Abstract
Trans-SNARE complexes catalyze fast synaptic vesicle fusion and bind complexin, but the function of complexin binding to SNARE complexes remains unclear. Here we show that in neuronal synapses, complexin simultaneously suppressed spontaneous fusion and activated fast Ca2+-evoked fusion. The dual function of complexin required SNARE binding, and additionally involved distinct N-terminal sequences of complexin that localize to the point where trans-SNARE complexes insert into the fusing membranes, suggesting that complexin controls the force that trans-SNARE complexes apply onto the fusing membranes. Consistent with this hypothesis, a mutation in the membrane insertion sequence of the v-SNARE synaptobrevin/VAMP phenocopied the complexin loss-of-function state without impairing complexin-binding to SNARE complexes. Thus, complexin probably activates and clamps the force-transfer from assembled trans-SNARE complexes onto fusing membranes.
Synaptic vesicle fusion is driven by assembly of trans-SNARE complexes (or SNAREpins) from syntaxin-1 and SNAP-25 on the plasma membrane, and synaptobrevin/ VAMP on the vesicle membrane [1-3]. Ca2+ then triggers fast synchronous synaptic vesicle fusion by binding to the Ca2+-sensor synaptotagmin [4-6]. Besides SNARE proteins and synaptotagmin, fast Ca2+-triggered fusion requires complexin [7]. Complexin is composed of short N-and C-terminal sequences and two central α-helices. Complexin binds to SNARE complexes via its central α-helix, which inserts in an anti-parallel orientation into a groove formed by synaptobrevin/VAMP and syntaxin-1 [8,9]. Although multiple approaches have revealed an essential role of complexin in synaptic fusion [7,10-15], the nature of this role remains unclear. In vertebrate autapses, deletion of complexin selectively impairs fast synchronous neurotransmitter release without changing asynchronous or spontaneous release [7,10]. In in vitro fusion assays, conversely, addition of complexin causes a general block of SNARE-dependent fusion, indicating that complexin is a SNARE clamp [11-14]. In Drosophila neuromuscular synapses, deletion of complexin produces a >20-fold increase in spontaneous release but only a small decrease in evoked release [15]. Thus, the role of complexin in fusion is unclear. Moreover, even the importance of complexin SNARE-complex binding remains uncertain [16,17]. Here we addressed these questions with two complementary approaches – RNAi-dependent knockdown of complexin with rescue, and replacing wild-type synaptobrevin with specific mutants using synaptobrevin knockout (KO) mice [18].
We knocked down complexin expression in cultured cortical neurons using an shRNA that targets both complexin-1 and -2, the only complexin isoforms significantly expressed in these neurons [19]. For this purpose, we used lentiviruses that simultaneously synthesize the complexin shRNA and either GFP, wild-type complexin-1, or 4M-mutant complexin-1 that is unable to bind to SNARE complexes (19,20). Lentivirus expressing GFP without the shRNA served a further control. This design enabled us to simultaneously analyze control neurons and complexin knock-down neurons expressing either GFP, wild-type complexin (as a rescue control), or 4M-mutant complexin. The shRNA strongly inhibits expression of complexin-1 and -2, whereas expression of other proteins, including SNARE proteins, was unchanged, and synapse numbers were unaltered (Figs. S1 and S2).
The complexin knock-down increased 3-4 fold the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs), without altering the mEPSC amplitude (Fig. 1A). In contrast, the complexin knock-down decreased 3-4 fold the amplitude of excitatory postsynaptic currents (EPSCs) evoked by isolated action potentials (Fig. 1B). Evoked EPSCs could not be restored by increasing the extracellular Ca2+-concentration, identifying complexin as a central component of the Ca2+-triggering machinery (Fig. S3). Both changes were rescued by wild-type but not by 4M-mutant complexin, confirming the specificity of the shRNA and the importance of SNARE binding. Finally, the complexin knock-down decreased the initial EPSCs during a 10 Hz stimulus train, but did not alter the total synaptic charge transfer evoked by the train, and even increased the delayed EPSCs following the train (Fig. S4). Thus, the complexin knock-down greatly impaired fast synchronous but not asynchronous synaptic vesicle fusion, and increased spontaneous fusion, thereby reconciling the divergent phenotypes observed in vertebrate autapses, Drosophila neuromuscular junctions, and in vitro fusion assays [7,10-15].
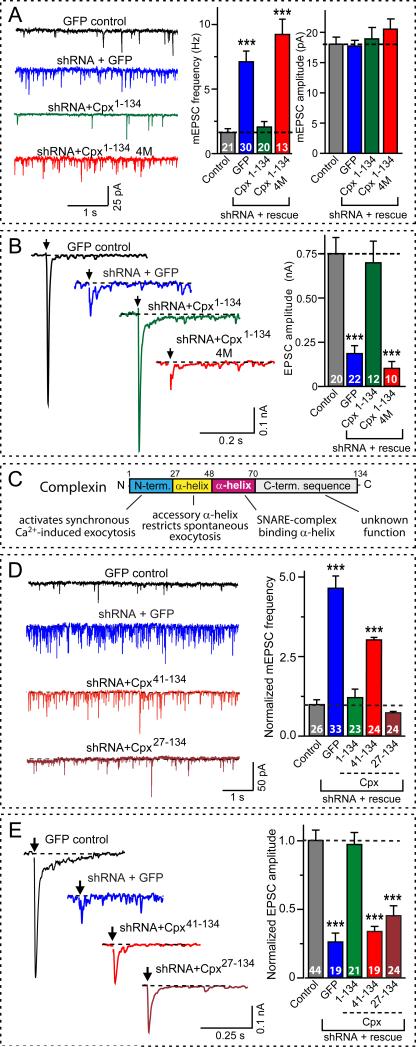
Figure 1. Complexin knock-down increases spontaneous fusion but suppresses fast Ca2+-evoked fusion.
A, Spontaneous fusion monitored as mEPSCs (left, representative traces; center and right, summary graphs of mEPSC frequencies and amplitudes, respectively). Recordings are from wild-type mouse neurons infected with with lentiviruses expressing GFP only (control), or an shRNA that suppresses both complexin-1 and -2, and additionally expresses either GFP, wild-type rat complexin-1 (Cpx1-134), or mutant rat complexin-1 with inactivated SNARE-binding sites (Cpx1-134 4M). For protein and synapse quantitations,see Figs. S1 and S2.
B, Ca2+-evoked fusion monitored as EPSCs triggered by isolated action potentials at 0.1 Hz (left = representative traces; right = mean amplitudes). Neurons were infected with lentiviruses as described in panel A. For responses elicited by 10 Hz stimulus trains, see Fig. S3.
C, Domain structure of complexin and assignment of domains based on the rescue analysis shown in panels D and E.
D and E, Complexin sequences required for rescue of the dual complexin loss-of-function phenotype: the increase in mEPSCs (D) and the decrease in EPSCs (E). Left, representative traces; right, summary graphs of mEPSC frequency (D) and EPSC amplitude (E), both normalized to control. Recordings were from mouse neurons infected with a lentiviruses expressing GFP only (control), or the complexin shRNA and either GFP or the indicated rat complexin-1 fragments.
Scale bars apply to all traces in a group. Summary graphs depict means±SEMs (see Table S1 for numerical electrophysiology data). Statistical significance was evaluated by ANOVA in comparison to control neurons (*** = p<0.001); total number of analyzed neurons in 4-6 independent cultures are shown in the bars.
We next examined which complexin sequences mediate spontaneous and evoked fusion (Fig. 1C). An N-terminal deletion of 40 residues (Cpx41-134) abolished all complexin function (Figs. 1D and 1E). This abolishment was not due to an impairment in SNARE-complex binding in competition with synaptotagmin because a complexin fragment containing only its central α-helical SNARE-binding sequence (Cpx41-86) still bound to SNARE complexes, and potently inhibited synaptotagmin-binding to SNARE complexes (Fig. S5). Thus, SNARE-complex binding in competition with synaptotagmin is necessary but not sufficient for complexin function, which also requires its N-terminal sequence. Additional dissection of this N-terminal sequence revealed that the non-structured N-terminal complexin sequence is essential for activating fast fusion, but not for clamping spontaneous fusion (Figs. 1D and 1E), indicating distinct sequence requirements for the dual activating/clamping functions of complexin.
To further analyze complexin function, we used synaptobrevin-deficient neurons that lack both spontaneous and evoked synaptic fusion [18]. Expression of wild-type synaptobrevin rescued the loss of fusion in synaptobrevin-deficient neurons. In contrast, 3A-mutant synaptobrevin that forms SNARE-complexes normally, but cannot support complexin binding to these SNARE complexes (Figs. S6 and S7), not only rescued spontaneous fusion, but increased it >2-fold above wild-type levels (Figs. 2A-2D). At the same time, 3A-mutant synaptobrevin decreased evoked fusion, and decelerated and desynchronized its timecourse (Figs. 2E-2G, S8 and S9). Moreover, although fusion elicited by a 10 Hz stimulus train was initially decreased in synapses expressing 3A-mutant synaptobrevin, fusion quickly recovered, such that the total synaptic charge transfer evoked by the stimulus train was normal, and the amount of delayed fusion after the stimulus train was enhanced (Figs. 2H-2J and S10). A second synaptobrevin mutation (the 6A-mutation) that impaired both SNARE-complex formation and complexin binding to SNARE complexes did not rescue the decrease in evoked release during either isolated action potentials or the stimulus train (Fig. 2). Thus, the block of complexin binding by the 3A-mutation caused a selective loss of synchronous fast fusion, different from the impairment of all fusion caused by inhibition of SNARE-complex assembly produced by the 6A-mutation.
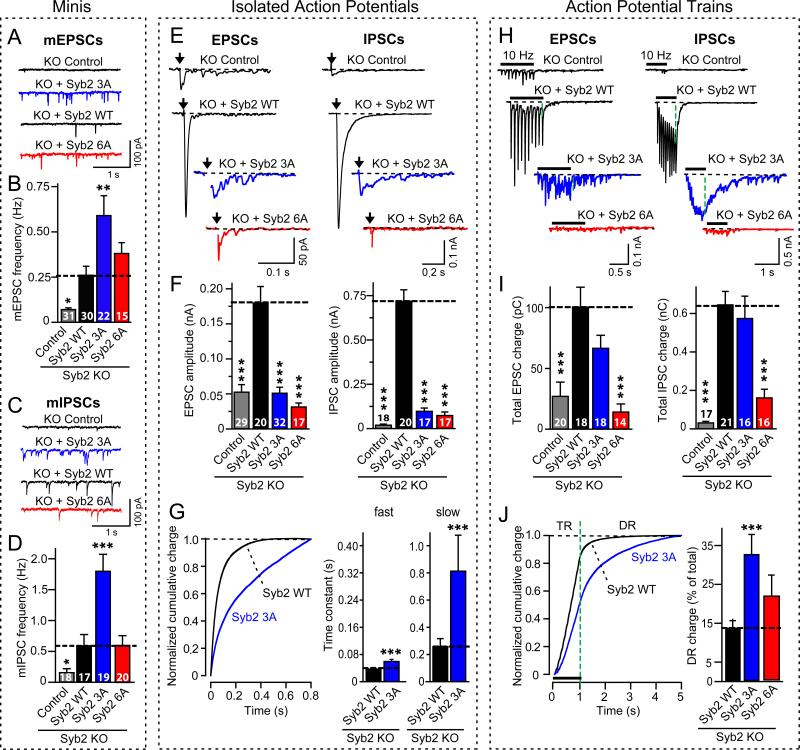
Figure 2. Blocking complexin-binding to SNARE complexes increases spontaneous fusion but suppresses fast Ca2+-evoked fusion.
Synaptobrevin-2 KO neurons were infected with lentiviruses expressing GFP only (control), or wild-type (Syb2 WT), 3A-mutant (Syb2 3A), or 6A-mutant synaptobrevin-2 (Syb2 6A). The 3A-mutation in synaptobrevin selectively blocks complexin-binding to SNARE complexes, whereas the 6A-mutation additionally impairs SNARE-complex assembly (Figs. S6 and S7).
A-D, Representative traces (A, C) and frequencies (B, D) of spontaneous mEPSCs (A,B) and mIPSCs (C,D). For mini amplitudes, see Fig. S8.
E and F, Representative traces (E) and mean amplitudes (F) of EPSCs (left) and IPSCs (right) evoked by isolated action potentials at 0.1 Hz.
G, Timecourse of isolated IPSCs monitored in synaptobrevin-2 KO neurons expressing wild-type (Syb2 WT) or 3A-mutant synaptobrevin-2 (Syb2 3A). The timecourse was analyzed as the cumulative normalized charge transfer (left), and fitted to a two-exponential equation yielding time constants of fast and slow release phases (right; see Fig. S9 illustrating scaled superimposed IPSC traces).
H and I, Representative traces (H) or mean synaptic charge transfer (I) of EPSCs (left) and IPSCs (right) evoked by 10 Hz action potential trains (see Fig. S10 for quantitations of charge transfers).
J, Timecourse of the cumulative IPSC charge transfer during the 10 Hz stimulus train. Left, plots of the cumulative normalized charge transfer allow quantitation of train release (TR) and delayed release (DR; shown only for neurons expressing wild-type or 3A-mutant synaptobrevin-2); right, bar diagram of the contribution of delayed release to the total synaptic charge transfer in neurons expressing wild-type (Syb2 WT), 3A-mutant (Syb3 3A), and 6A-mutant synaptobrevin-2 (Syb2 6A).
All scale bars apply to all traces in a series; all bar diagrams depict means±SEMs; statistical significance was evaluated by ANOVA in comparison to wild-type synaptobrevin-2: *** = p<0.001; total number of analyzed neurons in 5-6 independent cultures are shown in the summary bars).
The location of the N-terminal sequence of complexin at the point where SNARE complexes insert into the membrane suggests that the N-terminal complexin sequence may control the coupling of SNARE-complex assembly to membrane fusion. If so, complexin may act on SNARE sequences close to the membrane. We tested this hypothesis by mutating the juxta-membranous sequence of synaptobrevin/VAMP in three sets of alanine substitutions: K85A/R86A, R86A/K87A, and W89A/W90A (referred to as the 85-, 86-, and WA-mutation; Figs. S6 and S7).
The WA-mutation had no effect on SNARE-complex stability, complexin- or synaptotagmin-binding to SNARE complexes, or synaptobrevin targeting to synapses (Figs. S6, S7, and S11), but caused a 2-3 fold increase in mini frequency without a change in mini amplitude (Figs. 3A-3D and S12). Moreover, the WA-mutation produced a ~2-fold decrease in fast evoked fusion, with the remaining fusion being largely asynchronous because its kinetics was decelerated 2-3 fold (Figs. 3E-3G and S7). Again, as observed for complexin knock-down neurons and neurons expressing 3A-mutant synaptobrevin that lacked complexin binding, synaptic vesicle fusion induced by a 10 Hz stimulus train was only impaired during the initial synchronous responses. Later responses during the train's asynchronous phase were normal, and delayed release was enhanced (Figs. 3H-3J and S13), thus rendering the WA-mutation a weaker phenocopy of the synaptotagmin-1 KO, the complexin KO, the complexin knockdown, and the synaptobrevin 3A-mutation phenotype (7,15,21; Figs. 1-3). In contrast to the WA-mutation, the 85- and 86-mutations did not impair rescue of synaptic transmission by synaptobrevin in synaptobrevin-deficient neurons (Fig. S14), thus confirming the specificity of the WA-rescue phenotype.
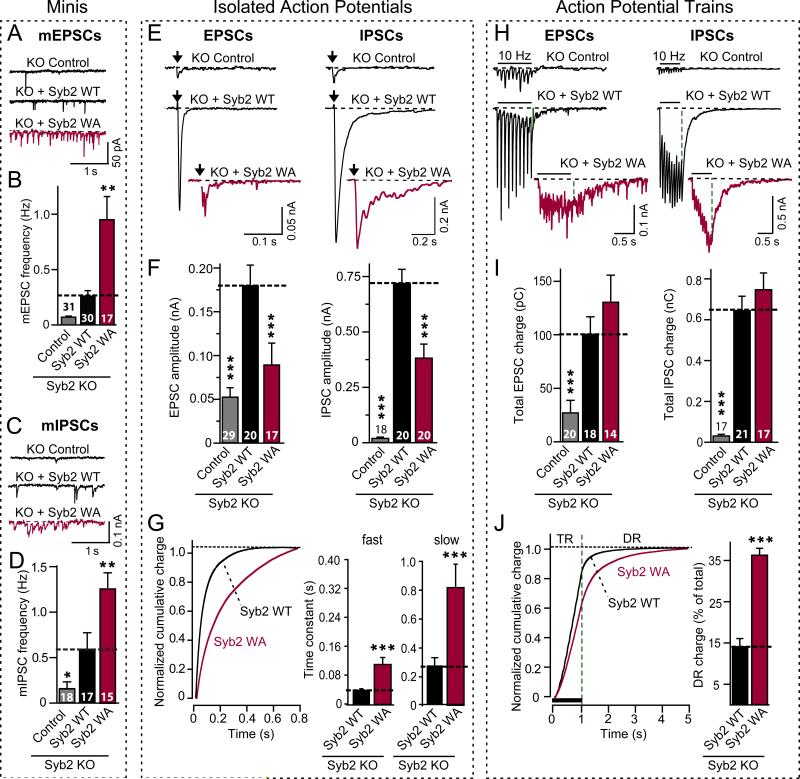
Figure 3. A mutation in the membrane-insertion sequence of synaptobrevin (WA-mutation) phenocopies the complexin knock-down.
Recordinges were performed in synaptobrevin-2 KO neurons expressing either GFP only (control), wild-type (Syb2 WT), or WA-mutant (Syb2 WA; see Figs. S6 and S7). A-D, Representative traces (A, C) and mean frequencies (B, D) of spontaneous mEPSCs (A,B) and mIPSCs (C,D). For synaptic targeting of WA-mutant synaptobrevin-2, see Fig. S11; for mini amplitudes, Fig. S12.
E and F, Representative traces (E) and mean amplitudes (F) of EPSCs (left) and IPSCs (right) evoked by isolated action potentials at 0.1 Hz.
G, Timecourse of isolated IPSCs, analyzed as cumulative normalized charge transfer (left), and fitted to a two-exponential equation yielding time constants of fast and slow phases of release (right; see Fig. S9 for scaled superimposed IPSC traces).
H and I, Representative traces (H) and mean synaptic charge transfer (I) of EPSCs (left) and IPSCs (right) evoked by 10 Hz action potential trains (see. Fig. S14 for quantitations of charge transfers).
J, Timecourse of the cumulative IPSC charge transfer during the 10 Hz stimulus train, analyzed as the cumulative normalized charge transfer (left; train release = TR; delayed release = DR) and illustrated as the fraction of delayed release of the total synaptic charge transfer (right).
All scale bars apply to all traces in a series; all bar diagrams depict means±SEMs; statistical significance was evaluated by ANOVA in comparison to wild-type synaptobrevin-2: *** = p<0.001; total number of analyzed neurons in 5-6 independent cultures are shown in the bars in B, D, F, and I).
Here we have shown that in central synapses, complexin acted both as a clamp and as an activator of SNAREs. These functions required complexin binding to SNARE-complexes, and depended on distinct N-terminal complexin sequences that localize to the point where trans-SNARE complexes insert into the two fusing membranes. A mutation in the juxta-membranous sequence of synaptobrevin phenocopies the complexin loss-of-function phenotype. Thus, the simultaneous control of spontaneous and evoked fusion by complexin appears to involve the translation of the force generated by assembly of trans-SNARE complexes onto the two fusing membranes (Fig. 4), consistent with biochemical data (22). We postulate that after complexin bound to assembling SNARE complexes, its N-terminal sequence activates and clamps the force generated by SNARE-complex assembly. The very N-terminus of complexin might perform its activator function by pulling the complex closer to the membrane, possibly by binding to phospholipids, whereas the accessory N-terminal α-helix might clamp the complex by inserting into the space between the v- and t-SNAREs, or even substituting for one of the SNAREs in the C-terminal segment of the trans-SNARE complex (23). Once anchored on the SNARE complex, the N-terminal 40 residues of complexin both activate and clamp SNARE complexes to control fast Ca2+-triggered neurotransmitter release in a process that is conserved in all animals. Viewed in the broader picture, complexin and synaptotagmin therefore operate as interdependent clamp-activators of SNARE-dependent fusion, with synaptotagmin exploiting the activator effect of complexin and reversing its clamping function [11,20,21]. In this molecular pas-de-deux, the functions of both proteins are intimately linked: their phenotypes are identical both as activators and as clamps, and one does not operate without the other.
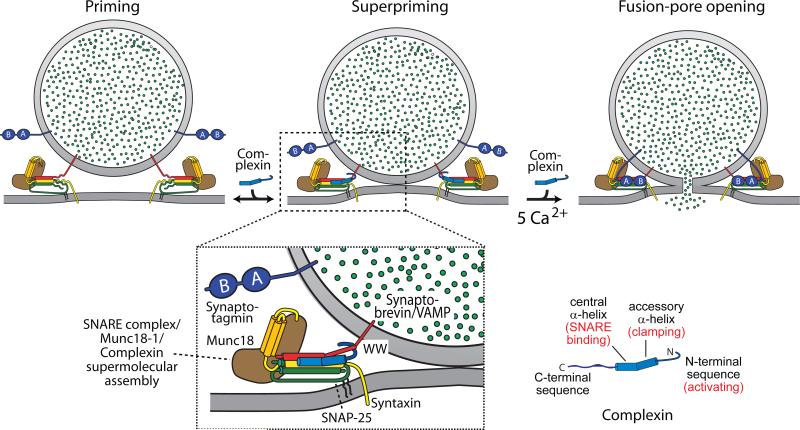
Figure 4. Complexin action in SNARE-dependent fusion.
During fusion a vesicle goes through three stages: priming, superpriming, and fusion pore opening (top panels). Complexin is proposed to suppress spontaneous fusion by inserting into the assembling trans-SNARE complex, and to activate evoked fusion by directly or indirectly interacting with the membrane-insertion sequence of SNARE proteins in the trans-complex (bottom panel). Complexin binding to trans-SNARE complexes simultaneously stabilizes SNARE complex assembly, blocks completion of assembly, and inhibits the transfer of the force generated by SNARE-complex assembly onto the fusing membranes. Synaptotagmin subsequently triggers fusion by reversing the complexin block on activated SNARE complexes in addition to its Ca2+-dependent phospholipid-binding activity.
Supplementary Material
Acknowledgments
We thank J. Rizo and L. Chen for advice and critical comments. This study was supported by an investigatorship to T.C.S. from the Howard Hughes Medical Institute.
REFERENCES and NOTES
- 1.Sørensen JB. Trends Neurosci. 2005;28:453. doi: 10.1016/j.tins.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Scheller RH. Nat. Rev. Mol. Cell. Biol. 2006;7:631. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 3.Verhage M, Toonen RF. Curr. Opin. Cell Biol. 2007;19:402. doi: 10.1016/j.ceb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Geppert M, et al. Cell. 1994;79:717. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Chacon R, et al. Nature. 2001;410:41. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 6.Yoshihara M, Littleton JT. Neuron. 2002;36:897. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- 7.Reim K, et al. Cell. 2001;104:71. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, et al. Neuron. 2002;33:397. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 9.Bracher A, Kadlec J, Betz H, Weissenhorn W. J. Biol. Chem. 2002;277:26517. doi: 10.1074/jbc.M203460200. [DOI] [PubMed] [Google Scholar]
- 10.Xue M, et al. Nat. Struct. Mol .Biol. 2007;14:949. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraudo CG, Eng WS, Melia TJ, Rothman JE. Science. 2006;313:676. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 12.Giraudo CG, et al. J. Biol. Chem. 2008;283:21211. doi: 10.1074/jbc.M803478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Nat. Struct. Mol. Biol. 2006;13:748. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 14.Yoon TY, et al. Nat Struct Mol Biol. 2008;15:707. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntwork S, Littleton JT. Nat. Neurosci. 2007;10:1235. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 16.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Structure. 2008;16:308. doi: 10.1016/j.str.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan R, Dai H, Rizo J. Biochemistry. 2008;47:1474. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- 18.Schoch S, et al. Science. 2001;294:1117. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 19.For a description of the experimental procedures, see Supplementary Online Materials.
- 20.Tang J, et al. Cell. 2006;126:1175. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Maximov A, Südhof TC. Neuron. 2005;48:547. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Hu K, Carroll J, Rickman C, Davletov B. J. Biol. Chem. 2002;277:41652. doi: 10.1074/jbc.M205044200. [DOI] [PubMed] [Google Scholar]
- 23.Giraudo CG, et al. Science. 2009 this issue. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.