Abstract
In the efforts aimed at improving the quality of in-vitro-matured human oocytes, the dynamic balance and roles of pro-/antioxidants merit further consideration. In-vitro maturation (IVM) is emerging as a popular technology at the forefront of fertility treatment and preservation. However, standard in-vitro culture conditions exert oxidative stress or an imbalance between oxidants and antioxidants. Reactive oxygen species (ROS) are oxygen-derived molecules formed as intermediary products of cellular metabolism. By acting as powerful oxidants, ROS can oxidatively modify any molecule, resulting in structural and functional alterations. ROS are neutralized by an elaborate defence system consisting of enzymatic and non-enzymatic antioxidants. This review captures the inherent and external factors that may modulate the oxidative stress status of oocytes. It discusses the suspected impacts of oxidative stress on the gamut of events associated with IVM, including prematuration arrest, meiotic progression, chromosomal segregation, cytoskeletal architecture and gene expression. In-vivo and in-vitro strategies that may overcome the potential influences of oxidative stress on oocyte IVM are presented. Future studies profiling the oxidative stress status of the oocyte may permit not only the formulation of a superior IVM medium that maintains an adequate pro-/antioxidant balance, but also the identification of predictors of oocyte quality.
Keywords: antioxidants, in-vitro maturation, oocyte, oxidative stress, reactive oxygen species
Introduction
Fertility preservation and infertility treatment have emerged at the forefront of reproductive health, thus introducing the concept of maturing immature oocytes in vitro that otherwise would have been lost in the physiological process (Chian et al., 2004a; Rao and Tan, 2005; Dal Canto et al., 2006; Jurema and Nogueira, 2006). Indeed, not all follicles respond to the effects of gonadotrophin stimulation with many oocytes never maturing and thus remaining in an immature state prior to their eventual demise. The retrieval of immature oocytes, together with their subsequent culture and maturation in vitro, not only eliminates the complications of stimulated cycles but also provides a source of invaluable and scarce oocytes. The in-vitro maturation (IVM) of oocytes is relevant to the fields of both infertility treatment and basic research. Multiple investigators have tapped the various aspects of the IVM process to determine the sequence of changes that take place in the oocyte as well as the factors that influence them. Over the years, the majority of research findings conclude that successful IVM necessitates not only nuclear but also cytoplasmic maturation (Fulka et al., 1998; Smith, 2001; Ali et al., 2006). In vivo, oocyte maturation is a complex process that is influenced by the interplay of regulatory factors (among which are gonadotrophins and a growing list of secreted molecules), interactions between the oocyte and cumulus cells, and the biochemical state of the oocyte (Canipari, 2000; Eppig, 2001; Tanghe et al., 2002; Combelles et al., 2004; Gilchrist et al., 2004). Much success has been achieved in terms of successful nuclear maturation in vitro but the cytoplasmic maturation lags behind, thereby reflecting an asynchrony between the two processes (Smith, 2001; Trounson et al., 2001; Ali et al., 2006). In an effort to simulate the in-vivo conditions that prevail in the pre-ovulatory antral follicle, a plethora of human studies have tested different formulations of culture media; to date, varied (and overall disappointing) results have been obtained in terms of human oocyte maturation, fertilization and pregnancy outcomes post IVM (Smitz et al., 2001; Chian et al., 2004b; Mikkelsen, 2005).
Inspite of the advantages of no ovarian hyperstimulation syndrome, relative ease of treatment and low cost, various challenges hinder the selection of oocyte IVM as an established mode of assisted reproduction. It is evident that the quality of in-vitro-matured oocytes is suboptimal since embryos derived from them display increased developmental abnormalities (Nogueira et al., 2000; Schramm et al., 2003). One aspect, among a multitude of others, that may be responsible for altered cell functions ex vivo is the generation of oxidative stress (OS) within the cell culture medium. OS arises whenever the balance between pro- and antioxidants is disturbed. As demonstrated for spermatozoa and preimplantation embryos, the increased OS that is generated in the in-vitro media affects the outcomes of assisted reproduction treatments in various ways (Guerin et al., 2001; Agarwal et al., 2006a). The generation of pro-oxidants (such as reactive oxygen species) is an invariable phenomenon in external culture, and it is possible that OS also influences oocyte development in vitro. If so, it may be envisaged that the oxidative insult exerted on developing oocytes could contribute to the low outcomes of human IVM. Prior research has explored methods to prevent any potential cell damage due to OS, but a final consensus for supplementing media with antioxidants in order to enhance oocyte quality is yet to be reached. There is an immediate need to understand the gamut of OS-related factors that may influence oocyte development.
With respect to potential reasons for the continued poor outcomes of IVM, this review highlights the suspected impact of OS on the oocyte maturation process in vitro. More precisely, literature is presented on investigated relationships between oocyte quality and various markers of OS including antioxidant protection. The suggested functions of a pro- and antioxidant balance during IVM is also reviewed, and major inconsistencies and gaps in the knowledge will be presented, together highlighting the need for further studies into not only the roles but also the regulation of OS during IVM. With an increasing body of evidence on all factors likely to influence oocyte maturation, it may then be possible to develop culture systems optimal for IVM. The focus of this review will be placed predominantly on in-vitro studies, and in-vivo findings will be presented whenever directly pertinent to current understanding and optimization of oocyte IVM. Given the limited number of studies and/or conclusive findings in oocytes, this study will, at times, present any relevant information from the oocyte’s next of kin, that is the spermatozoon or preimplantation embryo. Invaluable insights may then be gained and promising avenues of research opened onto the potential importance of OS during IVM.
Rationale for IVM as a next popular procedure for assisted reproduction
IVM constitutes the in-vitro advancement of an oocyte from the diplotene stage of prophase I (germinal vesicle, or GV) to metaphase II (MII), along with cytoplasmic maturation that encompasses a broad set of still ill-defined cellular events, all of which are essential for the fertilization and early development of the embryo (Smitz et al., 2001; Trounson et al., 2001; Ali et al., 2006). Arising from a growing pool of antral follicles, a number of oocytes are typically retrieved and available for IVM treatment. In the ovary, many follicles become recruited during the development of any given cohort, but, after the normal selection of the dominant follicle, the rest of the growing follicles undergo atresia and become lost permanently unless retrieved for IVM (Carrell et al., 2003). IVM has many benefits over conventional assisted reproduction techniques. While some ovarian priming is currently employed during IVM cycles, it is very minimal (Jurema and Nogueira, 2006). Thus, in comparison to conventional IVF for which ovarian stimulation is a routine protocol with a concerning risk for ovarian hyperstimulation syndrome (Aboulghar and Mansour, 2003; Fauser and Devroey, 2003), negative side effects are inherently reduced during IVM. Furthermore, IVM patients benefit in terms of finance, time and energy, all of which are significant issues faced by routine IVF programmes (Chian et al., 2004a; Papanikolaou et al., 2005; Jurema and Nogueira, 2006). As IVM of immature oocytes represents a safer, cheaper and comparatively simpler procedure than conventional techniques, it promises to be one of the most practiced techniques in the future if successful.
Despite its significant advantages, IVM remains an experimental procedure. The main challenge resides in the relatively low reproductive outcomes associated with IVM (Jurema and Nogueira, 2006). Recent reports document improved fertility outcomes after IVM, with live birth rates of 30% in donor oocyte programmes and a clinical pregnancy rate of 21.9% in patients with polycystic ovary syndrome (PCOS) for whom IVM proves to be an important treatment strategy (Cha et al., 2005; Holzer et al., 2007). A few groups have begun offering IVM as a treatment option to a highly selected group of patients; nonetheless, IVM is far from representing a widely available and well-accepted technology. Technically, hurdles still complicate the routine retrieval of immature oocytes although recent advances have significantly optimized its timing and protocols (Mikkelsen, 2005; Papanikolaou et al., 2005; Jurema and Nogueira, 2006). Biologically, IVM is limited by key deficiencies at the level of the oocyte and its adequate development in vitro. On one hand, oocytes vary significantly in their quality or competence to support development to term, and on the other hand, there is insufficient laboratory ability to foster such competency. Both of these phenomena highlight the current and dire need to improve understanding of oocyte development. Then and only then will techniques be developed to select, isolate, handle and support the development of immature oocytes in vitro. A growing number of reports, largely in animal models, point towards biological differences between oocytes matured in vitro and in vivo, whether at the level of gene expression patterns, spindle architecture or metabolism (Knijn et al., 2002; Lonergan et al., 2003a; Sanfins et al., 2003, 2004; Sutton et al., 2003; Zheng et al., 2005; Li et al., 2006). Multiple factors or deficiencies likely contribute to the overall poor quality of in-vitro-matured oocytes and, as presented in this review, one additional culprit may be oxidative stress. Overall, IVM is a rather understudied area of basic research and protocol development, particularly when compared with the remarkable strides made in the realms of IVF and in-vitro embryo culture. Clearly the time has come to devote more attention to IVM together with the identification of potential cellular targets for use in ongoing optimization efforts. The remainder of this article will highlight the currently emerging body of knowledge on OS during oocyte maturation, an understanding that, with further investigations, promises to contribute to the coming of IVM as a next popular procedure in assisted reproduction techniques.
Oxidative stress: in general terms and in the in-vivo maturing milieu of the oocyte
A reactive oxygen species (ROS) is an entity that is generated during various cellular metabolic reactions as well as indirectly from the cell’s surroundings. It comprises mainly superoxide anions, hydrogen peroxide, and hydroxyl radicals (Agarwal et al., 2005). A superoxide anion is formed when molecular oxygen acquires an additional electron. Hydrogen peroxide arises in various metabolic reactions catalysed by oxidases or superoxide dismutase (from superoxide), and it also further generates hydroperoxyl and hydroxyl radicals. Free radicals and peroxides are highly reactive and they may cause widespread injury to biomolecules (Yagi, 1993). Conversely, ROS can be beneficial to somatic cell functions (cell proliferation, differentiation, for example), and the redox state of cells regulates signalling pathways (Kamata and Hirata, 1999; Droge, 2002; Poli et al., 2004).
Under optimal conditions, the balance between ROS and antioxidants is maintained. However, increased generation of ROS overwhelms the antioxidant capacity of the system thus leading to OS. There is an exhaustive set of co-operating antioxidants (both enzymatic and non-enzymatic) that modulate the concentrations of pro-oxidants present in the cell (Brigelius-Flohe, 1999; Chelikani et al., 2004; Johnson and Guilivi, 2005; Luberda, 2005). Beyond superoxide dismutase (SOD), which catalyses the dismutation of superoxide into hydrogen peroxide and oxygen (Johnson and Guilivi, 2005), is a next line of defence that further neutralizes hydrogen peroxide into harmless components. Catalase (CAT) and glutathione peroxidase (GPx) are two central enzymes capable of converting hydrogen peroxide into water and oxygen (Brigelius-Flohe, 1999; Chelikani et al., 2004). Also extensively used as a biomarker of OS is the ratio between the oxidized form and reduced form (GSH) of glutathione. GSH functions in many cellular pathways including protection against oxidative damage (Luberda, 2005). It is not the intent of this review to introduce all other examples of known antioxidants; instead, the ones for which data exist with respect to IVM will be discussed below and in the context of their analyses.
Evidently, much attention has been placed on OS during embryo culture (Guerin et al., 2001) and, while much can be learnt from such knowledge, it is essential that oxidative damage be evaluated specifically in the human oocyte. The microenvironment within which the oocyte develops, that is the antral follicle, also warrants consideration. Notably, several studies have focused on human follicular fluid, a sample that is rather readily available and that represents the liquid milieu bathing and influencing the developing oocytes.
Some experimental evidence supports a link between pro-oxidants in the follicular fluid and predictors of oocyte quality. Indeed, one study showed a positive correlation between elevated ROS concentrations and pregnancy rates (Attaran et al., 2000). Similarly, another marker of OS, lipid peroxidation, was correlated with pregnancy rates, while no significant relationships were detected between lipid peroxidation and oocyte maturity, fertilization and embryo quality (Pasqualotto et al., 2004). Based on the oxidation properties of follicular fluid, a threshold of OS may be related to the number of mature oocytes retrieved and subsequent cleavage rates (Wiener-Megnazi et al., 2004). In a recent study, Das et al. (2006) reported high ROS follicular fluid concentrations that were correlated with poor oocyte quality and subsequent diminished embryo formation. Concentrations of three OS biomarkers (conjugated dienes, lipid hydroperoxide and thiobarbituric acid) were also determined in follicular fluid from human pre-ovulatory follicles (Jozwik et al., 1999). There was no evident relationship between pro-oxidant concentrations in follicular fluid and pregnancy rates (Jozwik et al., 1999). Clinically, 8-hydroxy-2-deoxyguanosine, an important OS marker in granulosa cells, displayed a negative correlation with embryo quality following IVF (Seino et al., 2002). Concentrations of 8-hydroxy-2-deoxyguanosine in individual follicular fluid samples were significantly elevated when an increased proportion of oocytes exhibited aberrant morphological features (Tamura et al., 2008). Thus, much uncertainty resides as to the exact predictive value of pro-oxidants (either in follicular fluid or granulosa cells) for pregnancy outcome, and even less is settled with respect to oocyte quality. Interestingly, several studies propose that the concentrations of ROS in follicular fluid may represent natural ranges of pro-oxidants that would be necessary for the normal development of the oocyte. A physiological amount of ROS may be indicative of healthy developing oocytes, whereas excessively high concentrations may denote a state of OS and compromized IVF outcomes (Attaran et al., 2000; Agarwal et al., 2003; Pasqualotto et al., 2004; Wiener-Megnazi et al., 2004). However, future work is necessary in order to support such a proposal.
Follicular fluid contains not only ROS but also antioxidants, with the total antioxidant capacity (TAC) itself having been investigated as a predictive marker of successful IVF (Pasqualotto et al., 2004). The beneficial effect of follicular fluid against oxidative damage may be in part due to an elevated activity of SOD isoenzymes, which act as important scavengers of free radicals; notably, a study in pig proposed a relationship between increased SOD activity in follicular fluid and an enhanced cytoplasmic maturation of the oocyte (Tatemoto et al., 2004). In contradiction, Sabatini et al. (1999) documented that significantly higher SOD activities were found in human follicular fluid samples associated with oocytes that failed to become fertilized. However, also in human but for a different antioxidant enzyme, certain physiological concentrations of selenium-dependent glutathione peroxidase activity in follicular fluid were positively correlated with fertilization rates (Paszkowski et al., 1995). The non-enzymatic antioxidant L-ascorbate was measured in human follicular fluid, but without any association with oocyte maturity as assessed solely by the presence or absence of a polar body (Paszkowski and Clarke, 1999). There was also no relationship between fertilization rates and follicular-fluid concentrations of the antioxidants carotenoids and α-tocopherol (Schweigert et al., 2003). Interestingly, TAC concentrations detected in human follicular fluids were significantly increased with fertilization but significantly reduced with embryo viability (Oyawoye et al., 2003). Taken together, studies on follicular fluid pro- and antioxidants do not yet portray an exact and concordant understanding of the potential influences of OS status on oocyte quality. It is important to note that there are several issues with respect to follicular fluid analyses conducted to date; these include the pooling of samples, sources of contamination, varied sample analyses and outcome measures, and confounding variables such as patient diagnosis.
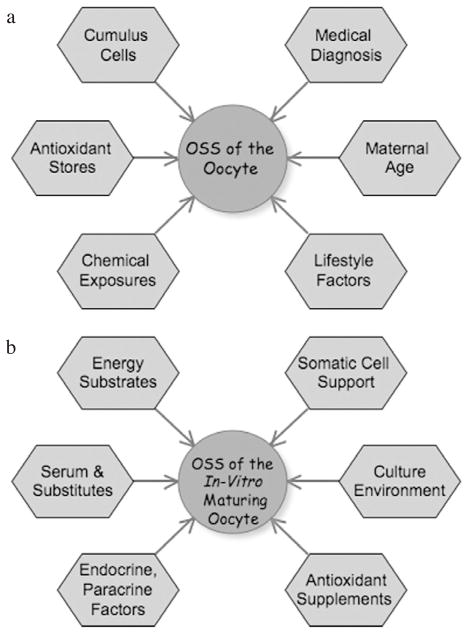
Yet the presence within the follicle of both pro- and antioxidants is relevant to IVM because one approach may be, for example, to model culture media on the natural antioxidant composition of follicular fluid (Sabatini et al., 1999; Kodaman and Behrman, 2001). However, simply reproducing in vitro the exact physiological ratios of antioxidants may represent a rather oversimplified solution. Indeed, the oxidative stresses faced by the maturing oocyte are different in vitro than in the Graafian follicle. The intrafollicular environment also diverges significantly from current IVM conditions, since one must consider the superimposed metabolic and secretory activities of granulosa cells in the follicle as well as the dynamic microenvironment directly surrounding the follicle (e.g. vasculature). In vivo, the large amounts of free radicals generated during cell metabolism are likely to be largely taken care of by the built-in antioxidant defences of the follicle. In vitro, it may then be the antioxidant compounds, with which IVM culture media are supplemented, that play the role of free radical scavengers. The next two sections now focus on potential influences (both intrinsic and extrinsic) on the OS status of oocytes not only at the start but also during IVM (Figure 1).
Figure 1.
Factors that may influence the oxidative stress status (OSS) of oocytes (a) prior to in-vitro maturation (IVM) and (b) upon IVM. Further experimentation is needed to buttress any direct or indirect relationships between OS and each of the parameters; nonetheless, all of these parameters merit consideration in studies aimed at optimizing culture conditions for IVM and the development of good-quality oocytes.
Intrinsic factors influencing the oxidative stress status of oocytes
Oocyte stores
How oxidatively challenged the cultured oocyte is depends on its own properties to cope with IVM-associated OS. Several studies have also examined the expression of various antioxidants through the culture period in mammalian oocytes and cumulus–oocyte–complexes (COC). Indeed, it is as important to define the antioxidant defences that COC begin with as it is to establish the use and potential regulation of builtin antioxidants during and post IVM. Consideration should also be given as to whether antioxidants become prematurely replenished and/or depleted during IVM. For the embryo to acquire developmental potential, it may also be important that antioxidants are stored in the oocyte (as mRNA transcripts or proteins) during its growth and maturation phases. It is thus imperative to define the antioxidant stores of cultured oocytes, not only in relation to the maturation process but also from the early stages of embryonic development through to the activation of the zygotic genome.
As aerobic cells generate ROS as a result of normal cellular metabolism, it is not surprising that oocytes would express and rely upon several antioxidant systems. These include GSH and the classical enzymatic antioxidants, among which are SOD, CAT and GPx. Importantly, there is a precedent in somatic cells for regulation of these antioxidants at both the pre-translational (Forsberg et al., 1996) and post-translational levels (Brown et al., 2004; Rhee et al., 2005); therefore, studies must consider all potential levels of expression. This is particularly relevant for maturing oocytes in which post-transcriptional and translational modes of regulation are prevalent (Oh et al., 2000; Eichenlaub-Ritter and Peschke, 2002; De La Fuente, 2006). At this point and as outlined below, additional studies are still needed in order to close the current gaps and settle inconsistencies in oocyte expression patterns, for all antioxidant molecules and across levels of expression (mRNA, protein and enzymatic activity).
Transcripts encoding GPx, Cu/Zn-SOD, Mn-SOD and γ-glutamylcysteine synthetase (another protective enzyme that is central to GSH synthesis) have been shown to be present not only in mature MII mouse oocytes but also in failed-to-fertilize human MII oocytes (El Mouatassim et al., 1999). All four transcripts were detected in immature mouse oocytes, but ones for GPx and Mn-SOD were not in immature human oocytes. El Mouatassim et al. (1999) further documented their developmental regulation via polyadenylation of the two transcripts during human oocyte maturation. In cattle, Lequarre et al. (2001) also reported transcripts for Mn-SOD and Cu/Zn-SOD in immature and in-vitro-matured oocytes. Interestingly, transcripts for catalase were not detected in either GV or MII stages of mouse and human oocytes (El Mouatassim et al., 1999). This is in contrast to Harvey et al. (1995) who reported catalase mRNA in mouse and cow fertilized eggs, and CAT (along with SOD and GPx) enzymatic activity in both immature and in-vitro-matured bovine oocytes (Cetica et al., 2001). Additional variances exist in the antioxidant expression of oocytes, with one other instance being the absence of detectable GPx transcripts in equine oocytes (Harvey et al., 1995; El Mouatassim et al., 1999; Luciano et al., 2006). Caution must thus be taken whenever extrapolating findings on the oocyte stores of antioxidants across species. Beyond analyses of different developmental stages and species, variations in the sensitivity and detection procedures may underlie the equivocal expression patterns reported to date.
The presence of antioxidant enzymes across different mammalian species suggests that defence mechanisms are conserved and are likely to be important for the final steps of oocyte maturation. Furthermore, comparing in-vitro-matured oocytes from slaughterhouse bovine ovaries with those matured in vivo, the transcript abundance for three antioxidant genes (Mn-SOD, Cu/Zn-SOD, and sarcosine oxidase or SOX) was altered significantly (Lonergan et al., 2003b). In-vitro-matured oocytes expressed Cu/Zn-SOD, Mn-SOD and SOX transcripts at higher levels than in-vivo-matured oocytes, and this may signify an induction of transcripts in order to protect the oocyte from oxidative damage. Alternatively, these findings may denote an inappropriate degradation of maternal mRNA prior to eventual activation of the embryonic genome. Interestingly, mitochondrial Mn-SOD mRNA was expressed at higher levels in oocytes derived from small follicles of slaughterhouse ovaries (both before and after IVM) when compared with oocytes recovered at the LH surge and matured in vitro or with their in-vivo-matured counterparts (Lonergan et al., 2003b). Together, this comparison suggests differences in antioxidant expression (notably Mn-SOD) for oocytes that are known to vary in developmental competence. In this vein, it remains to be established whether immature oocytes differ in their antioxidant stores with respect to their species, follicular origins and developmental state.
It is interesting to note that denuded bovine oocytes possessed particularly high levels of SOD activity relative to CAT and GPx (Cetica et al., 2001), thereby suggesting the potentially prevalent roles of SOD. However, with little attention placed on the other antioxidant players in the pathways, future efforts should thus define which antioxidants are most pertinent to oocyte maturation. In this vein, Luciano et al. (2006) demonstrated that, during IVM, GSH increases in the equine oocyte but the GSH content of oocytes does not correlate with their later developmental competence. Consequently, OS factors may not play a pivotal role during oocyte maturation or, alternatively, there may be OS factors other than glutathione that impact the quality of an oocyte (Luciano et al., 2006). Clearly, the multitude of molecules with protective roles against free radicals must be considered in the oocyte as well as in any of the intrafollicular compartments whenever pertinent to oocyte development. Notably the oocyte is not alone in its developmental journey that is even during ex-vivo culture, a time when cumulus cells cannot be ignored.
Contribution of cumulus cells
Tanghe et al. (2003) investigated the contribution of ROS from bovine cumulus cells and reported that, upon incubation with spermatozoa, significantly greater concentrations of ROS were present in conditioned medium of cumulus-enclosed oocytes than in that of cumulus-denuded oocytes. Interestingly, the COC generated higher redox concentrations, which enhanced sperm penetration. While this finding is most pertinent to IVF, Tatemoto et al. (2000) reported that the cumulus cells play a role in protecting porcine oocytes from OS-induced apoptosis. In their study, COC and denuded oocytes were cultured for 44 hrs under the experimental influence of the hypoxanthine-xanthine oxidase (XOD) system. As a result, denuded oocytes showed greater DNA nuclear damage and apoptotic degeneration due to ROS production when compared with COC, which displayed minimal alterations in response to OS. Concomitant with this protective effect of cumulus cells during IVM, there was a significant increase in GSH concentrations present in cultured COC when compared with freshly isolated oocytes. In addition, the GSH content of denuded oocytes was markedly reduced, irrespective of the presence or absence of the XOD system (Tatemoto et al., 2000). A study performed on in-vitro- matured equine oocytes by Luciano et al. (2006) investigated the beneficial effect of reduced OS (by cysteamine addition) on the GSH content of the oocyte, early embryonic development and GPx mRNA concentrations in the COC. The addition of cysteamine did not affect GSH content in in-vivo- or in-vitro- matured oocytes but, interestingly, cumulus cells expressed GPx mRNA at greater levels in both in-vivo- and in-vitro-matured oocytes when compared with the absence of cysteamine. Due to their regulated expression of GPx mRNA, a protective role can thus be inferred for cumulus cells (Luciano et al., 2006). This study further demonstrated that GPx mRNA is detectable in cumulus cells but not in oocytes (either immature or mature) of the horse. The possibility of compartmentalization between the oocyte and its surrounding somatic cells is an interesting one, particularly in light of the complementary expression patterns previously described for CAT and GPx in seminal plasma and the somatic cells that support the development of male germ cells (Lapointe et al., 1998; Yeung et al., 1998; Zini et al., 2002). Cetica et al. (2001) monitored SOD, CAT and GPx enzymatic activities in not only bovine COC but also cumulus cells alone. When compared with denuded oocytes, cumulus cells displayed relatively high degrees of antioxidant activities at the start of culture, which diminished after IVM (Cetica et al., 2001). Concomitant to this decrease in the cumulus cells, activity levels rose in oocytes matured in vitro, precisely illustrating a potential co-operation in antioxidant protection between the oocyte and its surrounding cumulus cells.
Peroxiredoxins (PRDX), a group of antioxidant enzymes comprised of six isoforms in mammals, are also expressed in bovine oocytes from the time of maturation through to the early embryonic development in vitro (Leyens et al., 2004a). An up-regulated expression of PRDX6 transcript and protein was found post IVM in both oocytes and cumulus cells (Leyens et al., 2004a,b). Interestingly, the up-regulation of PRDX6 in oocytes necessitated physical contact with cumulus cells, while this was not the case for the increase in PRDX6 in cumulus cells that actually depended on oocyte-derived secreted factors (Leyens et al., 2004b). PRDX may not only prevent oxidative damage but also modulate further the maturation of the COC via paracrine signalling pathways central to cumulus expansion. This is based on the increase in PRDX6 transcripts in bovine cumulus cells upon exposure in vitro to the growth-differentiation factor GDF-9, a known enabling factor for cumulus expansion (Leyens et al., 2004b). Other PRDX types may also influence oocyte quality. Mourot et al. (2006) showed a positive correlation between concentrations of PRDX1 transcripts in bovine oocytes and their developmental competence when based on their origins from different follicle sizes. However PRDX1–PRDX5 remain to be investigated in cumulus cells.
Taken together, the aforementioned studies indicate that not only antioxidant defences fluctuate during IVM but also there is an apparent need to consider the two cellular compartments of the COC. Given the well-documented and vital roles of cumulus cells in oocyte development, oocyte maturation and the acquisition of developmental competence (Eppig, 2001; Tanghe et al., 2002; Gilchrist et al., 2004; Gilchrist and Thompson, 2007), an involvement of both cell types in modulating the OS status of the COC is not the least surprising. A growing body of evidence supports the existence and importance of bidirectional influences between the oocyte and cumulus cells. In this vein, much work demonstrates the essential roles of secreted factors and direct cell–cell interactions, among which are members of the transforming growth factor β (TGFβ) family, insulin-like growth factors and gap junctional communication (Gilchrist et al., 2004; Knight and Glister, 2006). Nutritional influences constitute another mechanism by which a COC functions as an interactive and dynamic 2-cell compartment (Russell and Robker, 2007; Thompson et al., 2007). In addition, and also related to metabolic demands, cumulus cells may protect the oocyte by providing antioxidants and/or regulating their utilization during IVM. Direct evidence is undoubtedly needed in order to buttress such possibilities.
In-vivo influences: from lifestyle factors, dietary supplements, age, to medical history
Beyond the built-in antioxidant stores present in and utilized during IVM by oocytes and cumulus cells, factors that may influence the OS status of the COC in the body are also relevant. Lifestyle factors cannot be ignored as they may further confound the heterogeneous quality of oocytes used in IVM cycles. Cigarette smoking generally results in enhanced generation of superoxide anions with ensued oxidative stress leading to, for instance, vascular injury (Rahman and Laher, 2007). In human follicular fluid, smoking is associated with a depletion of the antioxidant beta-carotene (Tiboni et al., 2004). With a reported link between smoking and adverse fertility outcomes (Younglai et al., 2005), negative influences of cigarette smoke on the OS status of oocytes merit consideration. DNA damage is increased in human cumulus cells exposed in vitro to the toxic effects of smoke under either basal or induced conditions of OS (Sinko et al., 2005). It is thus possible that the protective capacity of cumulus cells becomes compromised in patients who smoke. Also relevant is a reported imbalance of pro-/ antioxidants in follicular fluid from smokers, notably elevated lipid peroxidation and diminished total antioxidant capacity levels; therefore, the in-vivo developmental milieu of an oocyte from a smoker appears oxidatively altered when compared with a non-smoker (Paszkowski et al., 2002). Based on this early evidence, smokers would represent poor candidates for clinical IVM. However, if such patients were to be treated with IVM, tailored culture conditions could be employed and aimed at directly targeting some of the inherent deficiencies in the pro-/antioxidant balances of the resulting oocytes.
Exposure to environmental contaminants, such as endocrine disruptors, are increasingly implicated in cases when reproductive performance becomes compromised, either in the infertile or the general population (Giudice, 2006; Homan et al., 2007). Given the ability of steroids to regulate antioxidant expression (as discussed below), it is conceivable that endocrine disruptors may influence ovarian function and oocyte quality via modulation of the OS status of these systems. Of relevance are previous reports on the ability of bisphenol A (an oestrogen mimic known to impact oocytes) to induce OS in multiple organs, including the testis (Kabuto et al., 2004; Ooe et al., 2005). Several metals, such as iron, copper, chromium, cobalt, nickel and cadmium, can lead to the generation of free radicals (Valko et al., 2005) and, with some of these metals possibly being associated with female reproductive health (Agarwal and Allamaneni, 2004), any potential links among metals, OS and oocyte maturation merit investigation. Lastly, Kovacic and Jacintho (2001) also reviewed emerging evidence for OS often underlying the detrimental influences of toxins on reproductive function. Taken together, future studies should consider OS as one of the modes of action by which environmental exposures may compromise the quality of oocytes and, in turn, their successful use for IVM. Similarly, a continued evaluation of other lifestyle influences (such as weight, nutrition and stress) should be undertaken.
Mounting evidence supports links between nutrition and oocyte quality (Boland et al., 2001; Fouladi-Nashta et al., 2007), and diets rich and/or supplemented with antioxidants deserve consideration in the context of OS and oocyte maturation. It remains controversial as to whether dietary supplementation with vitamins may affect reproductive functions via OS. In female mice, some scientific evidence points towards the positive effects of alpha-tocopherol (a lipid peroxy-radical scavenger and chain-breaking antioxidant) in attenuating age-induced abnormal chromosomal alignments and segregation of MII spindles (Tarin et al., 1998, 2002a). However, in-vivo supplementation with vitamins that possess antioxidant activity do not all prove beneficial; for instance, Tarin et al. (2002b) demonstrated that oral intakes of vitamins C and E diminished ovarian function and, in turn, oocyte quality. Additional studies must be conducted so that safe and beneficial doses of antioxidant supplements may be recommended for human use (Rodrigo et al., 2007). Given that pro-oxidants (when present in controlled amounts) do play important roles in the body (Kamata and Hirata, 1999; Droge, 2002; Poli et al., 2004), a balance must be attained and antioxidant therapy must not lead into any risk of neutralizing ROS to a detrimental extent.
Maternal age is also pertinent to any studies of OS and oocyte maturation. The ‘free radical theory of ageing’ was put forth to explain some of the deficiencies in oocyte and embryo quality, known to prevail with advanced maternal age (Tarin, 1995, 1996). This theory proposes that oxidative damage accumulates in female gametes in part due to the extended number of years that arrested oocytes in resting ovarian follicles remain metabolically active. In addition, a decline in oocyte quality may be exacerbated further due to diminished antioxidant expression, such as SOD (both Cu/Zn and Mn isoforms) and catalase in granulosa cells from women of advanced age (Tatone et al., 2006). A gene microarray analysis also revealed lower concentrations of gene products associated with OS (notably Cu/Zn-SOD and thioredoxin) in immature oocytes from older when compared with younger mice (Hamatani et al., 2004). Tarin et al. (2004) documented diminished amounts of both glutathione S-transferases and thiols (GSH) in oocytes from old mice. Further experiments should test whether modifications in antioxidant stores exist between young and aged oocytes from other mammalian species, notably ones actually subject to significant declines in oocyte quality with age.
Some instances point towards potential relationships between medical status and OS in both the ovary and the early embryo, and thus conceivably during oocyte maturation. For instance, Forsberg et al. (1996) related diabetes to modified antioxidant expression patterns in mouse embryos. Together with others, their findings indicate that elevated OS may underlie the aetiology of embryonic teratogenesis in diabetic individuals (Ornoy, 2007). Using animal models, links have been documented between high glucose (as relevant to maternal diabetes) and induced OS (Pampfer, 2000) and compromised embryo quality (Leunda-Casi et al., 2002). More directly relevant to oocyte development is a reported link between OS and PCOS. Elevated concentrations of oxidized proteins were detected in sera of women with PCOS as compared with controls (Palacio et al., 2006). Sabuncu et al. (2001) and Fenkci et al. (2003) also demonstrated augmented OS in PCOS patients. Furthermore, ROS production is increased in mononuclear cells in lean and obese PCOS women, and it has been proposed that ROS-induced OS may be responsible for insulin resistance and hyperandrogenism in these patients (Yilmaz et al., 2005; Gonzalez et al., 2006). Yilmaz et al. (2005) also showed significantly lower antioxidant status and raised malonyl-dialdehyde concentrations in lean PCOS when compared with controls. The concentrations of these OS biomarkers reversed when patients were treated with rosiglitazone, a drug regulating glucose metabolism and known to improve ovulatory function in PCOS patients. Mechanistically, Dinger et al. (2005) showed an increased susceptibility of DNA to OS-induced damage in women with PCOS. Taken together, OS is implicated in the aetiology of PCOS, and because PCOS women are treated with IVM, any superimposed influences of OS on oocyte quality and the maturation process will need to be analysed directly in this patient population.
There are, thus, a plethora of factors that can govern the OS status of oocytes (Figure 1A), and the gamut of them ought to be considered in studies on oocyte quality and IVM. While this section focused on OS-related factors intrinsic to the oocyte (that is prior to the start of IVM), external sources of pro-/antioxidant imbalances must also be considered during the culture period (Figure 1B).
Extrinsic influences impacting the oxidative stress status of oocytes during IVM
Simulating the physiological environment for oocyte maturation in vitro represents one of the greatest challenges in the field of IVM. There are many difficulties encountered when designing a suitable media, among which are the complex and yet not completely defined metabolic needs of the developing human oocyte. There are also considerable variations in substrate demands across the different animal species that are the typical subjects of IVM studies (Krisher et al., 2007), and a myriad of constituents significantly influence the in-vivo growth of the follicle and, in turn, the oocyte. It is thus imperative to assess whether any of these factors, singly or in combination, affect oocyte maturation through OS.
IVM supplements and conditions that may indirectly target the oxidative stress status of oocytes
General supplementation strategies
In vitro, some IVM media formulations have been supplemented with oestradiol (typically employed at a 1 μg/ml concentration); however, such a use is not based on any empirical evidence since systematic and optimization studies have failed to establish an unequivocal need for oestradiol supplementation during IVM. Although the precise roles of oestrogen during oocyte maturation remain unsettled, it undeniably influences follicle development and its role in ovulation is central (Kolibianakis et al., 2005). In such capacity, it remains conceivable that oestrogen influences oocyte quality in yet undefined ways. Interestingly, oestradiol can act as an antioxidant to help minimize the deleterious effects of free radicals in several tissue types, including those of the cardiovascular, neural and ovarian systems (Murdoch, 1998; Nathan and Chaudhuri, 1998; Lund et al., 1999; Behl and Pandey, 2002; Thibodeau et al., 2002; Strehlow et al., 2003). In vitro, oestrogens possess antioxidant properties through their inhibition of 8-hydroxylation of DNA guanine bases (Markides et al., 1998). However, oestrogen also possesses pro-oxidant properties, and whether the steroid acts as an anti- or pro-oxidant depends on its concentration and structure. In high concentrations, oestrogen metabolites tend to produce antioxidant effects, whereas, at low concentrations, pro-oxidant activities prevail. Furthermore, if the oestrogen metabolite possesses a catechol structure such as 2- or 4-hydroxy-oestradiol, then a pro-oxidant activity will most likely arise (Markides et al., 1998). One may propose then that the dual roles of oestrogen in modulating OS may undermine the controversial effects of oestrogens on oocyte development, with perhaps threshold concentrations of oestrogens shifting the OS balance towards detrimental consequences. Further work should define the influences of oestrogen on oocyte maturation in vivo and in vitro, and previous studies suggest the potential need to pay particular attention to balances of pro- and antioxidants whenever oestrogen concentrations vary.
Another important constituent that may alter developmental responses in the oocyte is the presence or absence of serum in the culture medium. Serum acts as a protein provider, supplies nutrition to the COC and prevents zona hardening in order to support the continued development of the oocyte (van de Sandt et al., 1990; George and Johnson, 1993). There is still no consensus as to the positive or negative influences on the maturing oocyte of each type of serum supplementation (whether fetal cord serum, fetal bovine serum, maternal serum or synthetic serum substitutes). Furthermore, different sera contain different proteins that may interact with each other or additional media constituents. Mechanistically, serum constituents can have varied effects on the function of cultured cells, and one influence of serum supplementation may be linked to its innate ability to scavenge metal ions that themselves generate free radicals in the medium. As proposed from earlier studies on cultured embryos, serum also contains various growth factors, macronutrients and other constituents that may directly or indirectly neutralize ROS generated in the culture environment (Esfandiari et al., 2005). Bovine and equine oocytes also display elevated GSH concentrations when matured in the presence of bovine serum albumin (BSA) when compared with fetal calf serum (Luciano et al., 2006; Curnow et al., 2008). Interestingly, serum components may compromise GSH synthesis and/or the known antioxidant actions of BSA may permit elevated stores of GSH in the mature oocyte. Whether supplementation with serum or any of its substitutes may represent a good option for managing the OS status of oocytes during IVM remains unknown, and future studies should consider OS-related effects of serum.
As presented above, the cumulus cells are postulated to provide an antioxidant defence to the oocyte (de Matos et al., 1997; Tatemoto et al., 2000; Cetica et al., 2001; Bing et al., 2002; Maedomari et al., 2007). Luciano et al. (2005) also demonstrated the need for cumulus cells at near physiological concentrations of GSH in in-vitro maturing bovine oocytes. Interestingly, cumulus cells cannot simply be added to cultures of denuded oocytes because it is the presence of intact COC that have been proved beneficial to the oocyte (Luciano et al., 2005). This is not surprising given the pivotal roles of oocyte-secreted factors in sustaining adequate cumulus cell function and, in turn, oocyte developmental competence (Hussein et al., 2006). Consequently, it seems logical to recur to cumulus cells (whenever available) as natural protectors and supporters of the in-vitro-matured oocyte. A handful of studies also explored the potential use of somatic cells other than cumulus cells in co-culture systems aimed at supporting oocyte IVM; no unequivocal benefits have been reached to date (Combelles et al., 2005). The advantages of using a co-culture system may be multiple fold; co-culture may on one hand permit a replacement of missing cumulus cells (that is, whenever there is a need to mature denuded oocytes), and on another hand provide a further cellular support to maturing COC (not unlike the natural functions of granulosa cells within the Graafian follicle). Co-cultured somatic cells likely contribute many factors to the oocyte. When it comes to OS, experimentation awaits, but it may be envisaged that supplementation with somatic cells may assist in neutralizing any excessive concentrations of ROS and maintaining a proper balance of pro-/antioxidants in the maturing COC.
Energy substrates
Glycolysis and oxidative phosphorylation constitute the backbone of cellular metabolism. Metabolism is inherently interlinked with the nuclear and cytoplasmic maturation of the oocyte and ultimately its developmental competence. Energy substrates are required for oocyte maturation, with human and mouse oocytes preferring pyruvate followed by lactate (Biggers et al., 1967; Roberts et al., 2002); other inherent substrate preferences exist in the metabolism of commonly studied mammalian oocytes (Krisher et al., 2007). Three main energy substrates (pyruvate, lactate and glucose) are typically considered in metabolic investigations of the cultured COC along with existing differences between the oocyte and cumulus cell compartments (Krisher et al., 2007; Thompson et al., 2007). The source of metabolites will influence energy production via different metabolic pathways; however, the prospect for concomitant influences on the OS state of COC merits consideration.
Dumollard et al. (2007) discovered that, in mouse oocytes, glucose metabolism was not a vital factor in any redox pathways while pyruvate and lactate play important roles in these cells, and demonstrated key relationships between pyruvate, lactate, lactate dehydrogenase and the setting of the ooplasmic redox potential. Cekleniak et al. (2001) studied meiotic maturation in cumulus-free human oocytes in a glucose-free medium (P1) when compared with oocytes matured in standard tissue culture medium (TC199). The group stressed the fact that cumulus-denuded oocytes probably need a specific medium like P1 unlike cumulus-enclosed oocytes for which TC199 and Ham’s F10 are more suitable. The P1 medium may be better tailored to support the physiological and metabolic demands of the oocyte (Cekleniak et al., 2001). This study reflects that increased concentrations of glucose in the medium can be detrimental to oocyte IVM, and conceivably this may be the case because such medium could induce the generation of elevated concentrations of oxygen radicals via glycolysis and oxidative phosphorylation. Interestingly, this scenario is supported in the bovine model with elevated concentrations of glucose in the IVM medium compromising developmental competence along with increased ROS and reduced glutathione concentrations within the oocyte (Hashimoto et al., 2000a). Therefore, the OS status of oocytes ought to be considered whenever energy sources are modified during IVM. Interestingly, during preimplantation embryo culture, a ‘quiet’ metabolism was proposed to relate to embryo health (Leese, 2002; Leese et al., 2007). With metabolic rates kept basal would come diminished (or at least controlled) concentrations of pro-oxidants and, thus presumably, embryos of superior quality. Whether a similar rule may apply to in-vitro maturing oocytes is intriguing and particularly relevant to studies on OS.
Culture environment
In-vitro-matured oocytes are inevitably exposed to OS due to the generation of ROS originating not only from cellular metabolism but also external influences placed onto the media. A multitude of factors govern the generation of free radicals in the media such as oxygen concentration, light, oocyte handling and generally physicochemical parameters that may alter the overall metabolism of the oocytes themselves.
The O2 concentration in the female reproductive tract lumen is one-third (3–9%) (Mastroianni and Jones, 1965) of the standard in-vitro concentration (20%). Fischer and Bavister (1993) showed that the oxygen tension in the reproductive tracts of rabbits, hamsters and pigs were less than half of the oxygen tension present in the atmosphere. In the hamster and rabbit, the oxygen tension decreased significantly during the time of early embryonic development and implantation demonstrating that in-vivo embryo development occurs under low oxygen tension, in contrast to during in-vitro procedures (Fischer and Bavister, 1993). In general, the high oxygen concentrations associated with in-vitro conditions result in increased ROS generation and, in turn, increased OS (Agarwal et al., 2003, 2006a,b). During IVM, several experimental studies demonstrated both beneficial and deleterious effects of high and low oxygen tension. In the mouse, pig and cow, low oxygen tension appears favourable and more physiological for oocyte IVM and the subsequent development of resulting blastocysts (Hashimoto et al., 2000b; Iwamoto et al., 2005; Preis et al., 2007). In contrast, a few studies report opposite results. Park et al. (2005) found that high oxygen tension is beneficial for porcine IVM, causing increased rates of blastocyst formation; furthermore, when IVM and embryo culture were performed under high and low oxygen tension respectively, blastomere numbers were increased. Most recently, Banwell et al. (2007) tested various oxygen concentrations during mouse IVM (followed by embryo culture in 5% oxygen) together with the assessment of a set of outcome measures. The influences of oxygen concentrations appeared complex and dynamic, with no immediate effects on maturation, fertilization and early developmental rates, but a significant decrease in cell numbers for blastocysts arising from oocytes cultured under increasing oxygen concentrations. Nonetheless, implantation and fetal development remained unaffected by oxygen concentrations with the exception of fetal and placental weights. Interestingly, fetal outcomes that seemed compromised with low oxygen concentrations were comparable to those from in-vivo-matured oocytes. The comprehensive study by Banwell et al. (2007) reinforces some of the not-yet-grasped influences of oxygen during IVM. It must also be noted that potential additive effects (either positive or negative) should be considered when comparing IVM studies altering not only oxygen tension but also media composition.
Overall, it is apparent that additional experiments are needed to elucidate the existing controversy as to the exact effects of oxygen on human oocyte maturation. Notably, future studies need to address the oxygen requirements of oocytes specifically during maturation, that is distinctly from subsequent events of fertilization and embryonic development. Indeed, much attention has been placed on the embryo and its oxygen requirements, often at the expense of teasing apart and separating the respective influences of oxygen on the developing embryo versus the maturing oocyte. Unfortunately, laboratories’ abilities to test the true quality of human embryos, and thus of the progenitor oocytes, deserve significant improvements. Consequently, the task of assigning with certainty the influential roles of oxygen concentrations on oocyte maturation may not yet be possible. That acknowledged, it is reasonable to envisage that oocytes will mature best in an oxygen tension different from the one required for in-vitro embryo culture, much like the use of sequential culture conditions for embryos themselves. This stems from the distinct environments that oocytes and early embryos develop in vivo. It is thus imperative to define the physiological oxygen tension of the pre-ovulatory antral follicle, precisely at the time when the oocyte undergoes the penultimate events of oocyte maturation.
Also associated with IVM are specific OS sources that inevitably result from the isolation of immature oocytes from the body into an artificial environment. Indeed, IVM entails a significant amount of oocyte handling with drastic changes, from not only differing oxygen tensions but also light conditions. Immature oocytes are suddenly exposed to light while they normally develop in a dark environment. This is relevant to OS since light is known to result in an imbalance of pro- and antioxidants in somatic cells and embryos (Beehler et al., 1992; Nakayama et al., 1994). Most recently, a relationship was established in a mouse model between a type of light commonly used in the laboratory, increased ROS concentrations and compromised embryonic and fetal development (Takenaka et al., 2007). Taken together, these various lines of evidence show that oocytes are faced with considerable OS-related challenges. Future investigations must focus on the extent to which immature oocytes are prepared and/or adaptable to such stresses.
With respect to preparedness to undertake oocyte maturation, recent studies have examined the use of prematuration meiotic arrest strategies in order to permit oocyte capacitation. A myriad of chemical agents have been tested to date, and many appear to cause reversible arrest of meiosis and increase the maturation capacity either with or without subsequent embryonic development of the oocytes (Saeki et al., 1997; Thomas et al., 2004; Nogueira et al., 2006; Barretto et al., 2007; Vanhoutte et al., 2007). How exactly meiotic inhibitors, alone or in combination with other supplements, enhance the maturation, fertilization capability and/or blastocyst formation rate is not well understood. In this respect, the potential role of these inhibitors through the modulation of OS is tenable, particularly since a prolonged arrest with chemical inhibitors is likely to alter the biochemical state of the oocyte. Of relevance is the demonstration that meiotic inhibition prior to IVM was associated with changes in mRNA polyadenylation and gene expression of glucose-6-phosphate dehydrogenase (G6PDH) and Mn-SOD, both of which are oxidative response proteins (Gomez et al., 2004; Lequarre et al., 2004). When compared with controls, lower concentrations of Mn-SOD transcripts were reported upon meiotic arrest of bovine oocytes; after IVM, Mn-SOD concentrations decreased but remained higher in oocytes that were previously arrested when compared with non-arrested oocytes (Gomez et al., 2004). Oocyte concentrations of G6PDH increased after meiotic arrest, but when comparing IVM oocytes, ones that were not arrested expressed more G6PDH in the end (Gomez et al., 2004). In the pig, Romar and Funahashi (2006) reported lower GSH concentrations in oocytes that were meiotically arrested prior to IVM, thereby reflecting again an influence of prematuration arrest on the OS status of the oocyte. Protection against ROS may have become compromised in these arrested oocytes, and consequently oocyte quality appeared suboptimal when compared with untreated gametes (Romar and Funahashi, 2006). Although not yet ascertained, it may be argued that prolonging the period of meiotic arrest under artificial in-vitro conditions (for 24–48 h as tested thus far) and precisely ones that generally augment OS (such as elevated oxygen tension and lack of exogenous antioxidant support) may prove detrimental to oocyte quality. Upon increased OS exposures, arrested oocytes may become irreversibly damaged with perhaps exhaustive defects in cellular and molecular elements and/or premature depletion of the oocyte’s antioxidant stores. Conversely, it may be argued that the arrested oocyte has a chance to adapt to in-vitro culture conditions, in turn permitting the oocyte to withstand IVM better than if allowed to mature immediately after isolation from the intrafollicular milieu. The latter scenario represents a more likely possibility in light of the overall positive influences of meiotic arresters on oocyte quality. Nonetheless, it is conceivable that the use of meiotic inhibitors could be improved further provided the right balance of pro-/antioxidants is targeted and maintained. Therefore, future efforts need to focus on investigating the potential involvement of OS during periods of pre-IVM oocyte capacitation in vitro. It must also be recognized that a complex interplay of multiple mechanisms and factors likely influence the prematuration arrest of oocytes.
IVM supplements aimed at directly targeting the pro-/antioxidant balance of oocytes
Under normal conditions, the physiological system possesses an optimum defence of antioxidants, both enzymatic (SOD, CAT, GPx) and non-enzymatic (ascorbic acid, alpha-tocopherol, thiols). As a result, the activity of ROS is kept at a normal balance, so the insult is less. But the in-vitro system may lack or be deficient in such defences and it is this fact, together with the augmented stresses faced in vitro, that lead researchers to study the effects of supplementing the culture medium with various sources of antioxidants.
Antioxidants such as vitamin C (ascorbic acid) assist in the neutralization of free radicals, thereby preventing their oxidizing actions (Nishikimi and Yagi, 1996). Vitamin C also plays a role in the regeneration of vitamin E or alpha-tocopherol; the former is a salient lipid-soluble vitamin and alpha-tocopherol is its most potent form (Schneider, 2005). Importantly, vitamin E prevents oxidative damage such as lipid peroxidation caused by ROS. Both alpha-tocopherol and ascorbic acid are known to act as indispensable antioxidants in intra- as well as extracellular environments and they can block the release of pro-inflammatory factors released as a result of OS (Rodrigo et al., 2007).
A handful of pivotal studies have demonstrated the beneficial influences of antioxidant vitamins during oocyte IVM. An anti-apoptotic role of ascorbic acid was demonstrated in a study wherein isolated murine oocyte–granulosa cell complexes were cultured in a serum-free Waymouth medium with increasing concentrations of ascorbic acid. It was found that an intermediate concentration (0.5 mmol/l) of the vitamin significantly reduced apoptosis in mouse COC (Eppig et al., 2000). Dalvit et al. (2005) evaluated the vitamin E regeneration capability of ascorbic acid along with the role of both antioxidants during oocyte IVM. The investigators allowed the bovine COC to mature in the control medium 199 supplemented with alpha-tocopherol and/ or ascorbic acid; they also measured the concentration of alpha-tocopherol in the COC. It was found that the concentration of alpha-tocopherol naturally present in COC decreased by half during IVM but remained constant in the presence of ascorbic acid. COC matured in media supplemented with alpha-tocopherol showed higher concentrations of alpha-tocopherol, and the addition of vitamins C and alpha-tocopherol kept the concentrations of alpha-tocopherol elevated. Interestingly, the use of both antioxidants during IVM was found to significantly decrease the subsequent rate of blastocyst formation (Dalvit et al., 2005). Tao et al. (2004) further investigated the effects of L-ascorbic acid and alpha-tocopherol on the meiotic maturation of porcine oocytes, the cumulus expansion and DNA fragmentation of cumulus cells. Alpha-tocopherol at a concentration of 10 μmol/l and L-ascorbic acid at 250 μmol/l caused a greater proportion of denuded oocytes to progress to MII when compared with control groups. Interestingly, the effect of vitamins on meiotic progression was not observed in oocytes cultured in the presence of cumulus cells, given that COC have high spontaneous rates of GV breakdown and maturation to MII (Tao et al., 2004). Ascorbic acid 2-O-α-glucoside (AA-2G), a stable derivative of ascorbate, at the concentration of 250 μmol/l enhanced the cytoplasmic maturation of porcine oocytes. Providing a steady and continuous supply of ascorbic acid during IVM thus appears essential in preventing OS and, in turn, permitting adequate developmental competence upon fertilization (Tatemoto et al., 2001). A recent study also demonstrated a beneficial role of vitamin C in protecting MII mouse oocyte spindle structure and chromosomal alignment against an oxidant (hydrogen peroxide)-induced damage (Choi et al., 2007). Together, all of the above studies emphasize the beneficial roles of nonenzymatic vitamin antioxidants when used during IVM at certain concentrations (not too high or too low) and in adequate combinations. Upon addition to IVM media, vitamins C and E also appear to influence oocytes and cumulus cells differentially. With an understanding of the exact balance of vitamins that will best support oocyte maturation, appropriate supplementation with ascorbic acid and alpha-tocopherol promises to have wide application in the optimization of IVM culture media.
Beyond the sole testing of vitamins, Ali et al. (2003) evaluated the combinatorial effects of non-enzymatic as well as enzymatic antioxidant supplementation during bovine IVM with morula and blastocyst development as a final outcome measure. In their study, bovine oocytes were matured, fertilized (under 20% O2) and embryos cultured (under 7% O2) in vitro with or without supplementation with the antioxidants cysteine, N-acetyl-L-cysteine (NAC), catalase and SOD. Addition of 0.6 mmol/l cysteine with low glucose concentration in the IVM medium resulted in a significant improvement in the rate of morula and blastocyst formation. There was no additive beneficial effect of any of the other extracellular antioxidants used in their study. In contrast, addition of antioxidants during IVF significantly compromised bovine embryonic development (Ali et al., 2003). This study suggests that antioxidant requirements vary during oocyte maturation, fertilization and early embryo development as, presumably, ROS generation may also fluctuate during the various stages. Most recently, antioxidant supplementation with NAC and a NAC derivative during porcine IVM demonstrated the complexity of effects when it comes to any reduction in OS as well as the compensating expression of antioxidant enzymes in the oocyte (Whitaker and Knight, 2008).
Additional studies have examined the potential effects of thiol components on oocyte maturation. A medium containing thiol compounds can protect developing oocytes against oxidative damage. Such protective effect has been demonstrated in mammalian oocytes, and it appears related to the generation of GSH, which itself possesses known free radical scavenging properties and enhances cytoplasmic and nuclear maturation (de Matos and Furnus, 2000; Luberda, 2005; Hossein et al., 2007). For instance, increased concentrations of GSH in oocytes matured in the presence of cysteamine suggest that the beneficial effects of cysteamine on IVM and subsequent developmental competence after IVF are mediated by GSH. However, cysteamine may not always prove beneficial with even toxic effects at certain doses (Guyader-Joly et al., 1998), and recent efforts tested alternate and improved approaches to modulate intracellular GSH concentrations in the oocyte (Curnow et al., 2008). A thorough account of intracellular modulators of oocyte GSH is beyond the scope of this review given that they were the subjects of previously published studies (Guerin et al., 2001; Luberda, 2005).
The use and potential benefits of antioxidants during in-vitro culture is still under debate, and a considerable challenge resides in quantifying the exact amounts of pro- and antioxidants that best support the somatic and germ cells of the COC. With the human reproductive system endowed itself with such defences, it becomes obvious to add antioxidants to in-vitro culture media in order to simulate in-vivo conditions. However, the overall gain needs further research. From the sources of oocytes in the body to the physical and chemical properties of the culture environments, there are thus many factors that may influence the OS status of oocytes and, in turn, their quality. Despite a preliminary body of knowledge, much progress remains to be realized in determining the various regulators of OS during IVM. Future efforts should strive towards the development of a medium that sustain the best pro- and antioxidant balance during IVM and thus the formation of best quality oocytes.
Roles of OS during oocyte maturation
As much as a better understanding of the OS status and its regulation during IVM is needed, one must also consider whether and how OS may influence the process of oocyte maturation in vitro. The role of ROS in oocyte maturation has been investigated indirectly by antioxidant supplementations in the media or more directly by oxidative insults with modifications in pro-oxidant concentrations. The outcomes of these studies are quite variable and discordant, and much of this work was presented above in the context of factors influencing the OS status of oocytes during IVM. However, this section will now focus on reports that have started to address (although not yet conclusively) any mechanistic roles for OS in oocyte maturation, especially with respect to key nuclear and cytoplasmic features of the oocyte.
Meiotic progression
Reports to date document conflicting results as to the potential involvement of ROS in either promoting or inhibiting germinal vesicle breakdown. Takami et al. (1999) demonstrated that, in the mouse, cell-permeable antioxidants and lipoxygenase inhibitors with antioxidant effects blocked GV breakdown in COC and denuded oocytes. Also in support of ROS not hindering and even perhaps promoting the resumption of meiosis, the oxidizing agent tertiary-butyl-hydroperoxide (tBH) did not inhibit mouse IVM (Tarin et al., 1996). In contrast, Tamura et al. (2008) showed that elevated OS (with hydrogen peroxide) inhibited oocyte maturation while the free radical scavenger melatonin rescued and protected mouse oocytes from OS-induced meiotic arrest. During porcine IVM, OS (as induced by the hypoxanthine-XOD system) minimally reduced maturation rates to MII; however, chemical inhibition of SOD activity resulted in a further and significant reduction in meiotic progression (Tatemoto et al., 2004). These seemingly disparate results merit further investigation into whether OS play any role in meiotic progression or a very fine balance of pro-/antioxidants may be needed during IVM. Beyond nuclear events, cytoplasmic maturation also merits consideration. In this respect, SOD inhibition together with induced OS lead to compromised cytoplasmic maturation during porcine IVM, as judged by decreased intracellular GSH content and subsequently diminished rates of cleavage and blastocyst formation (Tatemoto et al., 2004).
Aneuploidy
Oocytes are faced with a relatively high risk of aneuploidy or chromosomal abnormalities with a significant proportion of errors occurring during oocyte maturation, notably during meiosis I (Hassold and Hunt, 2001). Associations between oocyte aneuploidy and several risk factors exist, among which are advanced maternal age, ovarian stimulation and in-vitro culture (Tarin, 1995; Van Blerkom and Davis, 2001; Bean et al., 2002). The exact aetiology for chromosomal errors remains unknown, although multiple factors are likely to influence the process. In their ‘free-radical theory of reproductive ageing’, Tarin et al. (1996) propose that OS can induce aneuploidy in mouse oocytes with evidence stemming from exposures to tBH during IVM. tBH resulted in changes in spindle morphology concomitant with defects in chromosome alignment and a higher incidence of aneuploidy. Tarin et al. (1998) also demonstrated a slightly lowered incidence of aneuploidy in oocytes from mice that were fed antioxidant supplements. In somatic cells, studies point towards perturbations in the cell cycle checkpoint downstream of increased OS and in association with aneuploidy (D’Angiolella et al., 2007). Despite some of the clues on OS influencing chromosomal errors, evidence is awaited for humans, a species highly vulnerable to aneuploidy. When considering oocyte aneuploidy, one cannot ignore the meiotic spindle, the cellular structure permitting proper chromosome alignment and separation during cell division.
Cytoskeletal architecture
Oocyte maturation requires the normal assembly and function of a meiotic spindle, and many studies link modifications in spindle architecture with gamete origins and culture conditions during IVM (Sanfins et al., 2003, 2004; Ibanez et al., 2005; Li et al., 2006). Among the multiple stresses that can influence spindle integrity is OS. Previous studies highlight the influences of GSH on spindle microtubules not only post maturation in hamster oocytes (Zuelke et al., 1997) but also during oocyte maturation in the bovine model (Curnow et al., 2008). In an in-vitro study, it was observed that OS results in concentration- and time-dependent alterations in both microtubule dynamics and chromosomal alignment in maturing mouse oocytes (Choi et al., 2007). It was further demonstrated that supplementation with the antioxidant vitamin C together with hydrogen peroxide partially reversed the oxidative damage incurred to the meiotic spindle (Choi et al., 2007). Tarin et al. (1996) also showed changes in spindle morphology upon OS exposure of in-vitro maturing mouse oocytes. A reduction in the oxygen tension used during mouse IVM resulted in subtle yet detectable alterations in spindle morphology together with defects in chromosome alignment (Hu et al., 2001). Importantly, a precedent exists in somatic cells for the sensitivity of the cytoskeleton (both microtubule- and microfilament-based) to OS (Bellomo and Mirabelli, 1992; Dalle-Donne et al., 2001). More recently, increased concentrations of free radicals resulted in differential disruptions of the three cytoskeletal elements present in cultured neurons (Allani et al., 2004). It has been postulated that the redox potential and mitochondrial activity of cells (including the oocytes) can modulate the dynamic properties and integrity of the cytoskeleton (Eichenlaub-Ritter et al., 2004). To begin tackling a mechanistic basis for this, Zhang et al. (2006a) recently showed a reduction in mitochondrial ATP production, notably downstream of OS and concomitantly with defects in mouse oocyte spindles. Permeability transition pores of the mitochondria were further implicated, thereby paving the way for future studies aimed at defining the exact and molecular relationships between OS and the cytoskeleton in the maturing oocyte.
DNA damage, mitochondrial defects, and apoptosis
Lipid peroxides cause damage to DNA by inducing oxidation of bases, primarily guanine by lipid peroxyl and alkoxyl radicals. The breakdown products of lipid hydroperoxides, when covalently bonded with the DNA bases, cause strand breaks. Elevated concentrations of 8-hydroxy-2-deoxyguanosine, lipoperoxides, and protein carbonyls (another OS measure) prevail in mouse ovaries subject to multiple rounds of ovarian stimulation (Chao et al., 2005). This study further linked repeated ovarian stimulation with induced oxidative DNA damage and mitochondrial DNA mutations in mouse oocytes. Recent reports considered DNA repair in oocytes and embryos, and specifically their ability to withstand OS-induced damage. Immature human oocytes possess transcripts for genes that can repair DNA damage linked to ROS (El Mouatassim et al., 2007; Ménézo et al., 2007), and future investigations may continue unravelling links between redox potential, antioxidants, DNA repair genes and tolerance to damage in the oocyte.
Recent studies have shown that the redox status of the cell, resulting from an accumulation of ROS and a decrease of antioxidant concentrations, is involved in inducing apoptotic cell death (Ott et al., 2007). In rodent ovarian follicles and corpora lutea, antioxidants play a similar role to gonadotrophins in protecting cells from apoptosis in vitro (Tilly and Tilly, 1995; Dharmarajan et al., 1999). Using resting follicles isolated from adult human ovaries, Zhang et al. (2006b) showed that OS can induce apoptosis in the female gamete in a time- and dose-dependent manner. Most recently, Tsai-Turton et al. (2007) demonstrated the existence of a relationship in rat follicles between chemically induced apoptosis and increased ROS together with reduced GSH concentrations. In IVF cycles, elevated proportions of ROS-producing and apoptotic follicular cells were demonstrated concomitantly with a reduced number of retrieved oocytes and compromised implantation rates (Jancar et al., 2007). In porcine maturing oocytes, Tatemoto et al. (2004) demonstrated that OS caused by the hypoxanthine-XOD system together with SOD inhibition resulted in oocyte DNA fragmentation and, thus presumably, apoptosis. Most recently, SOD and GPx enzymes were chemically inhibited during the second half of porcine IVM and enzyme activities, GSH concentrations and degree of DNA fragmentation were monitored (Whitaker and Knight, 2008). Whenever SOD was inhibited, DNA fragmentation increased and elevated GPx and CAT activities were accompanied by reduced DNA fragmentation. Supplementation with the antioxidant NACA (NAC-amide, a derivative of N-acetylcysteine) also diminished the extent of DNA fragmentation (Whitaker and Knight, 2008). One may then speculate that any lack of proper antioxidant defences and/or their premature use due to the production of ROS in the medium may be associated with increased damage to the oocyte DNA or accelerated apoptosis in an unabated manner.
Gene expression
When evaluating the detrimental effects of IVM on oocyte quality, a large emphasis has been placed on spindle defects and chromosomal errors. However, it has become increasingly apparent that a need exists to evaluate cellular effects beyond spindle architecture. With the rise and improved sensitivity of tools to profile gene expression, recent studies have begun to depict a dynamic regulation of gene expression in response to in-vitro culture conditions, not only in the cultured embryo but also the oocyte (Lonergan et al., 2006; Fair et al., 2007). In the efforts aimed at optimizing IVM culture conditions, the expression of antioxidants should be routinely considered. Also any manipulations of the oxidative balance should in turn evaluate broad effects on oocyte gene expression. Indeed, many factors related to OS may influence the expression of genes. For example, diminished oxygen concentrations stimulate the expression of hypoxia-inducible factor (HIF), a transcription factor that up- and down-regulates many target genes. In bovine embryos, oxygen can modulate gene expression through several redox-sensitive transcription factors including HIF, nuclear factor kappa B and activator protein-1 (Harvey et al., 2002, 2004). These transcription factors can in turn modulate apoptotic genes, create pro-inflammatory states and affect the redox status of the cell. Extrapolating from emerging evidence in embryos, future studies should focus on how OS may influence gene expression in the maturing oocyte, whether in adaptive, regulatory or detrimental manners. After examining some of the cellular and molecular mechanisms by which OS may influence oocyte maturation, there are a few precedents that point towards potential benefits of pro-oxidants. However, the potential benefits and/or detriments of both pro- and antioxidants remain uncertain during IVM. Only future studies may settle this controversy. Also, given that a multitude of factors are known to influence the maturation and quality of oocytes, OS must be approached as one potential additional modulator of oocyte maturation.
Concluding remarks
The practice of IVM holds great promises for assisted reproduction techniques, and future advances may allow a more common use of it given its cost effectiveness, relatively simple protocol, the prevention of hyperstimulation and other treatment advantages over conventional IVF protocols. It is undeniable that the choice of IVM as a routine tool for infertility treatment remains a real challenge. Among several other issues, the poor successes of the technique may be attributable to the presence of undetected factors or deficiencies in the culture media. In order to design the perfect in-vitro environment for oocyte maturation, it is natural and logical to aim at either preventing oxidative damage or supporting the proper pro-/antioxidant balance in both cultured cumulus cells and oocytes. Consideration must also be given to the wide set of factors that influence the OS status of oocytes prior to their retrieval from the ovary. Upon IVM, oocytes experience an increase in OS because of their cellular metabolism, the culture environment and the lack of protective antioxidant mechanisms that are naturally present in their intrafollicular habitat. The external environment (e.g. pO2, physicochemical properties) that is associated with the IVM procedure also plays an important role in the generation of OS, in turn potentially positively and/or negatively affecting overall gamete and embryo viability in vitro.
Although the effects of antioxidants on oocyte quality and assisted reproduction outcomes need rigorous investigation, they seem to constitute suitable additives during IVM. Many extra- and intrafollicular factors play a role in vivo, the gamut of which merits further investigation during IVM; it may also be relevant to pay particular attention to their potential mechanistic and/or regulatory link(s) with OS. Follicular biology is complicated and it demands further study in order to obtain good quality oocytes and, ultimately, acceptable pregnancy rates. A daunting challenge is to unravel the perfect culture medium that will simulate the in-vivo milieu, thereby meeting all the developmental requirements of the oocyte. It may be proposed that the proper balances of pro- and antioxidants should be aimed for within not only the maturing oocyte but also in its culture environment. Lastly, future efforts should be placed on understanding not only any mechanistic influences of OS during oocyte maturation but also the existence and roles of compensating and repair mechanisms within the oocyte.
Acknowledgments
The authors thank Jashoman Banerjee MD and Rajeshree Koli MD for their assistance with electronic database search, manual search of articles and cross references, literature review and in the initial preparation of this systematic review. This work was supported by a grant from the Research Programs Committee and funds from the Reproductive Research Centre, Cleveland Clinic, Cleveland, Ohio (SG) and by grants from the Vermont Genetics Network (grant number P20 RR16462, INBRE Program, NIH/NCRR), National Institute of Child Health and Development (R15 HD05645–01), and funds from Middlebury College, Middlebury, Vermont (CMHC).
Biography
 Catherine Combelles received a PhD in Cell, Molecular, and Developmental Biology in 2002 from the Tufts University School of Medicine (Boston, USA). She completed her postdoctorate at Harvard Medical School, during which she also trained and worked as a senior embryologist in the Center for Reproductive Medicine at the Brigham and Women's Hospital, Boston. In 2004, she joined the faculty at Middlebury College, a small liberal arts institution in Vermont where she teaches and conducts research with undergraduate students on the cellular and molecular determinants of oocyte quality. She has published book chapters and several articles in peer-reviewed journals.
Catherine Combelles received a PhD in Cell, Molecular, and Developmental Biology in 2002 from the Tufts University School of Medicine (Boston, USA). She completed her postdoctorate at Harvard Medical School, during which she also trained and worked as a senior embryologist in the Center for Reproductive Medicine at the Brigham and Women's Hospital, Boston. In 2004, she joined the faculty at Middlebury College, a small liberal arts institution in Vermont where she teaches and conducts research with undergraduate students on the cellular and molecular determinants of oocyte quality. She has published book chapters and several articles in peer-reviewed journals.
Footnotes
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update. 2003;9:275–289. doi: 10.1093/humupd/dmg018. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reproductive BioMedicine Online. 2004;9:338–347. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Current Opinion in Obstetrics and Gynecology. 2006;18:325–332. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility – a clinician’s perspective. Reproductive BioMedicine Online. 2005;11:641–650. doi: 10.1016/s1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility and Sterility. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Ali A, Benkhalifa M, Miron P. In-vitro maturation of oocytes: biological aspects. Reproductive BioMedicine Online. 2006;13:437–446. doi: 10.1016/s1472-6483(10)61450-2. [DOI] [PubMed] [Google Scholar]
- Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirements for bovine oocytes varies during in-vitro maturation, fertilization and development. Theriogenology. 2003;59:939–949. doi: 10.1016/s0093-691x(02)01125-1. [DOI] [PubMed] [Google Scholar]
- Allani PK, Sum T, Bhansali SG, et al. A comparative study of the effect of oxidative stress on the cytoskeleton in human cortical neurons. Toxicology and Applied Pharmacology. 2004;196:29–36. doi: 10.1016/j.taap.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Attaran M, Pasqualotto E, Falcone T, et al. The effect of follicular fluid reactive oxygen species on the outcome of in-vitro fertilization. International Journal of Fertility and Women’s Medicine. 2000;45:314–320. [PubMed] [Google Scholar]
- Banwell KM, Lane M, Russell DL, et al. Oxygen concentration during mouse oocyte in-vitro maturation affects embryo and fetal development. Human Reproduction. 2007;22:2768–2775. doi: 10.1093/humrep/dem203. [DOI] [PubMed] [Google Scholar]
- Barretto LS, Caiado Castro VS, Garcia JM, Mingoti GZ. Role of roscovitine and IBMX on kinetics of nuclear and cytoplasmic maturation of bovine oocytes in vitro. Animal Reproduction Science. 2007;99:202–207. doi: 10.1016/j.anireprosci.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilization in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Human Reproduction. 2002;17:2362–2367. doi: 10.1093/humrep/17.9.2362. [DOI] [PubMed] [Google Scholar]
- Beehler BC, Przybyszewski J, Box HB, Kulesz-Martin MF. Formation of 8-hydroxydeoxyguanosine within DNA of mouse keratinocytes exposed in culture to UVB and H2O2. Carcinogenesis. 1992;13:2003–2007. doi: 10.1093/carcin/13.11.2003. [DOI] [PubMed] [Google Scholar]
- Behl R, Pandey RS. FSH induced stimulation of catalase activity in goat granulosa cells in vitro. Animal Reproduction Science. 2002;70:215–221. doi: 10.1016/s0378-4320(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Bellomo G, Mirabelli F. Oxidative stress and cytoskeletal alterations. Annals of the New York Academy of Sciences. 1992;663:97–109. doi: 10.1111/j.1749-6632.1992.tb38653.x. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proceedings of the National Academy of Science of the United States of America. 1967;58:560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing YZ, Hirao Y, Iga K, et al. In-vitro maturation and glutathione synthesis of porcine oocytes in the presence or absence of cysteamine under different oxygen tensions: role of cumulus cells. Reproduction, Fertility and Development. 2002;14:125–131. doi: 10.1071/rd01127. [DOI] [PubMed] [Google Scholar]
- Boland MP, Lonergan P, O’Callaghan D. Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology. 2001;55:1323–1340. doi: 10.1016/s0093-691x(01)00485-x. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radical Biology and Medicine. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Brown NM, Torres AS, Doan PE, O’Halloran TV. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu, Zn superoxide dismutase. Proceedings of the National Academy of Science of the United States of America. 2004;101:5518–5523. doi: 10.1073/pnas.0401175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canipari R. Oocyte–granulosa cell interactions. Human Reproduction Update. 2000;6:279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Moskovtsev S, Chohan KR, Peterson CM. Ovarian folliculogenesis: emerging role of in-vitro maturation of oocytes and follicles in clinical practice. Clinical Obstetrics and Gynecology. 2003;46:239–253. doi: 10.1097/00003081-200306000-00006. [DOI] [PubMed] [Google Scholar]
- Cekleniak NA, Combelles CM, Ganz DA, et al. A novel system for in-vitro maturation of human oocytes. Fertility and Sterility. 2001;75:1185–1193. doi: 10.1016/s0015-0282(01)01789-7. [DOI] [PubMed] [Google Scholar]
- Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in-vitro maturation. IUBMB Life. 2001;51:57–64. doi: 10.1080/15216540119253. [DOI] [PubMed] [Google Scholar]
- Cha KY, Chung HM, Lee DR, et al. Obstetric outcome of patients with polycystic ovary syndrome treated by in-vitro maturation and in-vitro fertilization-embryo transfer. Fertility and Sterility. 2005;83:1461–1465. doi: 10.1016/j.fertnstert.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Chao HT, Lee SY, Lee HM, et al. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Annals of the New York Academy of Sciences. 2005;1042:148–156. doi: 10.1196/annals.1338.016. [DOI] [PubMed] [Google Scholar]
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Current Opinion in Obstetrics and Gynecology. 2004a;16:211–219. doi: 10.1097/00001703-200406000-00003. [DOI] [PubMed] [Google Scholar]
- Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reproductive BioMedicine Online. 2004b;8:148–166. doi: 10.1016/s1472-6483(10)60511-1. [DOI] [PubMed] [Google Scholar]
- Choi WJ, Banerjee J, Falcone T, et al. Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertility and Sterility. 2007;88:1220–1231. doi: 10.1016/j.fertnstert.2007.02.067. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Fissore RA, Albertini DF, Racowsky C. In-vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Human Reproduction. 2005;20:1349–1358. doi: 10.1093/humrep/deh750. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Carabatsos MJ, Kumar TR, et al. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Molecular Reproduction and Development. 2004;69:347–355. doi: 10.1002/mrd.20128. [DOI] [PubMed] [Google Scholar]
- Curnow EC, Ryan J, Saunders D, Hayes ES. Bovine in vitro oocyte maturation as a model for manipulation of the gamma-glutamyl cycle and intraoocyte glutathione. Reproduction, Fertility, and Development. 2008;20:579–588. doi: 10.1071/rd08041. [DOI] [PubMed] [Google Scholar]
- D’Angiolella V, Santarpia C, Grieco D. Oxidative stress overrides the spindle checkpoint. Cell Cycle. 2007;6:576–579. doi: 10.4161/cc.6.5.3934. [DOI] [PubMed] [Google Scholar]
- Dal Canto MB, Mignini Renzini M, Brambillasca F, et al. IVM – the first choice for IVF in Italy. Reproductive BioMedicine Online. 2006;13:159–165. doi: 10.1016/s1472-6483(10)60610-4. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Milzani A, et al. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radical Biology and Medicine. 2001;31:1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- Dalvit G, Llanes SP, Descalzo A, et al. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in-vitro maturation. Reproduction in Domestic Animals. 2005;40:93–97. doi: 10.1111/j.1439-0531.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- Das S, Chattopadhyay R, Ghosh S, et al. Reactive oxygen species level in follicular fluid – embryo quality marker in IVF? Human Reproduction. 2006;21:2403–2407. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Developmental Biology. 2006;292:1–12. doi: 10.1016/j.ydbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in-vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in-vitro maturation of bovine oocytes: role of cumulus cells. Biology of Reproduction. 1997;57:1420–1425. doi: 10.1095/biolreprod57.6.1420. [DOI] [PubMed] [Google Scholar]
- Dharmarajan AM, Hisheh S, Singh B, et al. Antioxidants mimic the ability of chorionic gonadotropin to suppress apoptosis in the rabbit corpus luteum in vitro: a novel role for superoxide dismutase in regulating bax expression. Endocrinology. 1999;140:2555–2561. doi: 10.1210/endo.140.6.6713. [DOI] [PubMed] [Google Scholar]
- Dinger Y, Akcay T, Erdem T, et al. DNA damage, DNA susceptibility to oxidation and glutathione level in women with polycystic ovary syndrome. Scandinavian Journal of Clinical and Laboratory Investigation. 2005;65:721–728. doi: 10.1080/00365510500375263. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dumollard R, Ward Z, Carroll J, Duchen MR. Regulation of redox metabolism in the mouse oocyte and embryo. Development. 2007;134:455–465. doi: 10.1242/dev.02744. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Peschke M. Expression in in-vivo and in-vitro growing and maturing oocytes: focus on regulation of expression at the translational level. Human Reproduction Update. 2002;8:21–41. doi: 10.1093/humupd/8.1.21. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reproductive BioMedicine Online. 2004;8:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- El-Mouatassim S, Bilotto S, Russo GL, et al. APEX/Ref-1 (apurinic/apyrimidic endonuclease DNA-repair gene) expression in human and ascidian (Ciona intestinalis) gametes and embryos. Molecular Human Reproduction. 2007;13:549–556. doi: 10.1093/molehr/gam038. [DOI] [PubMed] [Google Scholar]
- El Mouatassim S, Guerin P, Ménézo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Molecular Human Reproduction. 1999;5:720–725. doi: 10.1093/molehr/5.8.720. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O’Brien MJ, et al. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Molecular and Cellular Endocrinology. 2000;163:109–116. doi: 10.1016/s0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Esfandiari N, Falcone T, Agarwal A, et al. Protein supplementation and the incidence of apoptosis and oxidative stress in mouse embryos. Obstetrics and Gynecology. 2005;105:653–660. doi: 10.1097/01.AOG.0000152384.91385.71. [DOI] [PubMed] [Google Scholar]
- Fair T, Carter F, Park S, et al. Global gene expression analysis during bovine oocyte in-vitro maturation. Theriogenology. 2007;68 (Suppl 1):S91–S97. doi: 10.1016/j.theriogenology.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends in Endocrinology and Metabolism. 2003;14:236–242. doi: 10.1016/s1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertility and Sterility. 2003;80:123–127. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Journal of Reproduction and Fertility. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Forsberg H, Borg LA, Cagliero E, Eriksson UJ. Altered levels of scavenging enzymes in embryos subjected to a diabetic environment. Free Radical Research. 1996;24:451–459. doi: 10.3109/10715769609088044. [DOI] [PubMed] [Google Scholar]
- Fouladi-Nashta AA, Gutierrez CG, Gong JG, et al. Impact of dietary fatty acids on oocyte quality and development in lactating dairy cows. Biology of Reproduction. 2007;77:9–17. doi: 10.1095/biolreprod.106.058578. [DOI] [PubMed] [Google Scholar]
- Fulka J, Jr, First NL, Moor RM. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Molecular Human Reproduction. 1998;4:41–49. doi: 10.1093/molehr/4.1.41. [DOI] [PubMed] [Google Scholar]
- George MA, Johnson MH. Use of fetal bovine serum substitutes for the protection of the mouse zona pellucida against hardening during cryoprotectant addition. Human Reproduction. 1993;8:1898–1900. doi: 10.1093/oxfordjournals.humrep.a137956. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology. 2007;67:6–15. doi: 10.1016/j.theriogenology.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Animal Reproduction Science. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Infertility and the environment: the medical context. Seminars in Reproductive Medicine. 2006;24:129–133. doi: 10.1055/s-2006-944418. [DOI] [PubMed] [Google Scholar]
- Gomez E, Rodriguez A, Goyache F, et al. Retinoid-dependent mRNA expression and poly-(A) contents in bovine oocytes meiotically arrested and/or matured in vitro. Molecular Reproduction and Development. 2004;69:101–108. doi: 10.1002/mrd.20154. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2006;91:336–340. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Human Reproduction Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Guyader-Joly C, Guerin P, Renard JP, et al. Precursors of taurine in female genital tract: effects on developmental capacity of bovine embryo produced in vitro. Amino Acids. 1998;15:27–42. doi: 10.1007/BF01345278. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Human Molecular Genetics. 2004;13:2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Pantaleon M, et al. Oxygen-regulated gene expression in bovine blastocysts. Biology of Reproduction. 2004;71:1108–1119. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002;123:479–486. doi: 10.1530/rep.0.1230479. [DOI] [PubMed] [Google Scholar]
- Harvey MB, Arcellana-Panlilio MY, Zhang X, et al. Expression of genes encoding antioxidant enzymes in preimplantation mouse and cow embryos and primary bovine oviduct cultures employed for embryo coculture. Biology of Reproduction. 1995;53:532–540. doi: 10.1095/biolreprod53.3.532. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Minami N, Yamada M, Imai H. Excessive concentration of glucose during in-vitro maturation impairs the developmental competence of bovine oocytes after in-vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Molecular Reproduction and Development. 2000a;56:520–526. doi: 10.1002/1098-2795(200008)56:4<520::AID-MRD10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Minami N, Takakura R, et al. Low oxygen tension during in-vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Molecular Reproduction and Development. 2000b;57:353–360. doi: 10.1002/1098-2795(200012)57:4<353::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Reviews Genetics. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Holzer H, Scharf E, Chian RC, et al. In-vitro maturation of oocytes collected from unstimulated ovaries for oocyte donation. Fertility and Sterility. 2007;88:62–67. doi: 10.1016/j.fertnstert.2006.11.087. [DOI] [PubMed] [Google Scholar]
- Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Human Reproduction Update. 2007;13:209–223. doi: 10.1093/humupd/dml056. [DOI] [PubMed] [Google Scholar]
- Hossein MS, Kim MK, Jang G, et al. Effects of thiol compounds on in-vitro maturation of canine oocytes collected from different reproductive stages. Molecular Reproduction and Development. 2007;74:1213–1220. doi: 10.1002/mrd.20674. [DOI] [PubMed] [Google Scholar]
- Hu Y, Betzendahl I, Cortvrindt R, et al. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Human Reproduction. 2001;16:737–748. doi: 10.1093/humrep/16.4.737. [DOI] [PubMed] [Google Scholar]
- Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Developmental Biology. 2006;296:514–521. doi: 10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Ibanez E, Sanfins A, Combelles CM, et al. Genetic strain variations in the metaphase-II phenotype of mouse oocytes matured in vivo or in vitro. Reproduction. 2005;130:845–855. doi: 10.1530/rep.1.00558. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Onishi A, Fuchimoto D, et al. Low oxygen tension during in-vitro maturation of porcine follicular oocytes improves parthenogenetic activation and subsequent development to the blastocyst stage. Theriogenology. 2005;63:1277–1289. doi: 10.1016/j.theriogenology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Jancar N, Kopitar AN, Ihan A, et al. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. Journal of Assisted Reproduction and Genetics. 2007;24:91–97. doi: 10.1007/s10815-006-9103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Molecular Aspects of Medicine. 2005;26:340–352. doi: 10.1016/j.mam.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Jozwik M, Wolczynski S, Szamatowicz M. Oxidative stress markers in preovulatory follicular fluid in humans. Molecular Human Reproduction. 1999;5:409–413. doi: 10.1093/molehr/5.5.409. [DOI] [PubMed] [Google Scholar]
- Jurema MW, Nogueira D. In-vitro maturation of human oocytes for assisted reproduction. Fertility and Sterility. 2006;86:1277–1291. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sciences. 2004;74:2931–2940. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Knijn HM, Wrenzycki C, Hendriksen PJ, et al. Effects of oocyte maturation regimen on the relative abundance of gene transcripts in bovine blastocysts derived in vitro or in vivo. Reproduction. 2002;124:365–375. [PubMed] [Google Scholar]
- Kodaman PH, Behrman HR. Endocrine-regulated and protein kinase C-dependent generation of superoxide by rat preovulatory follicles. Endocrinology. 2001;142:687–693. doi: 10.1210/endo.142.2.7961. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Papanikolaou EG, Fatemi HM, Devroey P. Estrogen and folliculogenesis: is one necessary for the other? Current Opinion in Obstetrics and Gynecology. 2005;17:249–253. doi: 10.1097/01.gco.0000169101.83342.96. [DOI] [PubMed] [Google Scholar]
- Kovacic P, Jacintho JD. Reproductive toxins: pervasive theme of oxidative stress and electron transfer. Current Medicinal Chemistry. 2001;8:863–892. doi: 10.2174/0929867013372878. [DOI] [PubMed] [Google Scholar]
- Krisher RL, Brad AM, Herrick JR, et al. A comparative analysis of metabolism and viability in porcine oocytes during in-vitro maturation. Animal Reproduction Science. 2007;98:72–96. doi: 10.1016/j.anireprosci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biology of Reproduction. 1998;58:747–753. doi: 10.1095/biolreprod58.3.747. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Sturmey RG, Baumann CG, McEvoy TG. Embryo viability and metabolism: obeying the quiet rules. Human Reproduction. 2007;22:3047–3050. doi: 10.1093/humrep/dem253. [DOI] [PubMed] [Google Scholar]
- Lequarre AS, Traverso JM, Marchandise J, Donnay I. Poly(A) RNA is reduced by half during bovine oocyte maturation but increases when meiotic arrest is maintained with CDK inhibitors. Biology of Reproduction. 2004;71:425–431. doi: 10.1095/biolreprod.103.026724. [DOI] [PubMed] [Google Scholar]
- Lequarre AS, Feugang JM, Malhomme O, et al. Expression of Cu/Zn and Mn superoxide dismutases during bovine embryo development: influence of in-vitro culture. Molecular Reproduction and Development. 2001;58:45–53. doi: 10.1002/1098-2795(200101)58:1<45::AID-MRD7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Leunda-Casi A, Genicot G, Donnay I, et al. Increased cell death in mouse blastocysts exposed to high D-glucose in vitro: implications of an oxidative stress and alterations in glucose metabolism. Diabetologia. 2002;45:571–579. doi: 10.1007/s00125-001-0752-y. [DOI] [PubMed] [Google Scholar]
- Leyens G, Knoops B, Donnay I. Expression of peroxiredoxins in bovine oocytes and embryos produced in vitro. Molecular Reproduction and Development. 2004a;69:243–251. doi: 10.1002/mrd.20145. [DOI] [PubMed] [Google Scholar]
- Leyens G, Verhaeghe B, Landtmeters M, et al. Peroxiredoxin 6 is upregulated in bovine oocytes and cumulus cells during in-vitro maturation: role of intercellular communication. Biology of Reproduction. 2004b;71:1646–1651. doi: 10.1095/biolreprod.104.030155. [DOI] [PubMed] [Google Scholar]
- Li Y, Feng HL, Cao YJ, et al. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertility and Sterility. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Fair T, Corcoran D, Evans AC. Effect of culture environment on gene expression and developmental characteristics in IVF-derived embryos. Theriogenology. 2006;65:137–152. doi: 10.1016/j.theriogenology.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Rizos D, Gutierrez-Adan A, et al. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reproduction in Domestic Animals. 2003a;38:259–267. doi: 10.1046/j.1439-0531.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Gutierrez-Adan A, Rizos D, et al. Relative messenger RNA abundance in bovine oocytes collected in vitro or in vivo before and 20 hr after the preovulatory luteinizing hormone surge. Molecular Reproduction and Development. 2003b;66:297–305. doi: 10.1002/mrd.10357. [DOI] [PubMed] [Google Scholar]
- Luberda Z. The role of glutathione in mammalian gametes. Reproductive Biology. 2005;5:5–17. [PubMed] [Google Scholar]
- Luciano AM, Goudet G, Perazzoli F, et al. Glutathione content and glutathione peroxidase expression in in-vivo and in-vitro matured equine oocytes. Molecular Reproduction and Development. 2006;73:658–666. doi: 10.1002/mrd.20469. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Lodde V, Beretta MS, et al. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione. Molecular Reproduction and Development. 2005;71:389–397. doi: 10.1002/mrd.20304. [DOI] [PubMed] [Google Scholar]
- Lund SA, Murdoch J, Van Kirk EA, Murdoch WJ. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: relevance to luteal function. Biology of Reproduction. 1999;61:388–392. doi: 10.1095/biolreprod61.2.388. [DOI] [PubMed] [Google Scholar]
- Maedomari N, Kikuchi K, Ozawa M, et al. Cytoplasmic glutathione regulated by cumulus cells during porcine oocyte maturation affects fertilization and embryonic development in vitro. Theriogenology. 2007;67:983–993. doi: 10.1016/j.theriogenology.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Markides CS, Roy D, Liehr JG. Concentration dependence of prooxidant and antioxidant properties of catecholestrogens. Archives of Biochemistry and Biophysics. 1998;360:105–112. doi: 10.1006/abbi.1998.0934. [DOI] [PubMed] [Google Scholar]
- Mastroianni L, Jr, Jones R. Oxygen tension within the rabbit Fallopian tube. Journal of Reproduction and Fertility. 1965;147:99–102. doi: 10.1530/jrf.0.0090099. [DOI] [PubMed] [Google Scholar]
- Ménézo Y, Jr, Russo G, Tosti E, et al. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. Journal of Assisted Reproduction and Genetics. 2007;24:513–520. doi: 10.1007/s10815-007-9167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen AL. Strategies in human in-vitro maturation and their clinical outcome. Reproductive BioMedicine Online. 2005;10:593–599. doi: 10.1016/s1472-6483(10)61666-5. [DOI] [PubMed] [Google Scholar]
- Mourot M, Dufort I, Gravel C, et al. The influence of follicle size, FSH-enriched maturation medium, and early cleavage on bovine oocyte maternal mRNA levels. Molecular Reproduction and Development. 2006;73:1367–1379. doi: 10.1002/mrd.20585. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ. Inhibition by oestradiol of oxidative stress-induced apoptosis in pig ovarian tissues. Journal of Reproduction and Fertility. 1998;114:127–130. doi: 10.1530/jrf.0.1140127. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Noda Y, Goto Y, Mori T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology. 1994;41:499–510. doi: 10.1016/0093-691x(94)90086-x. [DOI] [PubMed] [Google Scholar]
- Nathan L, Chaudhuri G. Antioxidant and prooxidant actions of estrogens: potential physiological and clinical implications. Seminars in Reproductive Endocrinology. 1998;16:309–314. doi: 10.1055/s-2007-1016289. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Yagi K. Biochemistry and molecular biology of ascorbic acid biosynthesis. Sub-cell Biochemistry. 1996;25:17–39. doi: 10.1007/978-1-4613-0325-1_2. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Ron-El R, Friedler S, et al. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biology of Reproduction. 2006;74:177–184. doi: 10.1095/biolreprod.105.040485. [DOI] [PubMed] [Google Scholar]
- Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in-vitro-matured oocytes. Fertility and Sterility. 2000;74:295–298. doi: 10.1016/s0015-0282(00)00642-7. [DOI] [PubMed] [Google Scholar]
- Oh B, Hwang S, McLaughlin J, et al. Timely translation during the mouse oocyte-to-embryo transition. Development. 2000;127:3795–3803. doi: 10.1242/dev.127.17.3795. [DOI] [PubMed] [Google Scholar]
- Ooe H, Taira T, Iguchi-Ariga SM, Ariga H. Induction of reactive oxygen species by bisphenol A and abrogation of bisphenol A-induced cell injury by DJ-1. Toxicological Sciences. 2005;88:114–126. doi: 10.1093/toxsci/kfi278. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reproductive Toxicology. 2007;24:31–41. doi: 10.1016/j.reprotox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Oyawoye O, Abdel Gadir A, Garner A, et al. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Human Reproduction. 2003;18:2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- Palacio JR, Iborra A, Ulcova-Gallova Z, et al. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clinical and Experimental Immunology. 2006;144:217–222. doi: 10.1111/j.1365-2249.2006.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampfer S. Peri-implantation embryopathy induced by maternal diabetes. Journal of Reproduction and Fertility Supplement. 2000;55:129–139. [PubMed] [Google Scholar]
- Papanikolaou EG, Platteau P, Albano C, et al. Immature oocyte in-vitro maturation: clinical aspects. Reproductive BioMedicine Online. 2005;10:587–592. doi: 10.1016/s1472-6483(10)61665-3. [DOI] [PubMed] [Google Scholar]
- Park JI, Hong JY, Yong HY, et al. High oxygen tension during in-vitro oocyte maturation improves in-vitro development of porcine oocytes after fertilization. Animal Reproduction Science. 2005;87:133–141. doi: 10.1016/j.anireprosci.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Pasqualotto EB, Agarwal A, Sharma RK, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertility and Sterility. 2004;81:973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Clarke RN. The Graafian follicle is a site of L-ascorbate accumulation. Journal of Assisted Reproduction and Genetics. 1999;16:41–45. doi: 10.1023/A:1022597629622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski T, Clarke RN, Hornstein MD. Smoking induces oxidative stress inside the Graafian follicle. Human Reproduction. 2002;17:921–925. doi: 10.1093/humrep/17.4.921. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clinica Chimica Acta. 1995;236:173–180. doi: 10.1016/0009-8981(95)98130-9. [DOI] [PubMed] [Google Scholar]
- Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Current Medicinal Chemistry. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- Preis KA, Seidel GE, Jr, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in-vitro maturation. Molecular Reproduction and Development. 2007;74:893–903. doi: 10.1002/mrd.20655. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Current Vascular Pharmacology. 2007;5:276–292. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- Rao GD, Tan SL. In-vitro maturation of oocytes. Seminars in Reproductive Medicine. 2005;23:242–247. doi: 10.1055/s-2005-872452. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, et al. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxidants and Redox Signaling. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Roberts R, Franks S, Hardy K. Culture environment modulates maturation and metabolism of human oocytes. Human Reproduction. 2002;17:2950–2956. doi: 10.1093/humrep/17.11.2950. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundamental and Clinical Pharmacology. 2007;21:111–127. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- Romar R, Funahashi H. In-vitro maturation and fertilization of porcine oocytes after a 48 h culture in roscovitine, an inhibitor of p34cdc2/cyclin B kinase. Animal Reproduction Science. 2006;92:321–333. doi: 10.1016/j.anireprosci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Human Reproduction Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- Sabatini L, Wilson C, Lower A, et al. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in-vitro fertilization. Fertility and Sterility. 1999;72:1027–1034. doi: 10.1016/s0015-0282(99)00411-2. [DOI] [PubMed] [Google Scholar]
- Sabuncu T, Vural H, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clinical Biochemistry. 2001;34:407–413. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- Saeki K, Nagao Y, Kishi M, Nagai M. Developmental capacity of bovine oocytes following inhibition of meiotic resumption by cycloheximide or 6-dimethylaminopurine. Theriogenology. 1997;48:1161–1172. doi: 10.1016/s0093-691x(97)00349-x. [DOI] [PubMed] [Google Scholar]
- Sanfins A, Plancha CE, Overstrom EW, Albertini DF. Meiotic spindle morphogenesis in in-vivo and in-vitro matured mouse oocytes: insights into the relationship between nuclear and cytoplasmic quality. Human Reproduction. 2004;19:2889–2899. doi: 10.1093/humrep/deh528. [DOI] [PubMed] [Google Scholar]
- Sanfins A, Lee GY, Plancha CE, et al. Distinctions in meiotic spindle structure and assembly during in-vitro and in-vivo maturation of mouse oocytes. Biology of Reproduction. 2003;69:2059–2067. doi: 10.1095/biolreprod.103.020537. [DOI] [PubMed] [Google Scholar]
- Schneider C. Chemistry and biology of vitamin E. Molecular Nutrition and Food Research. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM, VandeVoort CA. Causes of developmental failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Human Reproduction. 2003;18:826–833. doi: 10.1093/humrep/deg144. [DOI] [PubMed] [Google Scholar]
- Schweigert FJ, Steinhagen B, Raila J, et al. Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing IVF. Human Reproduction. 2003;18:1259–1264. doi: 10.1093/humrep/deg249. [DOI] [PubMed] [Google Scholar]
- Seino T, Saito H, Kaneko T, et al. 8-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in-vitro fertilization-embryo transfer program. Fertility and Sterility. 2002;77:1184–1190. doi: 10.1016/s0015-0282(02)03103-5. [DOI] [PubMed] [Google Scholar]
- Sinko I, Morocz M, Zadori J, et al. Effect of cigarette smoking on DNA damage of human cumulus cells analyzed by comet assay. Reproductive Toxicology. 2005;20:65–71. doi: 10.1016/j.reprotox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Smith GD. In-vitro maturation of oocytes. Current Women’s Health Reports. 2001;1:143–151. [PubMed] [Google Scholar]
- Smitz J, Nogueira D, Albano C, et al. Improving in-vitro maturation of oocytes in the human taking lessons from experiences in animal species. Reproduction in Domestic Animals. 2001;36:11–17. doi: 10.1046/j.1439-0531.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circulation Research. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Human Reproduction Update. 2003;9:35–48. doi: 10.1093/humupd/dmg009. [DOI] [PubMed] [Google Scholar]
- Takami M, Preston SL, Toyloy VA, Behrman HR. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. American Journal of Physiology. 1999;276:E684–688. doi: 10.1152/ajpendo.1999.276.4.E684. [DOI] [PubMed] [Google Scholar]
- Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proceedings of the National Academy of Science of the United States of America. 2007;104:14289–14293. doi: 10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. Journal of Pineal Research. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Tanghe S, Van Soom A, Mehrzad J, et al. Cumulus contributions during bovine fertilization in vitro. Theriogenology. 2003;60:135–149. doi: 10.1016/s0093-691x(02)01360-2. [DOI] [PubMed] [Google Scholar]
- Tanghe S, Van Soom A, Nauwynck H, et al. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Molecular Reproduction and Development. 2002;61:414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- Tao Y, Zhou B, Xia G, et al. Exposure to L-ascorbic acid or alpha-tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reproduction in Domestic Animals. 2004;39:52–57. doi: 10.1046/j.1439-0531.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Molecular Human Reproduction. 1996;2:717–724. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- Tarin JJ. Aetiology of age-associated aneuploidy: a mechanism based on the ‘free radical theory of ageing’. Human Reproduction. 1995;10:1563–1565. doi: 10.1093/humrep/10.6.1563. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Gomez-Piquer V, Pertusa JF, et al. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Molecular Reproduction and Development. 2004;69:402–410. doi: 10.1002/mrd.20180. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Garcia-Perez MA, Cano A. Effect of dietary supplementation with a mixture of vitamins C and E on fertilization of tertiary butyl hydroperoxide-treated oocytes and parthenogenetic activation in the mouse. Theriogenology. 2002a;57:869–881. doi: 10.1016/s0093-691x(01)00687-2. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Molecular Reproduction and Development. 2002b;61:385–397. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Vendrell FJ, Ten J, Cano A. Antioxidant therapy counteracts the disturbing effects of diamide and maternal ageing on meiotic division and chromosomal segregation in mouse oocytes. Molecular Human Reproduction. 1998;4:281–288. doi: 10.1093/molehr/4.3.281. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Vendrell FJ, Ten J, et al. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Molecular Human Reproduction. 1996;2:895–901. doi: 10.1093/molehr/2.12.895. [DOI] [PubMed] [Google Scholar]
- Tatemoto H, Muto N, Sunagawa I, et al. Protection of porcine oocytes against cell damage caused by oxidative stress during in-vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biology of Reproduction. 2004;71:1150–1157. doi: 10.1095/biolreprod.104.029264. [DOI] [PubMed] [Google Scholar]
- Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in-vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in-vitro maturation. Biology of Reproduction. 2001;65:1800–1806. doi: 10.1095/biolreprod65.6.1800. [DOI] [PubMed] [Google Scholar]
- Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In-vitro maturation: role of cumulus cells. Biology of Reproduction. 2000;63:805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, et al. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Molecular Human Reproduction. 2006;12:655–660. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- Thibodeau PA, Kachadourian R, Lemay R, et al. In-vitro pro- and antioxidant properties of estrogens. Journal of Steroid Biochemistry and Molecular Biology. 2002;81:227–236. doi: 10.1016/s0960-0760(02)00067-5. [DOI] [PubMed] [Google Scholar]
- Thomas RE, Thompson JG, Armstrong DT, Gilchrist RB. Effect of specific phosphodiesterase isoenzyme inhibitors during in-vitro maturation of bovine oocytes on meiotic and developmental capacity. Biology of Reproduction. 2004;71:1142–1149. doi: 10.1095/biolreprod.103.024828. [DOI] [PubMed] [Google Scholar]
- Thompson JG, Lane M, Gilchrist RB. Metabolism of the bovine cumulus–oocyte complex and influence on subsequent developmental competence. Society of Reproduction and Fertility Supplement. 2007;64:179–190. doi: 10.5661/rdr-vi-179. [DOI] [PubMed] [Google Scholar]
- Tiboni GM, Bucciarelli T, Giampietro F, et al. Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. International Journal of Immunopathology and Pharmacology. 2004;17:389–393. doi: 10.1177/039463200401700319. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Nakamura BN, Luderer U. Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biology of Reproduction. 2007;77:442–451. doi: 10.1095/biolreprod.107.060368. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Current Medicinal Chemistry. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P. Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Human Reproduction. 2001;16:757–764. doi: 10.1093/humrep/16.4.757. [DOI] [PubMed] [Google Scholar]
- van de Sandt JJ, Schroeder AC, Eppig JJ. Culture media for mouse oocyte maturation affect subsequent embryonic development. Molecular Reproduction and Development. 1990;25:164–171. doi: 10.1002/mrd.1080250209. [DOI] [PubMed] [Google Scholar]
- Vanhoutte L, De Sutter P, Nogueira D, et al. Nuclear and cytoplasmic maturation of in-vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Human Reproduction. 2007;22:1239–1246. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]
- Whitaker BD, Knight JW. Mechanisms of oxidative stress in porcine oocytes and the role of anti-oxidants. Reproduction Fertility and Development. 2008;20:694–702. doi: 10.1071/rd08037. [DOI] [PubMed] [Google Scholar]
- Wiener-Megnazi Z, Vardi L, Lissak A, et al. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in-vitro fertilization. Fertility and Sterility. 2004;82 (Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Yagi K. Lipid Peroxides and Antioxidants. CRC Press; New York: 1993. [Google Scholar]
- Yeung CH, Cooper TG, De Geyter M, et al. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in-vitro fertilization. Molecular Human Reproduction. 1998;4:835–839. doi: 10.1093/molehr/4.9.835. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Biri A, Karakoc A, et al. The effects of rosiglitazone and metformin on insulin resistance and serum androgen levels in obese and lean patients with polycystic ovary syndrome. Journal of Endocrinological Investigation. 2005;28:1003–1008. doi: 10.1007/BF03345339. [DOI] [PubMed] [Google Scholar]
- Younglai EV, Holloway AC, Foster WG. Environmental and occupational factors affecting fertility and IVF success. Human Reproduction Update. 2005;11:43–57. doi: 10.1093/humupd/dmh055. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu XQ, Lu S, et al. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Research. 2006a;16:841–850. doi: 10.1038/sj.cr.7310095. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li XH, Ma X, et al. Redox-induced apoptosis of human oocytes in resting follicles in vitro. Journal of the Society of Gynecologic Investigation. 2006b;13:451–458. doi: 10.1016/j.jsgi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zheng P, Patel B, McMenamin M, et al. Effects of follicle size and oocyte maturation conditions on maternal messenger RNA regulation and gene expression in rhesus monkey oocytes and embryos. Biology of Reproduction. 2005;72:890–897. doi: 10.1095/biolreprod.104.035881. [DOI] [PubMed] [Google Scholar]
- Zini A, Fischer MA, Mak V, et al. Catalase-like and superoxide dismutase-like activities in human seminal plasma. Urological Research. 2002;30:321–323. doi: 10.1007/s00240-002-0283-0. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biology of Reproduction. 1997;57:1413–1419. doi: 10.1095/biolreprod57.6.1413. [DOI] [PubMed] [Google Scholar]