Abstract
There is an urgent need for new drugs against malaria, which takes millions of lives annually. Cysteine proteases are potential new drug targets, especially when current drugs are showing resistance. Falcipains and vivapains are well characterized cysteine proteases of P. falciparum and P. vivax, respectively. Studies with cysteine protease inhibitors and manipulating cysteine proteases specific genes have suggested their roles in hemoglobin hydrolysis. In P. falciparum, falcipain-2 and falcipain-3 are major hemoglobinases that hydrolyze host erythrocyte hemoglobin in the parasite food vacuole. It is confirmed that disruption of the falcipain-2 gene led to a transient block in hemoglobin hydrolysis, and disruption of falcipain-3 gene was not possible, suggesting that protease is essential for erythrocytic parasites. On the other hand, vivapain-2, vivapain-3 and vivapain-4 are important cysteine proteases of P. vivax, which shared a number of features with falcipain-2 and falcipain-3. A recent study indicates that vivapains and aspartic protease of P. vivax works collaboratively to enhance the parasites’ ability to hydrolyze host erythrocyte hemoglobin. Studies also indicate that falcipains and vivapains also hydrolyse the erythrocyte cytoskeleton proteins and involved in rupture of red blood cell. Structural and biochemical analysis of falcipains and vivapains showed that they have unique domains for specific functions. Overall, the complexes of cysteine proteases with small and macromolecular inhibitors provide structural insight to facilitate the drug design. Therefore, giving due importance to the cysteine proteases, this review will briefly focus the recent advancement in the field of cysteine proteases of human malaria parasites.
Keywords: Malaria, Cysteine protease, Hemoglobin hydrolysis
Introduction
P. falciparum and P. vivax are the most predominant human malaria species worldwide, causes million of deaths and illnesses each year. The regions where malaria is mostly endemic include Africa, Asia, and South America (Sachs and Malaney 2002). A number of drugs are currently available to control malaria (Fidock 2010, however, treatment is getting complicated by drug resistance, toxicity, high cost. Recently study indicate that the drug resistance against new effective drug, artemisnin is also emerging (Wongsrichanalai and Meshnick 2008; Dondorp et al. 2009), and we definitely need new effective drug to treat malaria. Therefore, the development of other classes of effective antimalarial drug, especially compounds that act against novel biochemical targets, is required. Among potential new targets for antimalarial chemotherapy are Plasmodium proteases.
Advancement including the sequencing of plasmodium genome (http://plasmodb.org) and development of new tools for manipulating Plasmodium genes (Crabb 2002), have improved our knowledge of the cysteine proteases of parasites. Malaria parasite relies on human hemoglobin hydrolysis to supply amino acids for protein synthesis and to maintain osmotic stability (Rosenthal et al. 2002; Rosenthal 2004). Cysteine proteases are involved in hemoglobin hydrolysis and have been validated as promising drug targets (Rosenthal et al. 2002; Rosenthal 2004). Recent report by Ch’ng et al. (2010) using cysteine protease inhibitors, demonstrate that clan CA cysteine proteases of P. falciparum are also involved in chloroquine mediated programmed cell death.
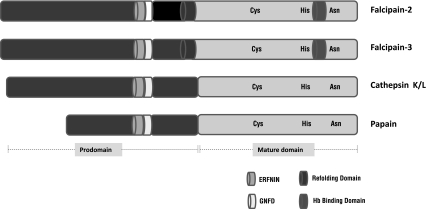
Schematic of different cysteine protease family include, falcipain-2, falcipain-3, vivapain, cathepsin, papain are given in Fig. 1.
Fig. 1.
Falcipains and vivapains have unique features. Falcipains and vivapains are papain family cysteine proteases. The active site residues (Cys, His, Asn) are conserved within papain family. Falcipains and vivapains have unique N-terminus extension act as a refolding domain and C terminus insert as a hemoglobin (Hb) binding domain. The prodomains of falcipains and vivapains have ERFNIN and GNFD motifs, conserved in papain, cathepsin K and cathepsin L
Among the four P. falciparum cysteine proteases, falcipain-2 and falcipain-3 appear to be the principal food vacuolar hemoglobinases (Rosenthal 2004; Rosenthal et al. 2002). Disruption of the falcipain-2 gene led to accumulation of undegraded hemoglobin in the food vacuole, confirming that this enzyme participates in hemoglobin hydrolysis (Sijwali and Rosenthal 2004). And disruption of falcipain-3 could not be achieved, but the gene was replaced with a tagged functional copy, indicating that falcipain-3 is essential for erythrocytic parasites (Sijwali et al. 2006). Falcipain-2 and falcipain-3 share 67% sequence identity, and contribute more or less equally to the digestion of hemoglobin in the food vacuole.
Vivapains are major cysteine proteases of P. vivax, vivapain-2, vivapain-3 and vivapain-4 genes shared number of features with falcipain-2 and falcipain-3 (Fig. 1; Table 1).
Table 1.
Summary of characterized cysteine proteases of P. falciparum and P. vivax
| Clan | Family | Protease | Gene | ChaActivityb | Function | Stage of gene expression | |
|---|---|---|---|---|---|---|---|
| Immunoblotting | Proteomic screen | ||||||
| CA | C1 | Falcipain-1 | PF14_0553 | 14 CP | Not essential | R/T/S | Sp |
| CA | C1 | Falcipain-2 | PF11_0165 | 11 CP | Hb hydrolysis | T | T |
| CA | C1 | Falcipain-2′ | PF11_0161 | 11 CP | Hb hydrolysis | T | |
| CA | C1 | Falcipain-3 | PF11_0162 | 11 CP | Hb hydrolysis | T/S | T/G |
| CA | C1 | Vivapain-2 | PVX_091405 | 09 CP | Hb hydrolysis | T | |
| CA | C1 | Vivapain-3 | PVX_091410 | 09 CP | Hb hydrolysis | T | |
| CA | C1 | Vivapain-4 | XP_001615272 | 09 CP | Hb hydrolysis | R/T/S/G | T |
CP cysteine proteases, Hb hemoglobin, R ring, T trophozoite, S schizont, G gametocyte, Sp sporozoite
aChromosome number
bGeneral properties of proteases that have been purified and characterized
Although P. vivax is less virulent than P. falciparum, but widely distributed human malaria parasite and it causes extensive morbidity (Mendis et al. 2001). Together, these two parasites are responsible for > 90% of episodes of human malaria (Rogerson and Carter 2008). The comprehensive studies of P. vivax cysteine proteases have been mainly hampered due to technical shortcomings. Notably, unlike the case with P. falciparum, the continuous in vitro culture of P. vivax is not available, and animal models are only limited to primates. It is equally important that the development of drugs against plasmodial cysteine proteases considers targets in both of the two prevalent human malaria parasites. This review will survey available information on major falcipains and vivapains.
Brief history of cysteine proteases
Cysteine proteases are named due to the function of a catalytic cysteine that mediates protein hydrolysis via nucleophilic attack on the carbonyl carbon of a peptide bond. Cysteine proteases are divided into different clans, which further subdivided into families based on sequence identities and similarities (Barrett and Rawlings 2001). Clan CA proteases use catalytic Cys, His, Asn residues, in Clan CA, Family C1 comes under papain family cysteine proteases. This review is deal with cysteine proteases of human malaria parasite, comes under Family C1 (Table 1).
Cysteine proteases as hemoglobinases
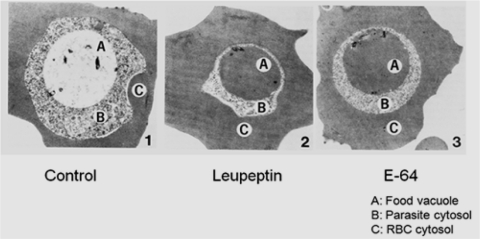
Published studies with cysteine protease inhibitors have provided valuable information regarding their functions in malaria parasites. The effects of leupeptin and E-64 against cultured P. falciparum parasites revealed that cysteine proteases are major hemoglobinases (Fig. 2).
Fig. 2.
Cysteine protease inhibitors inhibit hemoglobin hydrolysis; Pictures showing electron microscopy of infected erythrocytes with P. falciparum. Parasites treated with two cysteine proteases inhibitors, leupeptin and E-64, showed dark food vacuole due to accumulation of undegraded hemoglobin. Figure has been taken from Rosenthal et al. (2002)
The above evidence supports the critical role for cysteine proteases in hemoglobin hydrolysis at the trophozoite stage of malaria parasite. In blood stage, parasites multiply asexually, reaching large numbers in the circulation, and causing the disease. During this stage, parasites take up erythrocyte cytosol through cytostome, and transport to an acidic food vacuole, where parasites degrade hemoglobin (Francis et al. 1997).
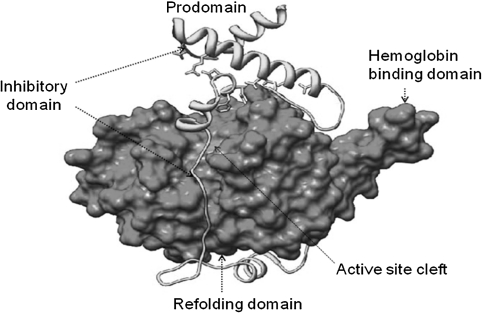
The three falcipains, falcipain-2, falcipain-2′ (a nearly identical copy of falcipain-2), falcipain-3 are the major hemoglobinases in P. falciparum. In case of P. vivax, vivapain-2, vivapain-3 and vivapain-4, are described as major hemoglobinases. Disruption of falcipain-2 gene results in reduced hemoglobin hydrolysis in the trophozoite stage and accumulation of undegraded hemoglobin in the parasite food vacuole. The parasite still able to survive, it seems to be compensated by over expression of falcipain-2′ and falcipain-3 in later stages of the P. falciparum life cycle (Sijwali and Rosenthal 2004). Further, it was confirmed by Pandey et al. that falcipain-2 can captures hemoglobin by a specific motif (Pandey et al. 2005) (Fig. 3).
Fig. 3.
Structure of Pro-falcipain-2: Purple color showing the mature domain of falcipain-2, solved by X-ray crystallography (Pandey et al. 2009; Wang et al. 2006). The mature domain has active site (catalytic triad residues in orange), hemoglobin binding domain, and refolding domain are labeled. The prodomain (cyan) runs up the face of the mature enzyme before forming helices containing the conserved ERFNIN and GNFD motifs (yellow). (Color figure online)
Interestingly, this motif was also present in other malarial proteases like falcipain-3, vivapain-2, vivapain-3, vivapain-4, indicating that this motif may be used by these enzymes for binding with hemoglobin (Fig. 1). Biochemical analysis of falcipain-2, falcipain-3 as well as vivapain-2, vivapain-3, vivapian-4 showed that they are efficient hemoglobinases. These enzymes efficiently hydrolyze human hemoglobin in acidic food vacuole of parasite (Shenai et al. 2000; Sijwali et al. 2001; Na et al. 2004; Na et al. 2010). Recent study indicated that plasmepsin-4, an aspartic protease of P. vivax, acted synergistically with vivapain-2 and vivapain-3, in the hydrolysis of hemoglobin (Moon et al. 2011). This result suggests that plasmepsin-4 and vivapains works collaboratively to enhance the parasites’ ability to degrade hemoglobin.
It has recently been suggested that hemoglobin hydrolysis is not a highly ordered process, but rather proceeds with rapid cleavage by enzymes at multiple sites (Subramanian et al. 2009). In fact, the motif of falcipain-2 specifically binds to hemoglobin and the secondary structure of motif is well conserve (Pandey et al. 2005; Kerr et al. 2009a). The detail role of a motif in hemoglobin binding is further described later in “Function of different domains of malarial proteases” section.
Expression of proteases by erythrocytic parasites
The enzymatic activities of falcipains (falcipain-2 and falcipain-3) are responsible for more than 90% of the cysteine protease activity that is identified in trophozoite lysates of P. falciparum (Shenai et al. 2000). Both of these enzymes differ in their timing of expression, as falcipain-2 is expressed about 12 h earlier than falcipain-3 (Sijwali and Rosenthal 2004). Falcipain-2 was predominant in early and late trophozoites, whereas, falcipain-3 could be seen in late trphozoites and early schizonts. Using immunoelectran microscopy and labelling with specific antibodies, it has been shown that falcipain-2 first appeared in food vacuole of early trophozoites, whereas, falcipain-3 appeared at late trophozoites/early schizonts. Using transient transfection and electrophoretic mobility shift assays, it has been demonstrated that the presence of stage specific nuclear factors probably modulate the expression of falcipain genes (Sunil et al. 2008).
Investigations for the localization for vivapains have been highly limited not only because of low parasitemia in the patients’ blood, but also due to failure of experimental maintenance of the parasite. Recently, vivapain-4 has been shown to express in all intraerythrocytic stages of P. vivax (Na et al. 2010). The majority of vivapain-4 localization appeared to be limited to the food vacuole with dark hemozoin pigment, while some protein was also labelled diffusely in the parasite cytoplasm (Na et al. 2010). As mentioned earlier, vivapains has hydrolytic activity against cytosketal proteins, and their cytoplasmic distributions may suggest their cytoplasmic roles such as cytosketal remodelling, erythrocytes rupture, and hemoglobin transport.
Cysteine proteases involved in erythrocyte rupture
Using cysteine proteases inhibitors, it has been shown to inhibit the rupture of red blood cell. Therefore, cysteine protease activity is required for the release of merozoites. Earlier studies have shown that the accumulation of mature schizonts in cultures treated with leupeptin (Hadley et al. 1983; Debrabant and Delplace 1989). Later study has shown the action of E-64 in mature schizont stage of parasites (Salmon et al. 2001). E-64 blocked the lysis of the parasitophorous vacuole membrane and suggested that cysteine protease activity is requires for the hydrolysis of parasitophorous vacuole membrane associated proteins to mediate merozoite released (Salmon et al. 2001). Further, different studies have shown that the release of merozoites is a two step process, first requiring hydrolysis of proteins which are associated with the parasitophorous vacuole, second, hydrolysis of red blood cell membranes proteins (Wickham et al. 2003). Falcipain-2 has been shown to hydrolyse the erythrocyte cytoskeletal proteins band 4.1 and ankyrin. Similarly, vivapain-2, vivapain-3 and vivapain-4 have shown to hydrolyse spectrin, protein 3, actin, glycophorin A and protein 4.1 (Dua et al. 2001; Hanspal et al. 2002, Na et al. 2004, 2010). Further, using forward chemical genetic screening, a cysteine protease, dipeptidyl peptidase 3, has been shown to regulate the red blood cell rupture (Kapur et al. 2008).
Function of different domains of malarial proteases
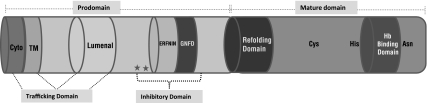
Falcipains and vivapains have two main domains, the prodomain and the mature domain. The prodomain is further divided into small sub-domains (Fig. 4).
Fig. 4.
Domains of falcipain-2: Schematic diagram showing different domains of falcipain-2. The prodomain is made up of cytoplasmic, transmembrane, lumenal and inhibitory domain. The mature domain has refolding domain and hemoglobin (Hb) binding domain, and catalytic triad residues (Cys, His, Asn). The conserved residues (Phe165, Phe 168) present in inhibitory domain are labeled with asterisks
Serial and truncation studies showed that 20 amino acid of luminal portion and a 10 amino acid stretch of the cytoplasmic portion of N-terminus of the prodomains from falcipain-2 and falcipain-3 were essential for food vacuole trafficking (Fig. 3) (Subramanian et al. 2007). Further, the C-terminus part of the prodomain is required for inhibition of the mature domain (Pandey et al. 2009). It has also been shown that a peptide with sequence corresponding to the prodomain of falcipain-2 inhibits parasite development (Korde et al. 2008). Recently, it has been shown that there are some crucial interactions (salt brides and hydrophobic interactions) between the prodomain and the mature domain. These interactions seem crucial for processing of falcipain-2 and falcipain-3 into active enzymes (Shrinivasan and Singh et al. 2011 pers. comm.). The N-terminal part of the mature domain is required for refolding (Sijwali et al. 2002; Pandey et al. 2004). The N-terminal part is functionally conserved within the falcipain-2, falcipain-3 and vivapain-2 and vivapain-3. Functional conservation was assessed by making chimeric constructs in which N-terminus part from falcipain-3, vivapain-2 and vivapain-3, fused to the mature domain of falcipain-2. All the chimera refolded into active proteases, with similar refolding efficiency, activity, and kinetic parameters to that of falcipain-2. This result indeed proved that within the malarial cysteine proteases, N-terminus part of the mature domain is functionally conserved as a refolding domain (Pandey et al. 2004).
Falcipains and vivapains subfamily proteases contain a unique motif near the C-terminus, which is present between highly conserved active site histidine and asparagine (Fig. 4). A motif of identical size (14 aa) is found in proteases of falcipain-2 subfamily, and made up of β-hairpin. Native PAGE, Biacore and gel filtration studies indicated that the motif mediates specific interactions with hemoglobin (Pandey et al. 2005). Enzyme without motif did show negligible activity against hemoglobin or globin. Data indicated that falcipain-2 capture hemoglobin via its C-terminus motif before subsequently cleaving the substrate at multiple sites (Pandey et al. 2005 and Subramanian et al. 2009). Further, the structure of falcipain indicates a protruding configuration for the motif, surrounded by a predominant negative charge (Wang et al. 2006), and may be charge residues are crucial for interaction with hemoglobin. It has also been demonstrated that falcipain-2 has relatively higher affinity for methemoglobin (kDa is 0.8 μM) than hemoglobin (kDa is 3.3 μM) (Hogg et al. 2006). Based on this observation it has been suggested that several factors contribute to the formation of methemoglobin during malarial infection, including an acidic pH of plasmodial food vacuole, oxidative damage in RBC (Akompong et al. 2000; Müller 2004), which causes an increase in methemoglobin content up to 20–42% in the plasmodial food vacuole. Thus, the higher affinity of falcipain-2 for methemoglobin looks like an adaptation to the specific conditions in the infected RBC. Based on solved structure of falcipain-2, it is proposed that hemoglobin, which has many charged residues, first binds at the motif through charge–charge interaction and bring closer to the active site before hydrolysis.
The specific role of the motif raise intriguing question. Why falcipains and vivapains contain unique motif that mediates the hydrolysis of hemoglobin? This mechanism might not have evolved simply to facilitate hemoglobin hydrolysis, because other papain-family enzymes without C terminal motifs still can hydrolyse hemoglobin. It looks like the utilization of a specific motif at this region to mediate enzyme–substrate interaction is an unusual but rather conserved feature of plasmodial cysteine proteases. It may be possible that an evolutionary bottleneck occurred in ancestral plasmodial cysteine proteases, and those enzymes might not be that efficient in hemoglobin hydrolysis. Therefore, plasmodial cysteine proteases might have evolved with introduction of specific motif for efficient hemoglobin hydrolysis.
Inhibition of falcipains and vivapains
Small molecule inhibitors (Leupeptin, E64 and Vinyl Sulfones)
Leupeptin, E-64 and vinyl sulfones are major cysteine proteases inhibitors binds to the active site (Kerr et al. 2009a, b). The active sites of both enzymes are located in a cleft between the structurally distinct domains of the papain like fold. The structures of falcipain-2 and falcipain-3 have been determined in complex with these small inhibitors (Kerr et al. 2009a). E-64 and leupeptin interact with residues in the S1, S2, and S3 subsites of falcipain-2 and falcipain-3, corresponding to the P1, and P2, and P3 position of the inhibitors (Fig. 5).
Fig. 5.
Chemical structures of E-64 and Leupeptin. The positions of P1, P2, P3 of the inhibitors that occupy the S1, S2, and S3 subsites of enzyme are labeled. Inhibitor and enzyme groups which are involved in covalent bond formation are shown in red. Figure has been taken from Kerr et al. (2009a). (Color figure online)
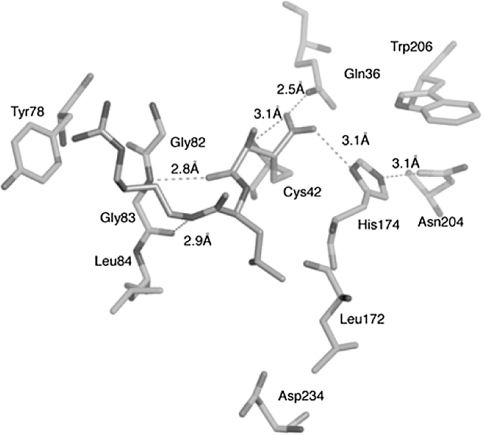
The inhibitors bind to the main chain of falcipain-2 and falcipain-3 by glycine (Gly 83 in falcipain-2 and Gly 92 in falcipain-3) residue that is highly conserved in the S3 subsite of clan CA cysteine proteases (Fig. 6).
Fig. 6.
Interaction between Falcipain-2 and E-64. The residues in the active site of falcipain-2 are colored blue and labeled, and E-64 is colored in grey. The interactions with enzyme and inhibitor are in pink. Figure has been taken from Kerr et al. (2009a). (Color figure online)
The active site cysteine of falcipain-3 forms a covalent, reversible hemithioacetal with the asymmetric carbonyl carbon of leupeptin (Kerr et al. 2009a). The interaction pattern of E-64 and leupeptin with all papain family enzymes are common. The carboxyl group of E-64 and carbonyl group of leupeptin occupy the oxyanoin hole formed by the conserved catalytic residues. Falcipain-2 and Falcipain-3 have a clear preference for substrates/inhibitors that contains a leucine at P2 position, and both leupeptin and E-64 contain a Leu at P2 position. Surprisingly, falcipain-3 has been shown to be much less efficient at hydrolyzing peptide substrates and more difficult to inhibit with small peptidyl based inhibitors as compare to falcipain-2 (Sijwali et al. 2001 and Ramjee et al. 2006). The effect of leupeptin and E-64 on vivapain-2, vivapain-3, indicated that the patterns and sensitivities are similar with falcipains. Notably, vivapain-2 was much more sensitive to E-64 than were falcipain-2 and falcipain-3 (Na et al. 2004) (Table 2).
Table 2.
Inhibitory kinetics of vivapains and falcipains (Table has been taken from Na et al. 2004)
| kass (M−1 s−1)a | |||||
|---|---|---|---|---|---|
| Vivapain-2 | Vivapain-3 | Falcipain-2 | Falcipain-3 | ||
| E-64 | 2,31,000 ± 17,700 | 5,690 ± 588 | 14,200 ± 475 | 5,870 ± 428 | |
| Z-Phe-Arg-FMK | 47,900 ± 5,090 | 3,680 ± 218 | 2,41,000 ± 20,100 | 60,200 ± 6,740 | |
| Mu-Phe-hPhe-FMK | 61,700 ± 6,180 | 540 ± 60 | 2,15,000 ± 5,760 | 5,26,000 ± 59,300 | |
| Mu-Leu-hPhe-FMK | 4,50,000 ± 16,800 | 79,200 ± 6,490 | 9,24,000 ± 26,200 | 58,50,000 ± 15,800 | |
| Mu-Phe-hPhe-VSph | 267 ± 14 | NAb | 4,890 ± 231 | 2,060 ± 41 | |
| Mu-Leu- hPhe-VSph | 88,200 ± 470 | 4,970 ± 370 | 1,02,000 ± 2,530 | 22,000 ± 1,420 | |
| Nmetpip-Phe-hPhe-VSPh | 96 ± 5 | 15 ± 004 | 1,230 ± 136 | 1,900 ± 43 | |
| Nmetpip-Leu-hPhe-VSPh | 53,600 ± 6,450 | 2,130 ± 158 | 26,300 ± 3,700 | 11,900 ± 762 | |
Mu morpholine urea, hPhe homophenylalanine, NmetpipN-methylpiperazine urea, VSPh phenylvinylsulphone
aGeometric mean ± SEM
bNo appreciable activity
Considering substrates and inhibitors, the vivapains had similar, but not identical specificities, and vivapain-3 was less active against all tested peptide substrates. But in all cases, inhibitors with Leu at the P2 position were preferred to corresponding peptides with Phe at this position (Na et al. 2004).
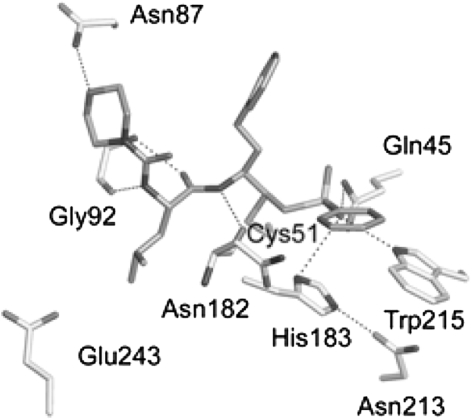
Like E-64 and leupeptin, vinyl sulphone (Mu-leu-Hph-VsPh) have been shown to be effective inhibitor of a number of papain family like cysteine proteases. Mu-Leu-Hph-VSPh is a potent irreversible inhibitor of falcipain-2 and falcipain-3 (Kerr et al. 2009a). The co-crystallization of falcipain-3 with Mu-Leu-Hph-VsPh indicate that inhibitor bind the respective S1 and S3 subsites and form an irreversible, covalent bond with the sulfur of the active site cysteine thiol in enzyme (Fig. 7).
Fig. 7.
Interaction of falcipain-3 with Morpholino urea-Leucine-Homophenylanyl-phenyl vinyl sulfone. The residues in the active site of falcipain-3 are colored yellow and labeled, and morpholino urea-Leucine-Homophenylanyl-phenyl vinyl sulfone is colored gray. The interactions with enzyme and inhibitor are in black dots . Figure has been taken from Kerr et al. (2009a). (Color figure online)
Falcipain-3 prefers for leu at P2 of Mu-Leu-Hph-VsPh. Given the hydrophobic nature of the P1 and P2 substituent’s in Mu-leu-Hph-VsPh, the active site of falcipain-3 is lined with number of residues that are able to make non-polar contacts with their respective inhibitor (Fig. 7).
Homology modelling of vivapain-4 suggests that overall topology is similar to falcipain-2, falcipain-3, vivapain-2 and vivapain-3 (Na et al. 2010). However, numbers of substitutions are recognized between vivapain-4 and other vivapains, including Ala 90, Gly154, Glu180 at the S2 pocket. Using the range of peptidyl inhibitors, the vivapains showed greater inhibition by E-64, but lower activity with vinyl sulphone inhibitors, than did the falcipains. Vivapain-2 was much more sensitive to peptidyl inhibitors. In some cases, data suggested that differences between the inhibition of vivapains and falcipains were large, suggesting that potent P. falciparum antimalarials may not be that effective against P. vivax.
Inhibition by macromolecules
Macromolecule inhibitors are polypeptide in nature, which are generally present inside the organisms. These endogenous cysteine proteases inhibitors have been described in a number of eukaryotic systems. Here we will discuss three major cysteine protease inhibitors, cystatin, chagasin, falstatin. Wang et al. found that falcipain-2 and chicken egg white cystatin, formed 1:1 complex (Fig. 8a).
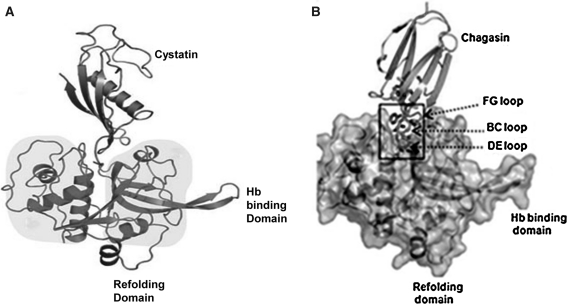
Fig. 8.
a 3D structure of falcipain 2–cystatin complex. Cystatin is colored in orange, and falcipain-2 protease is colored in green. Refolding domain and hemoglobin binding domain highlighted in pink and salmon, respectively (Figure has been taken from Wang et al. 2006). b Structure of falcipain 2–chagasin complex: overall structure of chagasin with falcipain-2, chagasin in red and falcipain 2 in golden color (Figure has been taken from Wang et al. 2007). (Color figure online)
The inhibitory constant (Ki) of cystatin for falcipain-2 and falcipain-3 are 6.5 and 100 nM, respectively (Wang et al. 2007). It is interesting that cystatin is more potent inhibitor of falcipain-2 than falcipain-3, which suggests that cystatin regulate both the falcipains with different rate. It might be important biologically, since their timing of expression is slightly different as discussed earlier. Cystatin largely interacts with falcipain-2 at prime sites where substrate-binding pockets are relatively shallow and less defined. Falcipain–cystatin complex can be exploited to design the potent inhibitor of malaria parasite. Recent study indicate that peptide based on cystatin binding residues blocked the activity of falcipain-2 and produced phenotypic effect led to accumulation of undegraded hemoglobin in the food vacuole (Rizzi et al. 2011).
Chagasin is a cysteine protease inhibitor that was first identified in Trypanosoma cruzi as the physiological regulator of cruzain, the major cysteine protease (Sanderson et al. 2003). Chagasin is also a potent inhibitor of falcipains, and demonstrates 1:1 binding with falcipain-2 (Fig. 8 b). The protease binding loops (BC, DE, and FG) in chagasin form a well-aligned wedge that fills the active site groove of falcipain-2 to obstruct substrate binding (Fig. 8 b) (Wang et al. 2007). The BC loop is one of the three signature motifs that contribute mainly in inhibiting the cysteine protease.
In case of malaria, falstatin has been recognized as an endogenous cysteine protease inhibitor in P. falciparum. Erythrocytic P. falciparum parasites express falstatin, a potent inhibitor of falcipains and many other cysteine proteases (Pandey et al. 2006). But it is unknown how falstatin regulate the P. falciparum cysteine proteases? The stage-specificity of falstatin expression and the effects of anti-falstatin antibodies on parasite development suggest that this inhibitor facilitates invasion (Pandey et al. 2006). Falstatin is a competitive and reversible inhibitor of falcipains, as demonstrated by increasing Km values but similar Vmax values with increasing concentrations of falstatin (Pandey et al. 2006). Further, our mutagenesis study indicates that there are some crucial residues present in falstatin may be important for inhibition of falcipains (Shrinivasan and Singh et al. personel communication).
Lead compounds as falcipain inhibitors
Recently, different heteroarylnitrile derivatives were studied as potential falcipain inhibitors, and suggested potential antiparasitic lead compounds. A heteroarylnitrile derivative, 5-substituted-2 cyanopyrimidine chemical class recently been emerging as the most potent and promising lead series (Coterón et al. 2010). Using sequential lead optimization process, these chemical class exhibiting nanomolar and subnanomolar inhibitors of falcipain-2 and falcipain-3 as recombinant form, whereas, micromolar range against culture parasites (Coterón et al. 2010). Recent development of a novel protease inhibitor peroxide hybrid with the concept of combination chemotherapy was used. By using a 1,2,4-trioxolane as a protease inhibitor carbonyl masking group, malaria parasite can be targeted. These compounds are designed to target the malaria parasite using two independent mechanisms of action. First, ferrous mediated release of a carbonyl protease inhibitor, and secondly, production of potentially toxic carbon radical (Gibbons et al. 2010) designed to target parasite. Ettari et al. (2009) previously mentioned the falcipain-2, inhibitors having a 1,4-benzodiazepine (1,4-BDZ) scaffold showed good potency.
Electrophilic vinyl ketone warhead as a potent inhibitor, which able to interact with thiol group of the cysteine active site by forming a covalent bond. Vinyl ketone was selected as lead compound with a good inhibitory activity against the target enzyme and the strong potency against cultured P. falciparum (IC50 9.3 μM) (Ettari et al. 2011).
Summary and conclusion
Understanding of the cysteine proteases of malaria parasites has increased markedly in recent years. Since cysteine proteases that play an important role in the parasite life cycle by degrading erythrocyte proteins, most notably hemoglobin, are attractive target for antimalarial chemotherapy. Falcipains and vivapains are the best characterized cysteine proteases of malaria parasite. Recent biochemical and molecular studies suggest that falcipain-2 and falcipain-3, and vivapain-2, vivapain-3 and vivapain-4 are major hemoglobinases, and also hydrolyze other red blood cell proteins. These enzymes appear to be potential targets for antimalarial chemotherapy. The structure and function of different domains, and their interaction with small and macromolecular inhibitors are studied in detail. The structure–function study of these enzymes and interaction with inhibitors will provides detail insights to develop rational design of inhibitor against these crucial proteases of human malaria parasites. Structure guided approaches should have great role in the design of potent and highly selective inhibitor. Efforts to optimize current inhibitors based on the structure–function of these cysteine proteases are currently underway.
Acknowledgments
Work in the author laboratory was supported by Department of Biotechnology, Govt. of India. We thank NIMR, for extramural funds for setting a new laboratory, and appreciate Dr. Valecha and Dr. Katoch for their support.
References
- Akompong T, Ghori N, Haldar K. In vitro activity of riboflavin against the human malaria parasite, Plasmodium falciparum. Antimicrob Agents Chemother. 2000;44:88–96. doi: 10.1128/AAC.44.1.88-96.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ, Rawlings ND. Evolutionary lines of cysteine peptidases. Biol Chem. 2001;382:727–733. doi: 10.1515/BC.2001.088. [DOI] [PubMed] [Google Scholar]
- Ch’ng JH, Kotturi SR, Chong AG, Lear MJ, Tan KS. A programmed cell death pathway in the malaria parasite Plasmodium falciparum has general features of mammalian apoptosis but is mediated by clan CA cysteine proteases. Cell Death Dis. 2010;1:e26. doi: 10.1038/cddis.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coterón JM, Catterick D, Castro J, Chaparro MJ, Díaz B, Fernández E, et al. Falcipain inhibitors: optimization studies of the 2-pyrimidinecarbonitrile lead series. J Med Chem. 2010;16:6129–6152. doi: 10.1021/jm100556b. [DOI] [PubMed] [Google Scholar]
- Crabb BS. Transfection technology and the study of drug resistance in the malaria parasite Plasmodiumfalciparum. Drug Resist Updat. 2002;5:126–130. doi: 10.1016/S1368-7646(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Debrabant A, Delplace P. Leupeptin alters the proteolytic processing of P126, the major parasitophorous vacuole antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1989;33(2):151–158. doi: 10.1016/0166-6851(89)90029-7. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua M, Raphael P, Sijwali PS, Rosenthal PJ, Hanspal M. Recombinant falcipain-2 cleaves erythrocyte membrane ankyrin and protein 4.1. Mol Biochem Parasitol. 2001;116:95–99. doi: 10.1016/S0166-6851(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Ettari R, Micale N, Schirmeister T, Gelhaus C, Leippe M, Nizi E, Di Francesco ME, Grasso S, Zappalà M. Novel peptidomimetics containing a vinyl ester moiety as highly potent and selective falcipain-2 inhibitors. J Med Chem. 2009;7:2157–2160. doi: 10.1021/jm900047j. [DOI] [PubMed] [Google Scholar]
- Ettari R, Zappalà M, Micale N, Grazioso G, Giofrè S, Schirmeister T, Grasso S. Peptidomimetics containing a vinyl ketone warhead as falcipain-2 inhibitors. Eur J Med Chem. 2011;46:e2065. doi: 10.1016/j.ejmech.2011.02.058. [DOI] [PubMed] [Google Scholar]
- Fidock DA. Drug discovery: priming the antimalarial pipeline. Nature. 2010;465:297–298. doi: 10.1038/465297a. [DOI] [PubMed] [Google Scholar]
- Francis SE, Sullivan DJ, Jr, Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- Gibbons P, Verissimo E, Araujo NC, Barton V, Nixon GL, et al. Endoperoxide carbonyl falcipain 2/3 inhibitor hybrids: toward combination chemotherapy of malaria through a single chemical entity. J Med Chem. 2010;53:8202–8206. doi: 10.1021/jm1009567. [DOI] [PubMed] [Google Scholar]
- Hadley T, Aikawa M, Miller LH. Plasmodiumknowlesi: studies on invasion of rhesus erythrocytes by merozoites in the presence of protease inhibitors. Exp Parasitol. 1983;55:306–311. doi: 10.1016/0014-4894(83)90027-9. [DOI] [PubMed] [Google Scholar]
- Hanspal M, Dua M, Takakuwa Y, Chishti AH, Mizuno A. Plasmodiumfalciparum cysteine protease falcipain-2 cleaves erythrocyte membrane skeletal proteins at late stages of parasite development. Blood. 2002;100:1048–1054. doi: 10.1182/blood-2002-01-0101. [DOI] [PubMed] [Google Scholar]
- Hogg T, Nagarajan K, Herzberg S, Chen L, Shen X, Jiang H, Wecke M, Blohmke C, Hilgenfeld R, Schmidt CL. Structural and functional characterization of Falcipain-2, a hemoglobinase from the malaria parasite Plasmodiumfalciparum. J Biol Chem. 2006;35:25425–25437. doi: 10.1074/jbc.M603776200. [DOI] [PubMed] [Google Scholar]
- Kapur AS, Ponder EL, Fonović UP, Yeoh S, Yuan F. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodiumfalciparum. Nat Chem Biol. 2008;4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- Kerr ID, Lee JH, Pandey KC, Harrison A, Sajid M, Rosenthal PJ, Brinen LS. Structures of falcipain-2 and falcipain-3 bound to small molecule inhibitors: implications for substrate specificity. J Med Chem. 2009;52(3):7–852. doi: 10.1021/jm8013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr ID, Lee JH, Farady CJ, Marion R, Rickert M, Sajid M, Pandey KC, Caffrey CR, Legac J, Hansell E, McKerrow JH, Craik CS, Rosenthal PJ, Brinen LS. Vinyl sulfones as antiparasitic agents and a structural basis for drug design. J Biol Chem. 2009;38:25697–25703. doi: 10.1074/jbc.M109.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde R, Bhardwaj A, Singh R, Srivastava A, Chauhan VS, Bhatnagar RK, Malhotra P. A prodomain peptide of Plasmodium falciparum cysteine protease (falcipain-2) inhibits malaria parasite development. J Med Chem. 2008;51:3116–3123. doi: 10.1021/jm070735f. [DOI] [PubMed] [Google Scholar]
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- Moon S, Kang J-M, Kim T-S, Yoon K, Sohn W-M, Na B-K. Plasmodium vivax: Collaborative roles for plasmepsin 4 and vivapains in hemoglobin hydrolysis. Exp Parasitol. 2011;128:127–132. doi: 10.1016/j.exppara.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Müller S. Redox and antioxidant systems of the malaria parasite Plasmodiumfalciparum. Mol Microbiol. 2004;5:305–1291. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- Na BK, Shenai BR, Sijwali PS, Choe Y, Pandey KC, et al. Identification and biochemical characterization of vivapains, cysteine proteases of the malaria parasite Plasmodium vivax. Biochem J. 2004;378:529–538. doi: 10.1042/BJ20031487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na BK, Bae Y-A, Zo Y-G, Choe Y, Kim S-H, et al. Biochemical Properties of a Novel Cysteine Protease of Plasmodiumvivax, Vivapain-4. Plos Neg Tropical Dis. 2010;4(10):e849. doi: 10.1371/journal.pntd.0000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KC, Sijwali PS, Singh A, Na BK, Rosenthal PJ. Independent intramolecular mediators of folding, activity, and inhibition for the Plasmodium falciparum cysteine protease falcipain-2. J Biol Chem. 2004;279:3484–3491. doi: 10.1074/jbc.M310536200. [DOI] [PubMed] [Google Scholar]
- Pandey KC, Wang SX, Sijwali PS, Lau AL, McKerrow JH, Rosenthal PJ. The Plasmodium falciparum cysteine protease falcipain-2 captures its substrate, hemoglobin, via a unique motif. Proc Natl Acad Sci USA. 2005;102:9138–9143. doi: 10.1073/pnas.0502368102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KC, Singh N, Arastu-Kapur S, Bogyo M, Rosenthal PJ. Falstatin, a cysteine protease inhibitor of Plasmodiumfalciparum, facilitates erythrocyte invasion. PLoS Pathog. 2006;11:e117. doi: 10.1371/journal.ppat.0020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KC, Barkan DT, Sali A, Rosenthal PJ. Regulatory elements within the prodomain of Falcipain-2, a cysteine protease of the malaria parasite Plasmodium falciparum. PLoS One. 2009;4(5):e5694. doi: 10.1371/journal.pone.0005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjee MK, Flinn NS, Pemberton TP, Quibell M, Wang Y, Watts JP. Substrate mapping and inhibitor profiling of falcipain-2, falcipain-3 and berghepain-2: implications for peptidase anti-malarial drug discovery. Biochem J. 2006;399:47–57. doi: 10.1042/BJ20060422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi L, Sundararaman S, Cendic K, Vaiana N, Korde R, Sinha D, Mohmmed A, Malhotra P, Romeo S. Design and synthesis of protein–protein interaction mimics as Plasmodium falciparum cysteine protease, falcipain-2 inhibitors. Eur J Med Chem. 2011;46(6):2083–2090. doi: 10.1016/j.ejmech.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Carter R. Severe vivax malaria: Newly recognized or rediscovered? PLoS Med. 2008;5:875–877. doi: 10.1371/journal.pmed.0050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal PJ. Cysteine proteases of malaria parasites. Intl J Parasitology. 2004;34:1489–1499. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rosenthal PJ, Sijwali PS, Singh A, Shenai BR. Cysteine proteases of malaria parasites: targets for chemotherapy. Curr Pharm Des. 2002;8:1659–1672. doi: 10.2174/1381612023394197. [DOI] [PubMed] [Google Scholar]
- Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- Salmon BL, Oksman A, Goldberg DE. Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis. Proc Natl Acad Sci USA. 2001;98:271–276. doi: 10.1073/pnas.011413198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson SJ, Westrop GD, Scharfstein J, Mottram JC, Coombs GH. Functional conservation of a natural cysteine peptidase inhibitor in protozoan and bacterial pathogens. FEBS Lett. 2003;542:12–16. doi: 10.1016/S0014-5793(03)00327-2. [DOI] [PubMed] [Google Scholar]
- Shenai BR, Sijwali PS, Singh A, Rosenthal PJ. Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase of Plasmodiumfalciparum. J Biol Chem. 2000;37:10–29000. doi: 10.1074/jbc.M004459200. [DOI] [PubMed] [Google Scholar]
- Sijwali PS, Rosenthal PJ. Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodiumfalciparum. Proc Natl Acad Sci USA. 2004;101(13):9–4384. doi: 10.1073/pnas.0307720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijwali PS, Shenai BR, Gut J, Singh A, Rosenthal PJ. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biochem J. 2001;360:481–489. doi: 10.1042/0264-6021:3600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijwali PS, Shenai BR, Rosenthal PJ. Folding of the Plasmodiumfalciparum cysteine protease falcipain-2 is mediated by a chaperone-like peptide and not the prodomain. J Biol Chem. 2002;26(277):14910–14915. doi: 10.1074/jbc.M109680200. [DOI] [PubMed] [Google Scholar]
- Sijwali PS, Koo J, Singh N, Rosenthal PJ. Gene disruptions demonstrate independent roles for the four falcipain cysteine proteases of Plasmodium falciparum. Mol Biochem Parasitol. 2006;150:96–106. doi: 10.1016/j.molbiopara.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Sijwali PS, Rosenthal PJ. Falcipain cysteine proteases require bipartite motifs for trafficking to the Plasmodium falciparum food vacuole. J Biol Chem. 2007;282:24961–24969. doi: 10.1074/jbc.M703316200. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Hardt M, Choe Y, Niles RK, Johansen EB, Legac J, Gut J, Kerr ID, Craik CS, Rosenthal PJ. Hemoglobin cleavage site-specificity of the Plasmodium falciparum cysteine proteases falcipain-2 and falcipain-3. PLoS One. 2009;4(4):e5156. doi: 10.1371/journal.pone.0005156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil S, Chauhan VS, Malhotra P. Distinct and stage specific nuclear factors regulate the expression of falcipains, Plasmodiumfalciparum cysteine proteases. BMC Mol Biol. 2008;14(9):47. doi: 10.1186/1471-2199-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Pandey KC, Somoza JR, Sijwali PS, Kortemme T, Brinen LS, Fletterick RJ, Rosenthal PJ, McKerrow JH. Structural basis for unique mechanisms of folding and hemoglobin binding by a malarial protease. Proc Natl Acad Sci USA. 2006;103(31):11503–11508. doi: 10.1073/pnas.0600489103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Pandey KC, Scharfstein J, Whisstock J, Huang RK, Jacobelli J, Fletterick RJ, Rosenthal PJ, Abrahamson M, Brinen LS, Rossi A, Sali A, McKerrow JH. The structure of chagasin in complex with a cysteine protease clarifies the binding mode and evolution of an inhibitor family. Structure. 2007;15(5):535–543. doi: 10.1016/j.str.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Wickham ME, Culvenor JG, Cowman AF. Selective inhibition of a two-step egress of malaria parasites from the host erythrocyte. J Biol Chem. 2003;278:37658–37663. doi: 10.1074/jbc.M305252200. [DOI] [PubMed] [Google Scholar]
- Wongsrichanalai C, Meshnick SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis. 2008;14(5):716. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]