Abstract
The last two decades have seen a paradigm shift in the understanding of ocular toxoplasmosis. Post-natally acquired infection with its atypical presentations, has emerged as a common form of the disease. We conducted a questionnaire-based survey to investigate the characteristics of atypical presentations and current treatment practices of ocular toxoplasmosis, in India. A written questionnaire was distributed to ophthalmologists at two major uveitis meetings, held in Hyderabad, India in January, 2009. It evaluated characteristics of atypical presentations of ocular toxoplasmosis and specific treatment-related issues in India. Of 37 respondents who completed the questionnaire, 28 (75.6%) found atypical presentations in less than one-fourth of ocular toxoplasmosis patients. Atypical presentations were mostly seen as primary retinitis lesion, and in healthy immuno-competent individuals. Most ophthalmologists (n = 28, 75.6%) thought viral retinitis to be the most common differential diagnosis for atypical ocular toxoplasmosis and relied on serological tests (n = 19, 51.3%) for the diagnosis. Twenty-three (62.1%) respondents treated all patients with active lesions. A diverse range of treatment regimens were used, trimethoprim-sulphamethoxazole combination being most common (n = 12, 32.4%). Corticosteroids were included in all regimens. Atypical presentations of ocular toxoplasmosis were identified by all ophthalmologists, participating in the survey, though not commonly by most. Treatment practices were diverse, reflecting the lack of consensus on this issue.
Keywords: Atypical, Ocular, Survey, Toxoplasmosis, Treatment
Introduction
Ocular toxoplasmosis (OT) is one of the most common manifestations of human toxoplasmosis, worldwide (Holland 2003). OT has often been associated with the typical lesion, defined as unilateral focal retinochoroiditis at the border of a pigmented scar with overlying vitritis (Smith and Cunningham 2002; Holland 2004). Such lesions have traditionally been associated with late manifestation and reactivation of congenital disease (Montoya and Liesenfeld 2004). A 2005 survey of ophthalmologists in the United States indicated nearly half of the respondents (227 of 459) thought congenital infection as the most common cause of OT in clinical practice (Lum et al. 2005). This has changed in last two decades. There is now clear evidence that post-natally acquired infection commonly causes ocular disease, and is even more prevalent than congenital OT (Holland 1999; Gilbert and Stanford 2000).
Since post-natally acquired OT need not be associated with a retinochoroidal scar (Smith, 2002), it can gives rise to a variety of ‘atypical’ lesions. Hence, we suspect that a large pool of OT lesions would belong to the ‘atypical’ category. Such lesions may be confused with other infectious and non-infectious conditions and need additional tests for accurate diagnosis, unlike the typical lesions (Fardeau et al. 2002; Mahalakshmi et al. 2006; Talabani et al. 2009).
Previous surveys on practice patterns in OT have been directed at management practices, number of patient visits and the ophthalmologist’s knowledge about the disease (Engstrom et al. 1991; Holland and Lewis 2002; Lum et al. 2005; Torun et al. 2008). They have not addressed the prevalence, characteristics and diagnosis of the atypical forms of OT in clinical practice. The overall sero-prevalence of anti-Toxoplasma gondii antibodies (IgG) in India (24.3%) (Dhumne et al. 2007), is similar to that in the United States (22.5%) (Jones et al. 2001), though significantly lower than other parts of the world (Silveira et al. 1988). However, there are no studies on the clinical presentations and treatment practices for OT, in India. Our survey, the first of its kind in the country, bridges the above gaps by focusing on the characteristics of atypical presentations and the various treatment practices for OT, in India.
Materials and methods
A written questionnaire (Table 1) was distributed to ophthalmologists at two meetings—a ‘special interest group’ meeting on OT at the Asia ARVO (Association for Research in Vision and Ophthalmology) and the annual meeting of the Uveitis Society of India—both held in Hyderabad, India on consecutive days in January, 2009. These meetings provided a captive survey group that was likely to have specific interest in OT, and also handled a significant number of OT cases. A given ophthalmologist could answer the questionnaire only once, in the two meetings. Respondents from countries other than India were excluded for this study. The study methodology was similar to previous surveys on practice patterns in OT that had been conducted in United States and Germany (Engstrom et al. 1991; Holland and Lewis 2002; Lum et al. 2005; Torun et al. 2008). There were 11 questions in all. Questions 1 and 2 evaluated the case load of uveitis and OT respectively, in the ophthalmologist’s practice. Questions 3–7 addressed the prevalence and clinical characteristics of atypical forms of OT. Typical OT was defined as unilateral focal retinochoroiditis at the border of a pigmented scar with overlying vitritis (Smith and Cunningham 2002; Holland 2004). Questions 8–11 dealt with specific issues, related to management of OT. Responses to all questions were sought in a multiple-choice format. An option of ‘others’ was given for select questions to allow for unique answers to those questions. As in a previous survey (Holland and Lewis 2002), ‘frequent treaters’ were the ophthalmologists treating more than 20 new OT patients in a year.
Table 1.
Results from questions on clinical case load, atypical presentations and treatment practices of ocular toxoplasmosis, in India
| Question | All respondents (n = 37) | Frequent treaters (n = 17) |
|---|---|---|
| How many new patients of uveitis are seen in your clinic in a month? | ||
| <20 | 11 (29.7%) | 0 (0%) |
| 20–50 | 21 (56.7%) | 12 (70.5%) |
| 51–100 | 4 (10.8%) | 4 (23.5%) |
| >100 | 1 (2.7%) | 1 (5.8%) |
| How many new patients with Ocular Toxoplasmosis are seen in your clinic in a year? | ||
| <5 | 5 (13.5%) | – |
| 5–20 | 15 (40.5%) | – |
| 21–50 | 17 (45.9%) | 17 (100%) |
| >50 | 0 (0%) | 0 (0%) |
| What is the percentage of ‘atypical’ presentations in your patients with Ocular Toxoplasmosis? (‘Typical’ would mean unilateral focal retinochoroiditis at the border of a pigmented scar with overlying vitritis) | ||
| <25% | 28 (75.6%) | 10 (58.8%) |
| 25–50% | 5 (13.5%) | 3 (17.6%) |
| 50–75% | 1 (2.7%) | 1 (5.8%) |
| >75% | 3 (8.1%) | 3 (17.6%) |
| Which is the most common form of atypical presentation, seen by you? | ||
| Full-thickness retino-choroiditis not associated with pigmented scar (primary lesion) | 30 (81.1%) | 13 (76.4%) |
| Punctate outer retinal toxoplasmosis | 0 (0%) | 0 (0%) |
| Neuro-retinitis | 7 (18.9%) | 4 (23.5%) |
| Other than above | 0 (0%) | 0 (0%) |
| What is the most common profile in your patients with atypical presentations? | ||
| Healthy, immuno-competent | 24 (64.8%) | 10 (58.8%) |
| Elderly | 4 (10.8%) | 3 (17.6%) |
| HIV | 8 (21.6%) | 4 (23.5%) |
| Non-HIV immuno-compromised | 1 (2.7%) | 0 (0%) |
| Which of the following diseases are most likely to cause diagnostic dilemma in patients with atypical presentations of Ocular Toxoplasmosis? | ||
| Viral retinitis | 28 (75.6%) | 12 (70.5%) |
| Ocular tuberculosis | 9 (24.3%) | 5 (29.4%) |
| Non-infectious uveitis | 0 (0%) | 0 (0%) |
| Others | 0 (0%) | 0 (0%) |
| What is your basis for diagnosis of Ocular Toxoplasmosis in patients with atypical presentations? | ||
| Clinical diagnosis | 7 (18.9%) | 7 (41.1%) |
| Polymerase chain reaction (PCR) | 9 (24.3%) | 4 (23.5%) |
| Serological tests (serum) | 19 (51.3%) | 4 (23.5%) |
| PCR and Goldman-Witmer co-efficient | 2 (5.4%) | 2 (11.7%) |
| What are your criteria for treatment of patients with Ocular Toxoplasmosis? | ||
| Treat all active lesions | 23 (62.1%) | 17 (100%) |
| Lesions threatening optic nerve/fovea/blood vessels | 0 (0%) | 0 (0%) |
| Significant drop in visual acuity | 0 (0%) | 0 (0%) |
| Lesions threatening optic nerve/fovea/blood vessels or significant drop in visual acuity | 14 (37.8%) | 0 (0%) |
| Which is your preferred anti-toxoplasma regimen? | ||
| Pyrimethamine + Sulphadiazine | 3 (8.1%) | 2 (11.7%) |
| Trimethoprim+Sulphamethoxazole | 12 (32.4%) | 7 (41.1%) |
| Pyrimethamine+Sulphadiazine+Clindamycin | 11 (29.7%) | 1 (5.8%) |
| Trimethoprim+Sulphamethoxazole+Clindamycin | 6 (16.2%) | 4 (23.5%) |
| Clindamycin | 2 (5.4%) | 1 (5.8%) |
| Azithromycin | 2 (5.4%) | 1 (5.8%) |
| Clindamycin+Azithromycin | 1 (2.7%) | 1 (5.8%) |
| What are your criteria for beginning corticosteroid therapy in patients with Ocular Toxoplasmosis? | ||
| At the time of starting antimicrobial therapy | 2 (5.4%) | 2 (11.7%) |
| After 48 h of starting antimicrobial therapy | 26 (70.2%) | 15 (88.2%) |
| Only after verifying clinical response to starting antimicrobial therapy (could be >48 h) | 9 (24.3%) | 0 (0%) |
| Other | 0 | 0 |
| Do not use corticosteroids | 0 | 0 |
| What are your criteria for stopping corticosteroid therapy in patients with Ocular Toxoplasmosis? | ||
| Continue anti-microbial therapy after ending corticosteroid therapy | 21 (56.7%) | 7 (41.1%) |
| Stop anti-microbial therapy, once corticosteroid dose reach lower ranges (e.g. <10 mg/day) | 12 (32.4%) | 8 (47.05%) |
| Stop anti-microbial and corticosteroid therapy simultaneously | 4 (10.8%) | 2 (11.7%) |
| Others | 0 (0%) | 0 (0%) |
Percentages mentioned in the table refer to the respondents, and not the distribution of lesion types or risk groups. Values in bold represent the most common response for the given question
Frequent treaters: Physicians treating more than 20 new patients with ocular toxoplasmosis, in a year
Survey results
A complete response was obtained from 37 ophthalmologists, practicing in India, 17 (45.9%) of who were identified as ‘frequent treaters’. The details are summarized in Table 1. Of the 37 respondents, 26 (70.3%) treated more than 20 new uveitis patients in a month. The average duration of practice, post-specialization was 8.6 years (median 12 years; range 3–40 years). Twenty of 37 respondents belonged to the southern states of India, seven to west, six to north and four to east and north-east. The details of the responses are provided in Table 1.
Atypical presentations
Atypical presentations of OT were identified in clinical practice by all ophthalmologists participating in the survey. Most respondents (n = 28, 75.6%) found atypical presentations in less than one-fourth of their patients with OT. Only 3 respondents found atypical presentations in more than 75% of their patients—all belonged to the ‘frequent treaters’ group. The most common form of atypical presentation, as seen by majority of respondents, was a primary lesion i.e. full-thickness retino-choroiditis not associated with pigmented scar (n = 30, 81.1%). Atypical presentations were seen in healthy, immunocompetent adults by most ophthalmologists (n = 24, 64.8%), as against HIV-infected (n = 8, 21.6%) or elderly individuals (n = 4, 10.8%). The most likely differential diagnosis for these presentations was identified as viral retinitis by majority of respondents (n = 28, 75.6%). Ocular tuberculosis (n = 9, 24.3%) was the next likely differential diagnosis. Most ophthalmologists (n = 19, 51.3%) relied on serological tests for diagnosis of OT in patients with atypical presentations. Only two respondents—both ‘frequent treaters’—used the Goldman–Witmer co-efficient for this purpose.
Treatment practices
Majority of respondents (n = 23, 62%) treated all patients with active lesions. The remaining treated only vision-threatening lesions. There was a great diversity in the choice of anti-toxoplasma treatment, both in the combined and the ‘frequent treaters’ groups. The two most common regimens were—a combination of trimethoprim and sulphamethoxazole (n = 12, 32.4%), and a combination of pyrimethamine, sulphadiazine and clindamycin (n = 11, 29.7%). All respondents used concurrent corticosteroid therapy. Most (n = 26, 70.3%) began corticosteroid therapy after 48 h of antimicrobial therapy and continued anti-microbial therapy after ending corticosteroid therapy (n = 21, 56.7%).
Discussion
The current survey focused on two key issues of OT in ophthalmic practice—atypical presentations and treatment practices, in India. To the best of our knowledge, it is the first such report from Asia (Medline search; key words: ocular toxoplasmosis, survey, clinical features, treatment), as all previous surveys on OT were from the Western Hemisphere (Engstrom et al. 1991; Holland and Lewis 2002; Lum et al. 2005; Torun et al. 2008). The respondents had reasonable clinical experience (average 8.6 years, post-specialization) and most (n = 26, 70.3%) had a significant case load of uveitis (>20 new patients per month).
Our interest in atypical presentations of OT was for two reasons—its association with post-natally acquired infection and the difficulty in differentiating these lesions, in the absence of sophisticated diagnostic tools, from other infectious and non-infectious conditions. The role of post-natally acquired infection in OT has gained prominence in the last two decades (Holland 1999). It was calculated that two-thirds of OT lesions in the United Kingdom were probably caused by post-natally acquired infection (Gilbert and Stanford 2000). In a recent report from France, acquired infections (23.5%) were found to be a more common cause of OT, than congenital infections (14.6%), though the origin of infection could not be determined in the majority (61.9%) of patients (Delair et al. 2008).
Post-natally acquired toxoplasmosis characteristically presents as focal chorioretinitis without associated old scars, particularly in early stage of systemic disease (Ronday et al. 1995; Montoya and Remington 1996). In the report from France, 87 (20.5%) of 425 eyes with OT had active chorioretinitis without scar(s) (Delair et al. 2008). Of these, 29 (33.3%) had serologically proven acquired infection, while 54 (62.1%) had infection of unknown origin. The clinical spectrum of atypical presentations of OT, in addition to primary retinitis lesions, also includes neuroretinitinis, primary outer retinal toxoplasmosis, retinal vasculitis, retinal and sub-retinal vascularisation and pigmentary retinopathy (Smith and Cunningham 2002). Such lesions, when not accompanied by an old scar (as seen in typical OT) may have similarity with posterior uveitis syndromes seen in other infectious and non-infectious conditions, and therefore, result in diagnostic dilemma. In our survey, all respondents reported atypical presentations of OT in their clinical practice, albeit in less than one-fourth of their patients with OT. We speculate that there could be under-reporting, either due to lack of awareness or lack of appropriate diagnostic facilities. Interestingly, three respondents reported atypical presentations in more than 75% of their patients with OT. All three were ‘frequent treaters’, associated with a high volume tertiary care center, with facilities for polymerase chain reaction and intra-ocular antibody testing.
The most common atypical presentation reported in this survey was a full-thickness retinochoroiditis not associated with a pigmented scar (primary ocular toxoplasmosis). It was reported by 30 (81%) respondents, though 7 (19%) respondents found neuro-retinitis to be the most common atypical presentation. Since primary OT was the most commonly reported form of atypical presentation in our survey, expectedly these lesions were found by most respondents in healthy, immunocompetent individuals. There are several reports of primary OT in healthy, immuno-competent individuals with acquired T. gondii infection (Ronday et al. 1995; Montoya and Remington 1996; Ongkosuwito et al. 1999), though the aggressive forms have been seen in pre-disposed populations like the elderly (Johnson et al. 1997), and HIV-infected individuals (Holland et al. 1988).
As OT is a self-limiting disease, many of the atypical presentations of OT are possibly not diagnosed correctly, and recover even with inappropriate therapy. Therefore, the actual incidence of atypical OT lesions may be higher than that reported in our survey. Viral retinitis and ocular tuberculosis, both of which can cause focal chorio-retinal lesions, were reported the most likely differential diagnoses for atypical OT. To accurately diagnose OT in such a situation, additional investigations would be needed (Fardeau et al. 2002; Mahalakshmi et al. 2006; Talabani et al. 2009). These include detection of toxoplasma DNA in ocular fluids with polymerase chain reaction and intra-ocular antibody testing for the Goldman-Witmer co-efficient. The use of only serological tests in such cases by most respondents in our survey reflects the non-availability of higher diagnostic facilities in most places. The value of serological tests in the diagnosis of primary OT is limited. In one series, serologic evidence of recently acquired infection was not present in half the cases of primary OT (Ongkosuwito et al. 1999). The lack of appropriate diagnostic techniques could thus also be responsible for under-detection of atypical OT in clinical practice in India. We did not investigate the respective roles of IgG and IgM titre testing in our questionnaire.
Our survey also evaluated treatment practices amongst Indian ophthalmologists. 62% of respondents (n = 23), including all the ‘frequent treaters’, treated all active lesions, irrespective of associated findings. The remaining (38%, n = 14) had specific criteria for treatment. These included lesions threatening optic nerve, fovea or blood vessels or a significant reduction in visual acuity. Our survey did not evaluate each of these criteria separately. This is in contrast to the surveys from the United States, where only 15% of the uveitis specialists (Holland and Lewis 2002), and 19% of general ophthalmologists treated all patients regardless of ocular findings (Lum et al. 2005). A more recent survey of members of the German Uveitis Society revealed that 45% would treat all peripheral lesions (typical/atypical), where as 100% would treat all central lesions in immuno-competent patients (Torun et al. 2008). Current evidences suggest that treatment for acute toxoplasmic retinochoroiditis is of no proven benefit as none of the currently available drugs have any effect on the bradyzoite stage of the parasite (Stanford et al. 2003). Early conversion from the tachyzoite to bradyzoite stage prevents action of the drugs on the parasite and fails to reduce the risk of recurrences.
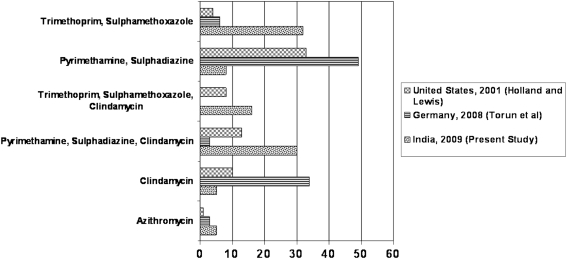
Six different anti-toxoplasmic drugs were used in seven different combinations by the respondents. The combination of trimethoprim and sulphamethoxazole (32.4%) was the most favored, and was marginally ahead of the combination of pyrimethamine, sulphadiazine and clindamycin (29.7%). Our finding differs from that in United States and Germany, where the combination of pyrimethamine and sulphadiazine was the most commonly used antibiotic regimen (Holland and Lewis 2002; Torun et al. 2008). Figure 1 compares the preferred antibiotic regimens in the present study to the reports from United States and Germany. Interestingly, the last survey from the United States on treatment practices mentioned a shift towards use of trimethoprim and sulphamethoxazole, increasing from 5 to 28%, between 1991 and 2001 (Holland and Lewis 2002). There are no convincing reports on the efficacy of various regimens. While Soheilian et al. (2005) reported no difference in the efficacies of trimethoprim/sulphamethoxazole versus pyrimethamine (25 mg, instead of traditional 50 mg) and sulphadiazine in terms of reduction in retinal lesion size and improvement in visual acuity, Rothova et al. (1993) had reported marginally superior performance of pyrimethamine in reducing the size of the retinal scar, but also had higher adverse events.
Fig. 1.
Bar chart comparing preferred antibiotic regimens in the present survey with previous surveys. Y-axis indicates the different antibiotic regimens used in the present survey, while X-axis indicates the percentage of respondents who preferred the given antibiotic regimen in different surveys
All respondents in our survey, used corticosteroids in their treatment regimens for OT. The use of corticosteroids in the treatment of OT has been debated (Bosch-Driessen and Rothova 1998). Given alone, it is known to cause fulminant ocular toxoplasmosis, and used as an adjunct to antiparasitic therapy, it has failed to alter the inflammatory activity of the disease (Rothova et al. 1993). However, it is generally accepted in clinical practice that corticosteroids should be a part of treatment regimens. In the 2005 survey from the United States, 80% of all respondents reported use of both anti-parasitic drugs and steroids in healthy, immunocompetent patients (Lum et al. 2005). The temporal association between anti-parasitic and corticosteroid therapy needs further exploration through controlled trials.
The present survey suffers from drawbacks typical of any survey-based study. The questionnaire could not be piloted and validated because of the small sample size. Most of the respondents belonged to the southern states of India, which could have skewed the results in that direction. Additionally, we could not investigate, the age distribution of atypical lesions, and whether treatment practices for atypical lesions differed from those for typical lesions Future surveys could look into such issues.
To summarize, though recognized universally, atypical presentations of OT are yet to have a significant presence in Indian clinical practice. Increased awareness about these presentations and better diagnostic facilities, might lead to more frequent diagnosis of OT in such cases. Treatment practices of OT are not uniform, though trimethoprim-sulphamethoxazole appears to be slightly more favored than other combinations.
Acknowledgments
Hyderabad Eye Research Foundation, Hyderabad.
References
- Bosch-Driessen EH, Rothova A. Sense and nonsense of corticosteroid administration in the treatment of ocular toxoplasmosis. Br J Ophthalmol. 1998;82:858–860. doi: 10.1136/bjo.82.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delair E, Monnet D, Grabar S, et al. Respective roles of congenital and acquired infections in presumed acquired ocular toxoplasmosis. Am J Ophthalmol. 2008;146:851–855. doi: 10.1016/j.ajo.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Dhumne M, Sengupta C, Kadival G, et al. National seroprevelance of Toxoplasma gondii in India. J Parasitol. 2007;93:1520–1521. doi: 10.1645/GE-1159.1. [DOI] [PubMed] [Google Scholar]
- Engstrom RE, Jr, Holland GN, Nussenblatt RB, et al. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 1991;111:601–610. doi: 10.1016/s0002-9394(14)73706-7. [DOI] [PubMed] [Google Scholar]
- Fardeau C, Romand S, Rao NA, et al. Diagnosis of toxoplasmic retinochoroiditis with atypical clinical features. Am J Ophthalmol. 2002;134:196–203. doi: 10.1016/S0002-9394(02)01500-3. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Stanford MR. Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol. 2000;84:224–226. doi: 10.1136/bjo.84.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland GN. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol. 1999;128:502–505. doi: 10.1016/S0002-9394(99)00263-9. [DOI] [PubMed] [Google Scholar]
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136:973–988. doi: 10.1016/j.ajo.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Holland GN. Ocular toxoplasmosis:a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004;137:1–17. [PubMed] [Google Scholar]
- Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 2002;134:102–114. doi: 10.1016/S0002-9394(02)01526-X. [DOI] [PubMed] [Google Scholar]
- Holland GN, Engstrom RE, Jr, Glasgow BJ, et al. Ocular toxoplasmosis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1988;106:653–667. doi: 10.1016/0002-9394(88)90697-6. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Greven GM, Jaffe GJ, et al. Atypical, severe toxoplasmic retinochoroiditis in elderly patients. Ophthalmology. 1997;104:48–57. doi: 10.1016/s0161-6420(97)30362-5. [DOI] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Wilson M, et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154:357–365. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- Lum F, Jones JL, Holland GN, et al. Survey of ophthalmologists about ocular toxoplasmosis. Am J Ophthalmol. 2005;140:724–726. doi: 10.1016/j.ajo.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Mahalakshmi B, Therese KL, Madhavan HN, et al. Diagnostic value of specific antibody production and nucleic acid amplification technique-nested polymerase chain reaction (nPCR) in clinically suspected ocular toxoplasmosis. Ocul Immunol Inflamm. 2006;14:105–112. doi: 10.1080/09273940500545692. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Remington JS. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin Infect Dis. 1996;23:277–282. doi: 10.1093/clinids/23.2.277. [DOI] [PubMed] [Google Scholar]
- Ongkosuwito JV, Bosch-Driessen EH, Kijlstra A, et al. Serologic evaluation of patients with primary and recurrent ocular toxoplasmosis for evidence of recent infection. Am J Ophthalmol. 1999;128:407–412. doi: 10.1016/S0002-9394(99)00266-4. [DOI] [PubMed] [Google Scholar]
- Ronday MJ, Luyendijk L, Baarsma GS, et al. Presumed ocular acquired toxoplasmosis. Arch Ophthalmol. 1995;113:1524–1529. doi: 10.1001/archopht.1995.01100120054009. [DOI] [PubMed] [Google Scholar]
- Rothova A, Meenken C, Buitenhuis HJ, et al. Therapy for ocular toxoplasmosis. Am J Ophthalmol. 1993;115:517–523. doi: 10.1016/s0002-9394(14)74456-3. [DOI] [PubMed] [Google Scholar]
- Silveira C, Belfort R, Jr, Burnier M, et al. Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am J Ophthalmol. 1988;106:362–364. doi: 10.1016/0002-9394(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Smith JR, Cunningham ET., Jr Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol. 2002;13:387–392. doi: 10.1097/00055735-200212000-00008. [DOI] [PubMed] [Google Scholar]
- Soheilian M, Sadoughi MM, Ghajarnia M, et al. Prospective randomized trial of trimethoprim/sulphamethoxazole versus pyrimethamine and sulphadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005;112:1876–1882. doi: 10.1016/j.ophtha.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Stanford MR, See SE, Jones LV, et al. Antibiotics for toxoplasmic retinochoroiditis: an evidence-based systematic review. Ophthalmology. 2003;110(5):926–931. doi: 10.1016/S0161-6420(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Talabani H, Asseraf M, Yera H, et al. Contributions of immunoblotting, real-time PCR and Goldman-Witmer co-efficient to the diagnosis of atypical toxoplasmic retinochoroiditis. J Clin Microbiol. 2009;47:2131–2135. doi: 10.1128/JCM.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torun N, Sherif Z, Garweg J, et al. Diagnosis and treatment of ocular toxoplasmosis: a survey of German speaking ophthalomologists. Ophthalmologe. 2008;105:1023–1028. doi: 10.1007/s00347-008-1694-2. [DOI] [PubMed] [Google Scholar]