Abstract
Prevalence of pigeon haemosporidians and effect of infection with Haemoproteus columbae on biochemical factors were investigated in 280 wild pigeons (Columba livia) captured from various locations in Khorasan province, in eastern of Iran, between April 2008 and June 2009. Infection prevalence with H. columbae and Leucocytozoon was detected 50 and 2%, respectively. However, no pigeon showed infection with Plasmodium. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin were the parameters which showed significant differences between parasitized and non parasitized pigeons and high ALT and AST activities and reduced serum albumin level (hypoalbominemia) were observed in pigeons infected with Haemoproteus. High level of AST, ALT, and hypoalbuminemia may be due to impairment of liver cells caused by hepatic migration of parasite for its life cycle.
Keywords: Pigeon haemosporidians, Prevalence, Biochemical factors, Iran
Introduction
Bird haemosporidians are characterized by some unique, frequently fascinating properties and therefore they are important as models, of protistological studies (Valkiunas 2005). Bird haemosporidians are distributed worldwide and the families of Haemoproteidae, Plasmodiidae, and Leucocytozoidae are distributed in all zoogeographical regions and use blood-sucking dipteran insects as vectors (Valkiunas 2005). It should be kept in mind, however, geographic location and genetic are the most important factors that determine resistance to bird haemosporidians, although the age of the bird, strain of the parasite, and stress may also play roles in the pathogenesis of blood parasites (Jones 2006). The role of haemosporidioses in the pathology of birds in captivity cannot be currently correctly estimated due to poor organization of the veterinary inspection of haemosporidians in many zoos, aviaries, and private collections.
It is noteworthy that due to the worldwide distribution, practical importance, and scientific appeal of the research conducted with birds and blood-sucking dipteran insects, haemosporidians may be excellent objects for scientific research. Therefore, this research was conducted to determine the prevalence of the pigeon haemosporidians and effect of infection with Haemoproteus columbae on haematological and biochemical factors in pigeons in Khorasan province of Iran.
Materials and methods
The Blood samples from wild pigeons (Columba livia) were collected in tubes containing EDTA as an anticoagulant for preparation of thin blood smear and into plain tubes for serum separation and biochemical analysis.
Blood smears were fixed with methanol for 5 min, stained with Giemsa at a dilution of 5% in buffer solution for 30 min. The smears were later examined using an oil emersion lens (100×) for the presence of haemosporidians.
The serum was separated after centrifugation at 1800×g for 10 min and stored at −20°C until analysis. The amounts of bilirubin, albumin, glucose, creatinine, uric acid, aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), and alanine aminotransferase (ALT) were measured by commercial kits [Pars Azmoon, Tehran, Iran] using an autoanalyser (Biotecnica, Targa 3000, Rome, Italy). Control serum (Randox control sera, Antrim, UK) was used for controlling measurement accuracy.
For statistical evaluation of results, SPSS 15 (SPSS for windows ver.15) was used. The Mann–Whitney test was used for comparison of measured factors between two groups.
Results
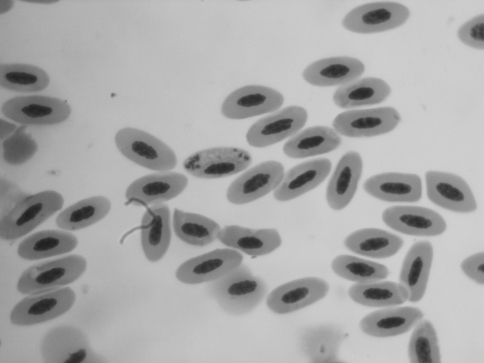
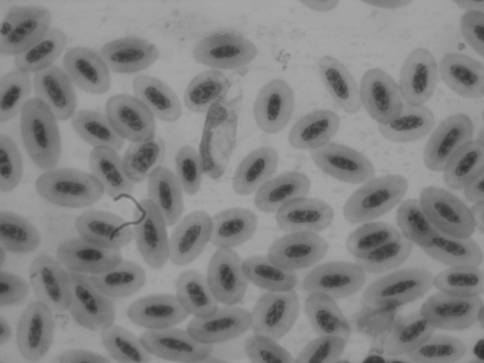
Two hundred and eighty blood samples from wild pigeons (Columba livia) captured in 5 different geographical locations in Khorasan province between April 2008 and June 2009. Thin blood smear examination showed that 50% of pigeons (140/280) were infected with H. columbae, 2% (6/280) Leucocytozoon and non showed infection with Plasmodium (Figs. 1, 2).
Fig. 1.
Haemoproteus columbae
Fig. 2.
Leucocytozoon
The results of Mann–Whitney test showed that significant differences were seen between infected and non infected pigeons for ALT, AST and albumin (Table 1). No significant difference was seen for other measured parameters between groups (Table 1).
Table 1.
Median (Min–Max) of different biochemical parameters in infected and no infected groups
| Parameters | No infected | Infected |
|---|---|---|
| GGT (IU/L) | 1.625 (0.22–19.50) | 4.625 (1.01–11.80) |
| ALT (IU/L)* | 21.95 (5.97–51.60) | 36.35 (26.3–67) |
| AST (IU/L)* | 80.47 (47.6–183) | 147.34 (57.4–308) |
| Bilirubin (mg/dl) | 0.2385 (0.026–4.77) | 0.084 (0.042–0.784) |
| Uric acid (mg/dl) | 5.57 (1.06–21.3) | 3.41 (0.78–9.43) |
| Creatinine (mg/dl) | 0.353 (0.195–0.802) | 0.3215 (0.113–0.405) |
| Albumin (g/dL)* | 0.452 (0.102–0.789) | 0.706 (0.553–0.926) |
| Glucose (mg/dl) | 348 (221–479) | 355 (282–401) |
*Significant effect of group (P < 0.05)
Discussion
Our research showed that H. columbae was the most prevalent Haemoparasites (50%) in pigeon in Khorasan province. This finding is higher than that reported by Razmi and Andalibian (2006) in khorasan province and Esmaeili et al. (2006) in Bushehr. It is probably due to the rich fauna of vectors in this region and existence of the total heat for the ability of the parasite to complete its development in the invertebrate host (Valkiunas, 2005).
The proportion of Leucocytozoon in this region was 2%. It is the first time that this parasite was reported from this region. It has to be noted that the Leucocytozoon fauna is connected to the global region including the Holarctic, Ethiopian, and Oriental regions, where, a tendency to the increase of the prevalence of Leucocytozoon infection from the tropical latitudes to the high latitudes of the Holarctic (Greiner 1975; White and Bennett 1978; Valkiunas and Iezhova 1992; Valkiunas 2005). It is likely that this is related primarily to the decrease of the density of vector populations as well as to the inability of the parasite to complete its development in the vectors.
On the contrary, no infection was found with Plasmodium. The rich fauna of malaria parasites is found in the Ethiopian, Oriental, and Neotropical regions and these regions are a vast center of Plasmodium and infection rate reaches 20–50% in some regions of the African tropics (Crewe 1975). Absence of Plasmodium infection in this region is accounted for the poor fauna of vectors, and probably the lack of necessary total heat for the development in the invertebrate host.
ALT, AST, and albumin were the parameters which showed significant difference between parasitized and non parasitized pigeons and high increase in ALT and AST, and reduced serum albumin level (hypoalbominemia) was observed in pigeons infected with Haemoproteus.
AST and ALT are sensitive indicators of liver damage or injury from different types of disease (Coles 1980). High level of AST and ALT may be due to impairment of liver cells caused by hepatic migration of parasites for their life cycle and is characterized by injury to numerous liver cells (extensive hepatic necrosis). On the other hand, there were no significant differences in the other liver parameters (GGT and bilirubin) between the parasitized and non parasitized groups. It is apparent that GGT is produced in the bile ducts and may become elevated when there is a bile duct disorder. Therefore, there is not an impairment, or bile duct blockage within the liver in Haemoproteus infection.
Hypoalbuminemia may be due to impairment of liver cells caused by hepatic migration of parasites for their life cycle. Low albumin levels are also indicative of disruption of protein metabolism due to starvation or decreased synthesis of albumin in the liver because of acute phase response of inflammation (Kaneko et al. 2009; Fleck 1989). Many reports on the pathogenicity of avian Haemoparasites have been based on evidence of high parasitemia in sick birds (Bennett and Bishop 1988; Bennett et al. 1993) and few studies have compared the biochemical parameters between parasitized and non parasitized birds. Pigeon is one of the rare avian species that has been shown clinical disease following infection with Haemoproteus (Thrall 2004). However, Ots and Horak (1998) reported that albumin was not affected by the Haemoproteus infection in great tits. They concluded that Haemoproteus infection does not affect severe inflammatory response and infected individuals do not reveal symptoms of poor nutritional state.
Further investigations are needed to identify the effect of Haemoproteus infection on food intake, digestion, and absorption processes or acute phase inflammatory responses in pigeon.
Our results demonstrate that parasitized pigeons with Haemoproteus differed from non parasitized ones with respect to serum ALT, AST, and albumin level. It means that infection by Haemoproteus might have a health impact on the host pigeons, or parasitemia is secondary to poor health state of certain individuals.
Acknowledgments
This work supported by the Ferdowsi University of Mashhad and grant number 154. We thank Mr. H. Eshrati and Mr. Azari for his technical assistance during data collection.
References
- Bennett GF, Bishop MA. Influence of blood parasites on the body mass of passeriform birds. J Wildl Dis. 1988;24:339–343. doi: 10.7589/0090-3558-24.2.339. [DOI] [PubMed] [Google Scholar]
- Bennett GF, Peirce MA, Ashford RW. Avian haematozoa, mortality and pathogenicity. J N Histol. 1993;27:993–1001. doi: 10.1080/00222939300770621. [DOI] [Google Scholar]
- Coles EP. Veterinary clinical pathology. 3. Philadelphia: Saunders; 1980. [Google Scholar]
- Crewe SM (1975) Studies on the biology of malaria and other blood parasites of Nigerian birds. PhD thesis, University of Liverpool
- Esmaeili H, Nabian S, Shahi ferdous MM,Gholami H, Mokhtari MR (2006) Infestation rate of parasite in pigeons of Busheahr-Iran. 6 th EAZWV, Budapest, Hungary
- Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc. 1989;48:347–354. doi: 10.1079/PNS19890050. [DOI] [PubMed] [Google Scholar]
- Greiner EC. Prevalence and potential vectors of Haemoproteus in Nebraska mourning doves. J Wildl Dis. 1975;11:150–156. doi: 10.7589/0090-3558-11.2.150. [DOI] [PubMed] [Google Scholar]
- Jones MP. Selected infectious diseases of birds of prey. J Exot Pet Med. 2006;15:5–17. doi: 10.1053/j.jepm.2005.11.008. [DOI] [Google Scholar]
- Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals. 6. Amsterdam: Academic; 2009. [Google Scholar]
- Ots I, Horak P. Health impact of blood parasites in breeding great tits. Oecologia. 1998;116:441–448. doi: 10.1007/s004420050608. [DOI] [PubMed] [Google Scholar]
- Razmi GH, Andalibian A. Survey of Haemoproteus columbae in pigeon of Mashhad and Shirvan. J Res Develop. 2006;71:3. [Google Scholar]
- Thrall MA. Veterinary hematology and clinical chemistry. USA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- Valkiunas G. Avian malaria parasites and other haemosporidia. Boca Raton: CRC Press; 2005. [Google Scholar]
- Valkiunas G, Iezhova T. Parasitic protozoa of the blood of birds in the USSR. Haemoproteidae of Passeriformes, Charadriiformes and Anseriformes. Ibidem. 1992;2:52–73. [Google Scholar]
- White EM, Bennett GF. Avian Haemoproteidae, description of Haemoproteus stellaris n.sp. and a review of the haemoproteids of the swallow family Hirundinidae. Can J Zool. 1978;56:2110–2116. doi: 10.1139/z78-286. [DOI] [Google Scholar]