;284:4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 10.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PLR, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratico D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 12.Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer’s disease. J Alzheimer Dis. 2010;20(Suppl 2):S633–643. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younkin SG. Evidence that Aβ42 is the real culprit in Alzheimer’s disease. Ann Neurol. 1995;37:287–288. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- 14.Bitan G, Tarus B, Vollers SS, Lashuel HA, Condron MM, Straub JE, Teplow DB. A molecular switch in amyloid assembly: Met35 and amyloid β-protein oligomerization. J Am Chem Soc. 2003;125:15359–15365. doi: 10.1021/ja0349296. [DOI] [PubMed] [Google Scholar]
- 15.Näslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P, et al. Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci USA. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo YM, Kokjohn TA, Beach TG, Sue LI, Brune D, Lopez JC, Kalback WM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Roher AE. Comparative analysis of amyloid-β chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J Biol Chem. 2001;276:12991–12998. doi: 10.1074/jbc.M007859200. [DOI] [PubMed] [Google Scholar]
- 17.Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal binding and oxidation of amyloid-β within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 18.Boutte AM, Woltjer RL, Zimmerman LJ, Stamer SL, Montine KS, Manno MV, Cimino PJ, Liebler DC, Montine TJ. Selectively increased oxidative modifications mapped to detergent-insoluble forms of Aβ and β-III tubulin in Alzheimer’s disease. FASEB J. 2006;20:1473–1483. doi: 10.1096/fj.06-5920com. [DOI] [PubMed] [Google Scholar]
- 19.Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali FE, Separovic F, Barrow CJ, Cherny RA, Fraser F, Bush AI, Masters CL, Barnham KJ. Methionine regulates copper/hydrogen peroxide oxidation products of Aβ. J Pept Sci. 2005;11:353–360. doi: 10.1002/psc.626. [DOI] [PubMed] [Google Scholar]
- 21.Ejiri SI, Weissbach H, Brot N. Reduction of methionine sulfoxide to methionine by Escherichia coli. J Bacteriol. 1979;139:161–164. doi: 10.1128/jb.139.1.161-164.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oien DB, Moskovitz J. Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr Top Dev Biol. 2008;80:93–133. doi: 10.1016/S0070-2153(07)80003-2. [DOI] [PubMed] [Google Scholar]
- 23.Moskovitz J, Weissbach H, Brot N. Cloning the expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proc Natl Acad Sci USA. 1996;93:2095–2099. doi: 10.1073/pnas.93.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim G, Cole NB, Lim JC, Zhao H, Levine RL. Dual sites of protein initiation control the localization and myristoylation of methionine sulfoxide reductase A. J Biol Chem. 2010;285:18085–18094. doi: 10.1074/jbc.M110.119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadtman ER, Moskovitz J, Berlett BS, Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem. 2002;234:3–9. [PubMed] [Google Scholar]
- 26.Gabbita SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer’s disease brain. J Neurochem. 1999;73:1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 27.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal R, Oien DB, Ersen FY, Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res. 2007;180:765–774. doi: 10.1007/s00221-007-0903-6. [DOI] [PubMed] [Google Scholar]
- 29.Oien DB, Osterhaus GL, Latif SA, Pinkston JW, Fulks J, Johnson M, Fowler SC, Moskovitz J. MsrA knockout mouse exhibits abnormal behavior and brain dopamine levels. Free Radic Biol Med. 2008;45:193–200. doi: 10.1016/j.freeradbiomed.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, Brot N, Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol. 1995;177:502–507. doi: 10.1128/jb.177.3.502-507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung H, Kim AK, Jung SA, Kim SW, Yu K, Lee JH. The Drosophila homolog of methionine sulfoxide reductase A extends lifespan and increases nuclear localization of FOXO. FEBS Lett. 2010;584:3609–3614. doi: 10.1016/j.febslet.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci USA. 1998;95:14071–14075. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butterfield DA, Boyd-Kimball D. The critical role of methionine 35 in Alzheimer’s amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta. 2005;1703:149–156. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Hou L, Kang I, Marchant RE, Zagorski MG. Methionine 35 oxidation reduces fibril assembly of the amyloid β-(1–42) peptide of Alzheimer’s disease. J Biol Chem. 2002;277:40173–40176. doi: 10.1074/jbc.C200338200. [DOI] [PubMed] [Google Scholar]
- 35.Riek R, Guntert P, Döbeli H, Wipf B, Wüthrich K. NMR studies in aqueous solution fail to identify significant conformational differences between the monomeric forms of two Alzheimer peptides with widely different plaque-competence, Aβ(1–40)ox and Aβ(1–42)ox. Eur J Biochem. 2001;268:5930–5936. doi: 10.1046/j.0014-2956.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- 36.Yan Y, McCallum SA, Wang C. M35 oxidation induces Aβ40-like structural and dynamical changes in Aβ42. J Am Chem Soc. 2008;130:5394–5395. doi: 10.1021/ja711189c. [DOI] [PubMed] [Google Scholar]
- 37.Maiti P, Piacentini R, Ripoli C, Grassi C, Bitan G. Surprising toxicity and assembly behavior of amyloid β-protein oxidized to sulfone. Biochem J. 2010;443:323–332. doi: 10.1042/BJ20101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triguero L, Singh R, Prabhakar R. Comparative molecular dynamics studies of wild-type and oxidized forms of full-length Alzheimer amyloid β-peptides Aβ(1–40) and Aβ(1–42) J Phys Chem B. 2008;112:7123–7131. doi: 10.1021/jp801168v. [DOI] [PubMed] [Google Scholar]
- 39.Misiti F, Clementi ME, Giardina B. Oxidation of methionine 35 reduces toxicity of the amyloid β-peptide(1–42) in neuroblastoma cells (IMR-32) via enzyme methionine sulfoxide reductase A expression and function. Neurochem Int. 2010;56:597–602. doi: 10.1016/j.neuint.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Rahimi F, Maiti P, Bitan G. Photo-induced cross-linking of unmodified proteins (PICUP) applied to amyloidogenic peptides. J Vis Exp. 2009 doi: 10.3791/1071. http://www.jove.com/index/details.stp?id=1071. [DOI] [PMC free article] [PubMed]
- 41.Fradinger EA, Monien BH, Urbanc B, Lomakin A, Tan M, Li H, Spring SM, Condron MM, Cruz L, Xie CW, Benedek GB, Bitan G. C-terminal peptides coassemble into Aβ42 oligomers and protect neurons against Aβ42-induced neurotoxicity. Proc Natl Acad Sci USA. 2008;105:14175–14180. doi: 10.1073/pnas.0807163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiti P, Lomakin A, Benedek GB, Bitan G. Despite its role in assembly, methionine 35 is not necessary for amyloid β-protein toxicity. J Neurochem. 2010;113:1252–1262. doi: 10.1111/j.1471-4159.2010.06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jankowsky JL, Slunt HH, Gonzales V, Jenkins NA, Copeland NG, Borchelt DR. APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol Aging. 2004;25:885–892. doi: 10.1016/j.neurobiolaging.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Andersson M, Ostman A, Westermark B, Heldin CH. Characterization of the retention motif in the C-terminal part of the long splice form of platelet-derived growth factor A-chain. J Biol Chem. 1994;269:926–930. [PubMed] [Google Scholar]
- 45.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 46.Andersson A, Bohman S, Borg LA, Paulsson JF, Schultz SW, Westermark GT, Westermark P. Amyloid deposition in transplanted human pancreatic islets: a conceivable cause of their long-term failure. Exp Diabetes Res. 2008;2008:562985. doi: 10.1155/2008/562985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ringman JM, Fithian AT, Gylys K, Cummings JL, Coppola G, Elashoff D, Pratico D, Moskovitz J, Bitan G. Plasma methionine sulfoxide in persons with familial Alzheimer’s disease mutations. 2011 doi: 10.1159/000338546. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Ripoli C, Piacentini R, Riccardi E, Leone L, Bitan G, Grassi C. Synaptotoxic effects of different amyloid β-protein analogues in rodent hippocampal neurons and brain slices. 2011 doi: 10.1016/j.neurobiolaging.2012.06.027. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 50.Moskovitz J, Stadtman ER. Selenium-deficient diet enhances protein oxidation and affects methionine sulfoxide reductase (MsrB) protein level in certain mouse tissues. Proc Natl Acad Sci USA. 2003;100:7486–7490. doi: 10.1073/pnas.1332607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansel A, Kuschel L, Hehl S, Lemke C, Agricola HJ, Hoshi T, Heinemann SH. Mitochondrial targeting of the human peptide methionine sulfoxide reductase (MSRA), an enzyme involved in the repair of oxidized proteins. FASEB J. 2002;16:911–913. doi: 10.1096/fj.01-0737fje. [DOI] [PubMed] [Google Scholar]
- 52.Marchetti MA, Pizarro GO, Sagher D, Deamicis C, Brot N, Hejtmancik JF, Weissbach H, Kantorow M. Methionine sulfoxide reductases B1, B2, and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Invest Ophthalmol Vis Sci. 2005;46:2107–2112. doi: 10.1167/iovs.05-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oien DB, Shinogle HE, Moore DS, Moskovitz J. Clearance and phosphorylation of α-synuclein are inhibited in methionine sulfoxide reductase a null yeast cells. J Mol Neurosci. 2009;39:323–332. doi: 10.1007/s12031-009-9274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schenk DB, Seubert P, Grundman M, Black R. Aβ immunotherapy: Lessons learned for potential treatment of Alzheimer’s disease. Neurodegener Dis. 2005;2:255–260. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- 55.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 56.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 57.Goni F, Prelli F, Ji Y, Scholtzova H, Yang J, Sun Y, Liang FX, Kascsak R, Mehta P, Wisniewski T. Immunomodulation targeting abnormal protein conformation reduces pathology in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e13391. doi: 10.1371/journal.pone.0013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oien DB, Canello T, Gabizon R, Gasset M, Lundquist BL, Burns JM, Moskovitz J. Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys. 2009;485:35–40. doi: 10.1016/j.abb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bayer TA, Schafer S, Breyhan H, Wirths O, Treiber C, Multhaup G. A vicious circle: role of oxidative stress, intraneuronal Aβ and Cu in Alzheimer’s disease. Clin Neuropathol. 2006;25:163–171. [PubMed] [Google Scholar]
- 60.Moskovitz J, Berlett BS, Poston JM, Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci USA. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]