Abstract
Presynaptic nerve terminals release neurotransmitters repeatedly, often at high frequency, and in relative isolation from neuronal cell bodies. Repeated release requires cycles of SNARE-complex assembly and disassembly, with continuous generation of reactive SNARE-protein intermediates. Although many forms of neurodegeneration initiate presynaptically, only few pathogenic mechanisms are known, and the functions of presynaptic proteins linked to neurodegeneration, such as α-synuclein, remain unclear. Here, we find that maintenance of continuous presynaptic SNARE-complex assembly required a non-classical chaperone activity mediated by synucleins. Specifically, α-synuclein directly bound to the SNARE-protein synaptobrevin-2/VAMP2, and promoted SNARE-complex assembly. Moreover, triple knockout mice lacking synucleins developed age-dependent neurological impairments, exhibited decreased SNARE-complex assembly, and perished prematurely. Thus, synucleins may function to sustain normal SNARE-complex assembly in a presynaptic terminal during aging.
In presynaptic terminals, neurotransmitter release requires a tightly coordinated membrane fusion machinery whose central components are soluble NSF attachment protein receptor (SNARE) and Sec1/Munc18-like proteins (1-3). Terminals release neurotransmitters thousands of times per minute; during each release reaction, SNARE-complex assembly and disassembly generates highly reactive unfolded SNARE protein intermediates, rendering the terminals potentially vulnerable to activity-dependent degeneration. Indeed, much evidence points to presynaptic terminals as an initiation site for neurodegeneration (4-6), and knockout (KO) of at least one presynaptic chaperone protein, cysteine string protein-α (CSPα), causes fulminant neurodegeneration in mice (7). Synucleins are abundant presynaptic proteins that are expressed from three genes (α-, β- and γ-synuclein; 8). α-Synuclein is involved in neurodegeneration (9-11), and γ-synuclein may contribute to progression of many types of cancer (12). Synucleins may modify neurotransmitter release (13,14), but their physiological functions remain unknown. Strikingly, transgenic expression of α-synuclein abolishes the lethal neurodegeneration induced by KO of CSPα, whereas deletion of endogenous synucleins accelerates this neurodegeneration (15). CSPα KO mice exhibit decreased levels of the SNARE protein SNAP-25 and impaired SNARE-complex assembly, suggesting a link between SNARE-complex assembly and neurodegeneration. α-Synuclein rescues SNARE-complex assembly but not SNAP-25 levels (15). This result indicates that α-synuclein may enhance SNARE-protein function, and thereby compensate for the CSPα deletion. To address this hypothesis, we here examine the role of α-synuclein in SNARE-complex assembly and in the maintenance of continuous SNARE-cycling in presynaptic terminals over the lifetime of an animal.
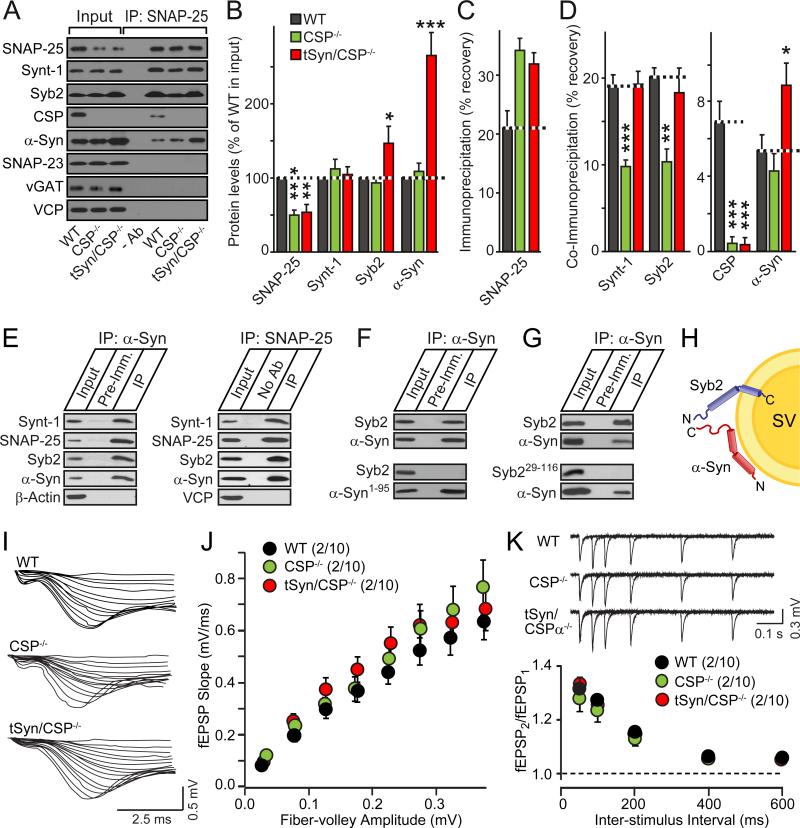
We immunoprecipitated SNARE complexes from brain homogenates using SNAP-25 antibodies. We measured the levels of SNARE proteins and α-synuclein in the input and immunoprecipitates from littermate wild-type (WT) mice, CSPα KO mice, and CSPα KO mice rescued by transgenic α-synuclein (15). As expected (15), transgenic α-synuclein rescued the SNARE-complex assembly deficit but not the ~50% decrease in SNAP-25 levels in CSPα KO mice (Figs. 1A-1D). Unexpectedly, however, endogenous and transgenic α-synuclein were co-immunoprecipitated with SNAP-25, independent of CSPα (Figs. 1A-1D; for additional controls, see Figs. S1A-S1C). Moreover, α-synuclein co-immunoprecipitated SNARE proteins from mouse brains (Figs. S1D and S1E) or when co-expressed in HEK293 cells (Fig. 1E). When each SNARE protein was co-expressed separately with α-synuclein (Figs. 1F, 1G, and S1F-S1H), only synaptobrevin-2 co-immunoprecipitated with α-synuclein. Direct α-synuclein binding to synaptobrevin-2 was confirmed by glutathione S-transferase pulldowns (Fig. S1I) and liposome-binding experiments (Fig. S2). Removal of either the C-terminal 44 residues from α-synuclein – which are not involved in phospholipid binding (17; Figs. S1J and S1K) – or deletion of the N-terminal 28 residues from synaptobrevin-2 – which are not involved in SNARE-complex formation (1-3) – blocked α-synuclein binding to synaptobrevin-2 (Figs. 1F and 1G). Thus, the C-terminus of α-synuclein binds to the N-terminus of synaptobrevin-2 on the synaptic vesicle surface (Fig. 1H).
Figure 1. α-Synuclein directly binds to synaptobrevin-2/VAMP2 in SNARE complexes.
(A-D) α-Synuclein associates with SNARE-complexes immunoprecipitated with SNAP-25 antibodies from wild-type (WT) and CSPα KO (CSP-/-) mouse brains containing or lacking transgenic α-synuclein (tSyn). Panels show representative immunoblots (A; -Ab = control IP from WT brain without primary antibody; Synt-1 = syntaxin-1; Syb2 = synaptobrevin-2; α-Syn = α-synuclein), and quantitations of input levels (B), immunoprecipitated SNAP-25 (C), and co-immunoprecipitated proteins (D), performed by quantitative immunoblotting using 125I-labeled secondary antibodies (means ± SEMs, *p<0.05; **p<0.01; ***p<0.001 per Student's t-test; n=3-5).
(E) Co-immunoprecipitation of α-synuclein with SNARE-complexes reconstituted in transfected HEK293T cells. Cell lysates were immunoprecipitated with antibodies to α-synuclein (left panel) or SNAP-25 (right panel), and analyzed by immunoblotting.
(F & G) α-Synuclein C-terminus directly binds to synaptobrevin-2 N-terminus. α-Synuclein was immunoprecipitated from HEK293T cells co-expressing full-length (α-Syn) or C-terminally truncated α-synuclein (α-Syn1-95) with full-length (Syb2) or N-terminally truncated synaptobrevin-2 (Syb229-116). Immunoprecipitates were analyzed by immunoblotting for α-synuclein and synaptobrevin-2 (see also Figs. S1 and S2).
(H) Diagram of the α-synuclein/synaptobrevin-2 complex on synaptic vesicles (SV).
(I-K) Neither CSPα KO nor transgenic α-synuclein overexpression detectably alters synaptic strength. Data show sample traces (I and K top) and summary graphs (J and K bottom; means ± SEMs) of extracellular recordings from input-output measurements (I and J) or paired-pulse facilitation experiments (K) in acute hippocampal slices (see also Fig. S3).
How does binding of α-synuclein to SNARE-complexes relate to its ability to rescue the neurodegeneration of CSPα KO mice? The activity-dependence of neurodegeneration in CSPα KO mice (7,18) suggests that α-synuclein may indirectly rescue the CSPα KO phenotype by inhibiting synaptic strength. However, electrophysiological measurements in acute brain slices from WT and CSPα KO mice, without or with transgenic α-synuclein expression, failed to reveal changes in synaptic strength (Figs. 1I-1K and S3). As an alternative hypothesis, we thus tested whether α-synuclein directly rescued the neurodegeneration of CSPα KO mice by promoting SNARE-complex assembly.
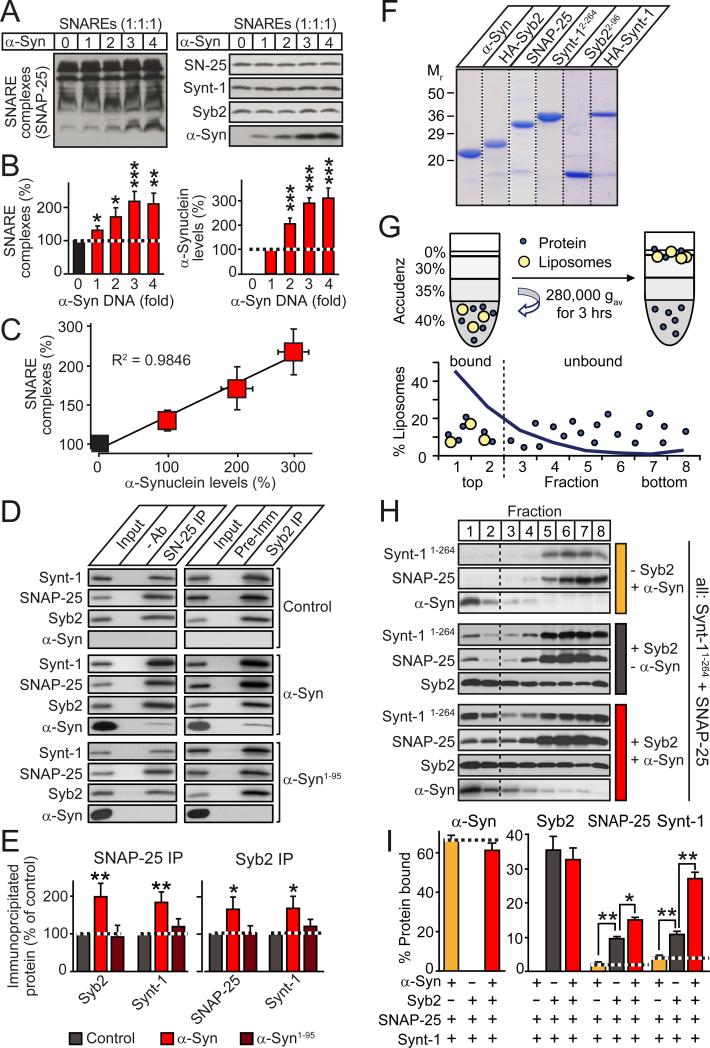
We co-transfected HEK293 cells with constant amounts of SNARE-protein, and increasing amounts of α-synuclein expression vectors, and measured SNARE-complex assembly as a function of α-synuclein, using immunoblotting of non-boiled samples as an assay (19; Figs. 2A and 2B). Strikingly, we observed a linear relationship between SNARE-complex assembly and α-synuclein levels (Figs. 2C and S4). Using co-immunoprecipitation assays, we confirmed that full-length but not C-terminally truncated α-synuclein promoted SNARE-complex assembly (Figs. 2D and 2E; for additional data, see Figs. S4 and S5).
Figure 2. α-Synuclein directly catalyzes SNARE-complex assembly.
(A-C) α-Synuclein promotes SNARE-complex assembly in a dose-dependent manner. HEK293T cells were co-transfected with constant amounts of syntaxin-1 (Synt-1), synaptobrevin-2 (Syb2), and SNAP-25, and increasing amounts of α-synuclein (α-Syn). Cell lysates were immunoblotted without (A) and with boiling (Fig. S4); SNARE-complexes and α-synuclein were quantified (B), and plotted as a function of each other (C).
(D & E) Full-length but not C-terminally truncated α-synuclein promotes SNARE-complex assembly. SNARE complexes were immunoprecipitated with antibodies to SNAP-25 (left) or synaptobrevin-2 (right) from HEK293T cells co-expressing SNARE proteins with emerald (control), full-length α-synuclein (α-Syn), or C-terminally truncated α-synuclein (α-Syn1-95), and analyzed by immunoblotting (D, representative blots; E, SNARE-complex quantitation).
(F) Coomassie-stained SDS gel of purified recombinant α-synuclein (α-Syn), H A-tagged synaptobrevin-2 (HA-Syb2), SNAP-25, C-terminally truncated syntaxin-1 (Synt-11-264) or synaptobrevin-2 (Syb21-96), and full-length HA-tagged syntaxin-1 (HA-Synt-1).
(G) In vitro SNARE-complex assembly assay. Liposomes containing or lacking reconstituted synaptobrevin-2 are mixed with SNAP-25 and C-terminally truncated syntaxin-1, with or without α-synuclein, and floated by density gradient centrifugation. SNARE-complex assembly is measured as co-flotation of SNAP-25 and syntaxin-1 with liposomes in the top two fractions.
(H & I) Distribution of SNAREs and α-synuclein in liposome flotation gradients, shown as representative immunoblots (H) and quantitations of liposome-bound proteins (I). Note that whereas α-synuclein (α-Syn) and synaptobrevin-2 are directly liposome-bound, SNAP-25 and syntaxin-1 (Synt-1) only bind to liposomes via SNARE-complex formation.
Data are means ± SEMs (*p<0.05; **p<0.01; ***p<0.001 by Student's t-test; n=3-8).
Because the effect of α-synuclein in transfected HEK293 cells may have been indirect, we examined the function of α-synuclein in a purely in vitro system. We reconstituted recombinant synaptobrevin-2 into liposomes (Fig. S2A-C), and measured SNARE-complex assembly with soluble recombinant syntaxin-1 and SNAP-25 onto these liposomes as a function of α-synuclein (Figs. 2F-2I). SNARE-complex assembly was monitored by flotation of liposomes on an Accudenz centrifugation gradient (Fig. 2G). Reconstituted and free synaptobrevin-2 were not separated prior to the assay; as a result, synaptobrevin-2 was distributed across the gradient, whereas α-synuclein – which quantitatively binds to phospholipids (17) – was exclusively present in the bound fractions at the top of the gradient (Fig. 2H). In this purified system, SNARE-complex assembly was strongly enhanced by α-synuclein (Fig. 2I).
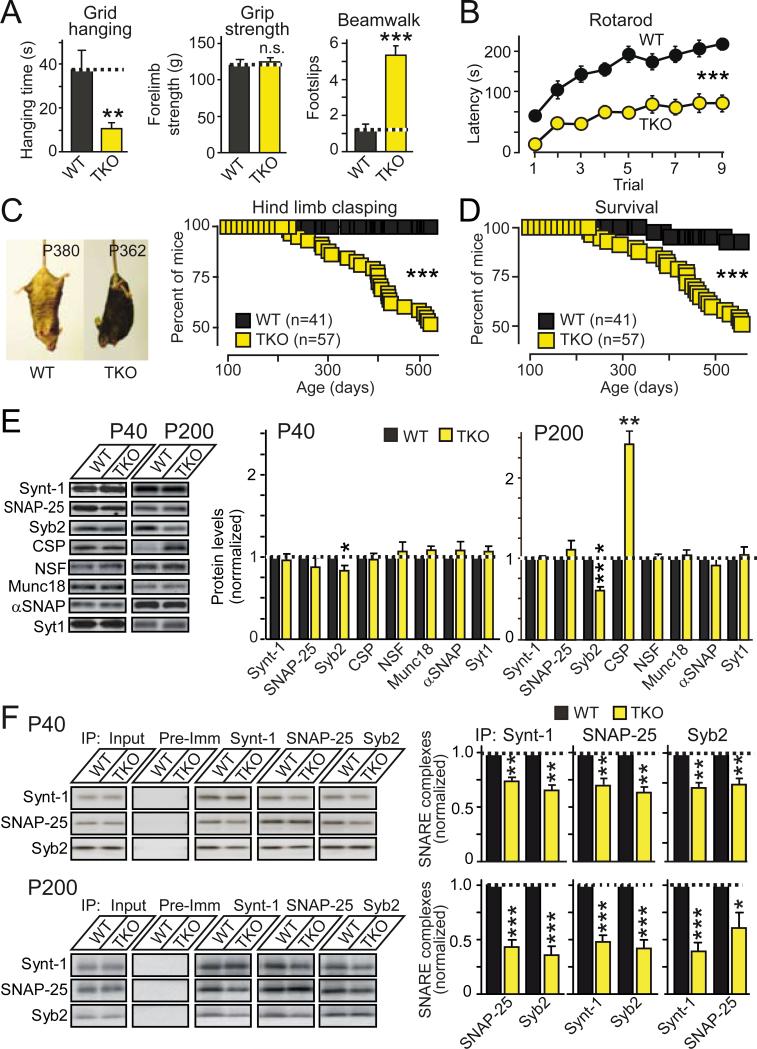
To assess the physiological significance of the observed biochemical activity of α-synuclein, we generated αβγ-synuclein triple knockout (TKO) mice. Similar to single and double α-, β-, and γ-synuclein KO mice (20-22), young TKO mice displayed no obvious phenotype. During aging, however, TKO mice developed severe neurological impairments (Figs. 3A-3C), and died prematurely (Fig. 3D). Although TKO mice did not display overt neurodegeneration or changes in synaptic strength (Figs. S6 and S7), and most synaptic proteins were unchanged, we observed a marked, age-dependent decrease in synaptobrevin-2 and a large increase in CSPα levels (Figs. 3E and S8), consistent with their functional interaction. Noticeably, TKO mice exhibited an age-dependent decrease in SNARE-complex assembly (Figs. 3F, and S9). Thus, synucleins are required for maintaining normal SNARE-complex assembly during aging in mice.
Figure 3. αβγ-Synuclein TKO mice exhibit impaired survival and decreased SNARE-complex assembly.
(A & B) Neurological impairments in αβγ-synuclein TKO mice (at P300). Data show (A) latency to fall off an inverted wire grid (grid-hanging time; WT, n=6; TKO, n=7), forelimb grip strength (measured using a digital grip strength meter; WT, n=9; TKO, n=11), and footslips during beamwalking (WT, n=9; TKO, n=11), and (B) the latency to fall off an accelerating rotarod (5 min trials; WT, n=9; TKO, n=11).
(C) Hind-limb clasping of WT and TKO mice as a function of age (left, representative images; right, clasping incidence; WT, n=41; TKO, n=57).
(D) Age-dependent mortality of WT and TKO mice (WT, n=41; TKO, n=57).
(E) Age-dependent changes in protein levels in TKO mice (left, representative blots; right, quantitations).
(F) SNARE-complex assembly analyzed by co-immunoprecipitation of SNAREs in brain lysates from WT and TKO mice at P40 (top) and P200 (bottom). Left panels show representative blots, right panels quantitations from multiple independent experiments (n=3-5; Syb2 = synaptobrevin-2; Synt-1 = syntaxin-1).
See Figs. S6-S9 for further data. Data are means ± SEMs, *p<0.05; **p<0.01; ***p<0.001 by Student's t-test (A, E and F), two-way ANOVA (B), or Mantel-Cox test (C).
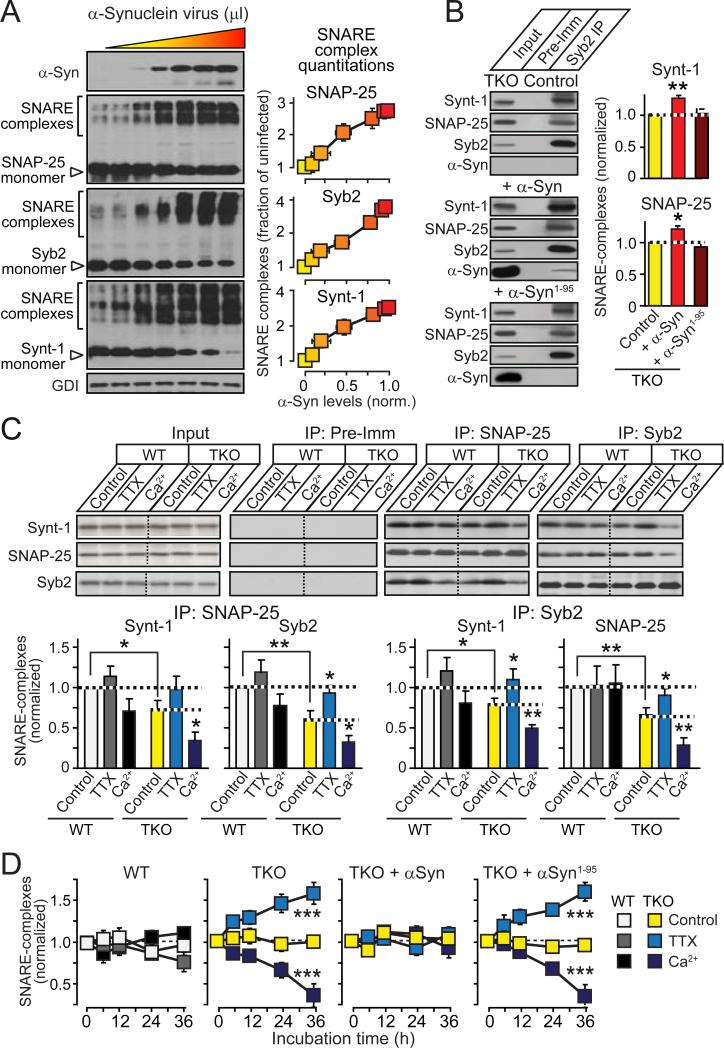
We next examined whether the SNARE-complex assembly deficit in TKO mice could be reversed by reintroduction of α-synuclein. Expression of increasing amounts of α-synuclein in cultured TKO neurons produced a corresponding dose-dependent increase in SNARE-complex assembly (Fig. 4A), similar to the HEK293 cell experiments (Figs. 2A-2C). The C-terminus of α-synuclein that bound to synaptobrevin-2 (Fig. 1) was required for its promotion of SNARE-complex assembly (Fig. 4B), even though it was not required for synaptic targeting of α-synuclein (Fig. S10), confirming that α-synuclein acts by binding to synaptobrevin-2.
Figure 4. α-Synuclein boosts SNARE-complex assembly during synaptic activity.
(A) Linear relationship between α-synuclein levels and SNARE-complex assembly in αβγ-synuclein TKO neurons infected at DIV4 with increasing amounts of lentivirus expressing α-synuclein, and analyzed by immunoblotting of non-boiled samples at DIV17 (n=3-6).
(B) Full-length (α-Syn) but not truncated α-synuclein (α-Syn1-95) enhances SNARE-complex assembly in TKO neurons, as measured by co-immunoprecipitation of syntaxin-1 (Synt-1) and SNAP-25 with synaptobrevin-2 (Syb2; control = plain virus; left, representative blots; right, quantitations; n=3-6).
(C) Activity-dependence of SNARE-complex assembly in cultured WT and TKO neurons. Neurons were incubated at DIV10 for 36 hrs in control medium, 0.5 μM tetrodotoxin (TTX), or 4 mM Ca2+ (Ca2+). SNARE-complex assembly was measured by co-immunoprecipitation of SNARE-proteins with SNAP-25 and synaptobrevin-2. Representative blots are shown on top (Pre-Imm = pre-immune control), and quantitations from multiple independent experiments on the bottom (n=3-6).
(D) Time course of activity-induced changes in SNARE-complex assembly in WT and TKO neurons, and in TKO neurons infected with lentiviruses expressing full-length (α-Syn) or C-terminally truncated α-synuclein (α-Syn1-95). Neurons were incubated in control medium, 0.5 μM TTX, or 4 mM Ca2+ for the indicated times, and SNARE-complex assembly was measured by immunoblotting for SNAP-25 in unboiled samples (n=3).
See Figures S10 and S11 for further data. Data are means ± SEMs, *p<0.05; **p<0.01; ***p<0.001 by Student's t-test (A-C) or one-way ANOVA (D).
The age-dependent impairment of SNARE-complex assembly in TKO mice suggests an activity-dependent phenotype. To test this hypothesis, we blocked or stimulated synaptic activity in cultured TKO and wild-type neurons for 36 hours, using tetrodotoxin (TTX, an inhibitor of voltage-gated Na+-channels), or increased ambient Ca2+-concentrations (4 mM). Cultured TKO neurons exhibited a significant SNARE-complex assembly deficit that was dramatically enhanced by increased synaptic activity, but rescued by decreased synaptic activity (Fig. 4C and S11). The activity-dependent phenotype in TKO neurons developed in a time-dependent manner, and was rescued by wild-type but not C-terminally truncated α-synuclein (Fig. 4D and S11C), consistent with the biochemical activity of α-synuclein (Fig. 2).
Here, we found that α-synuclein directly promotes SNARE-complex assembly by a non-enzymatic mechanism that involves simultaneous binding of α-synuclein to phospholipids via its N-terminus, and to synaptobrevin-2 via its C-terminus (Fig. S12). The SNARE-complex promoting function of α-synuclein becomes important during increased synaptic activity and aging, rendering its action akin to a proofreading activity that is essential for the continued maintenance of SNARE-mediated fusion over the lifetime of an animal. As a result, loss of synuclein function manifests in an age-dependent loss of neuronal function, as revealed in αβγ-synuclein TKO mice whose phenotype resembles the delayed onset and slow progression of a neurodegenerative disease. Relative loss of α-synuclein function, for example by sequestration of α-synuclein in Lewy bodies or by increased truncation of α-synuclein during aging (23-27), may thus contribute to neurodegenerative diseases such as Parkinson's disease and Lewy body dementia. Furthermore, the increase in γ-synuclein in many cancers (12) may support enhanced membrane traffic in transformed cells.
Supplementary Material
Summary sentence.
α-Synuclein, a protein implicated in neurodegeneration and cancer, is shown to bind to SNARE proteins, which mediate membrane fusion in synaptic transmission and cell growth, and to promote the assembly of SNARE proteins into SNARE-complexes during fusion.
Acknowledgments
We thank Dr. S. Chandra for advice. This work was supported by postdoctoral fellowships from the Human Frontiers Program (LT00527/2006-L to M.S. and LT000135/2009-L to T.T.) and the Deutsche Akademie der Naturforscher Leopoldina (BMBF-LPD 9901/8-161 to J.B.).
REFERENCES and NOTES
- 1.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 2.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;23:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer ML, Schulz-Schaeffer WJ. Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 6.Gray BC, Siskova Z, Perry VH, O'Connor V. Selective presynaptic degeneration in the synaptopathy associated with ME7-induced hippocampal pathology. Neurobiol Dis. 2009;35:63–74. doi: 10.1016/j.nbd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Chacon R, et al. The synaptic vesicle protein CSPα prevents presynaptic degeneration. Neuron. 2004;42:237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- 8.Clayton DF, George JM. The Synucleins: a family of proteins involved in synaptic function, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 9.Polymeropoulos MH, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 10.Spillantini MG, et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson's disease? Ann Neurol. 2008;64(Suppl 2):S3–15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad M, Attoub S, Singh MN, Martin FL, El-Agnaf OM. γ-Synuclein and the progression of cancer. FASEB J. 2007;21:3419–3430. doi: 10.1096/fj.07-8379rev. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, et al. α-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemani VM, et al. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Südhof TC. α-synuclein cooperates with CSPα in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Materials and methods are available as supporting material on Science Online.
- 17.Chandra S, Chen X, Rizo J, Jahn R, Südhof TC. A broken α-helix in folded α-synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 18.García-Junco-Clemente P, et al. Cysteine string protein-alpha prevents activity-dependent degeneration in GABAergic synapses. J Neurosci. 2010;30:7377–7391. doi: 10.1523/JNEUROSCI.0924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, et al. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abeliovich A, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 21.Chandra S, et al. Double-knockout mice for γ- and β-synucleins: effect on synaptic functions. Proc Nat Acad U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninkina N, et al. Neurons expressing the highest levels of γ-synuclein are unaffected by targeted inactivation of the gene. Mol Cell Biol. 2003;23:8233–8245. doi: 10.1128/MCB.23.22.8233-8245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spillantini MG, et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 24.Takeda A, et al. Abnormal accumulation of NACP/α-synuclein in neurodegenerative disorders. Am J Pathol. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- 25.Kahle PJ, et al. Selective insolubility of α-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol. 2001;159:2215–2225. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CW, et al. A precipitating role for truncated α-synuclein and the proteasome in α-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 27.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.