Abstract
The prevalence and bionomics of known Indian malaria vector anophelines was studied in three forest fringe villages in Sonitpur district of Assam, India. Anopheles philippinensis/nivipes (36.8%) and An. annularis (25.6%) were the most abundant of the seven vector anopheline species recorded. Densities of vector anophelines in general were high during monsoon season and low during winter months. An. dirus s.l. was the most anthropophagic (91.6%) of all the species collected whereas, An. annularis, An. fluviatilis s.l. and An. varuna were predominantly zoophagic. The highest and the lowest slide positivity rates (SPR) was recorded during monsoon (36%) and winter (12.5%) seasons, respectively. SPR was positively correlated with vector anopheline densities (r = 0.902), which itself was found to be correlated with mean minimum temperature and relative humidity (RH).
Keywords: Malaria, Forest fringe, Vector, Diversity, Seasonal density
Introduction
Malaria control in forest areas is still a challenge to mankind in many parts of Asia (Erhart et al. 2005). The disease transmission in these areas is intense due to the presence of highly efficient vectors like Anopheles dirus s.l., which have adapted themselves to the forest habitats (Guerra et al. 2006). The rural communities living in forest fringe areas are at high risk of malaria throughout the year. Deforestation and subsequent changes in land use pattern are often regarded as the major risk factors of malaria in these areas. There is frequent movement of humans to and from the forests, which leads to the transmission of malarial parasites from forests to the nearby villages (Kondrashin 1992; Verdrager 1995).
The occurrence of forest malaria in central and northeastern India leads to high rates of morbidity and mortality (Singh et al. 2003; Das et al. 2004). Northeastern India is endemic to malaria and contributes 10–12% of cases and more than 20% of deaths (Dev et al. 2003). The region is highly receptive to the disease and 30–40% of the population is estimated to be at risk (Dev et al. 2009). High relative humidity (RH) and temperature prevailing during most of the year create favourable conditions for the proliferation of malaria vectors. The relationships between malaria transmission and forests need to be understood so as to device strategies for management of malaria in forest and forest fringe areas (Guerra et al. 2006). However, the information on vector diversity and distribution in forest and forest fringe areas in northeastern India is limited due to the inaccessibility of these areas (Srivastava et al. 2001). Hence, the present studies were conducted to understand the diversity and seasonal densities of malaria vector anophelines in forest fringe villages of district Sonitpur, Assam in northeastern India.
Materials and methods
Study area
The studies were conducted during March 2004–February 2005 in three forest fringe villages namely, Bengenajuli, Sapairaumari Pathar and Nigam near the Assam–Arunachal Pradesh border in Sonitpur district of Assam State, India. Sonitpur district (92°20′E–93°45′E and 26°20′N–27°05′N), having an area of 5,324 km2 is situated on the north bank of the river Brahmaputra. The population of Bengenajuli (3,920), Sapairaumari Pathar (4,020) and Nigam (2,443) comprised mainly of ethnic tribes. Bengenajuli and Nigam were situated in the Himalayan foothills while Sapairaumari Pathar was located in plain forested area. The settlers migrated from elsewhere occupied these areas after clearing dense forests. The villagers were engaged in rice cultivation and collection of firewood to make a living. Entomological studies and active malaria surveillance were carried out on monthly basis during the study period, which was categorised into four seasons namely, pre-monsoon (March–May), monsoon (June–August), post-monsoon (September–November) and winter (December–February).
Entomological studies
Adult mosquitoes were collected using CDC miniature light-traps operated from 1800 to 0600 h. Four trap collections were made every month from each village during the study period. A total of 144 trap night collections were made from the three villages, 72 each from human dwellings (HD) and cattle sheds (CS). In each village, the location of the traps remained the same throughout the study period. The mosquitoes were identified using standard keys (Wattal and Kalra 1961; Nagpal et al. 2005) and the densities of known Indian malaria vectors were analysed in terms of season-wise trap catches. The host preference of the vectors was established using their relative abundance in traps set in HD and CS.
Active malaria surveillance
Collection of thick and thin blood smears was done on monthly basis from each village from persons having fever or other symptoms of malaria. A total of 4,300 blood samples were collected from the three villages having a total population of 10,383. The blood smears were stained with Giemsa stain and examined under the microscope for the presence of the parasites. The cases positive for malaria were treated with the help of the State Health Department. Epidemiological indices such as slide positivity rate (SPR), slide falciparum rate (SFR) and Plasmodium falciparum percentage (%Pf) were calculated.
Meteorological data
The data on temperature, RH and rainfall were obtained from the North Eastern Regional Institute of Water and Land Management (NERIWALM), Tezpur, Assam (India).
Data analysis
ANOVA was used for season-wise analysis of vector abundance whereas Student t test was used to compare mean vector densities between HD and CS. Stepwise linear regression was used to establish the relationships between malaria incidence, vector density (VD) and weather parameters.
Results
A total of 7,889 individuals of known Indian malaria vectors were observed in the light trap collections of which 2,259 and 5,630 numbers were collected from HD and CS, respectively. Six species of vector anophelines namely, Anopheles annularis, An. culicifacies s.l., An. fluviatilis s.l., An. minimus s.l., An. philippinensis/nivipes and An. varuna were recorded from all the three study areas while An. dirus s.l. was recorded from Bengenajuli and Nigam only (Table 1). Sapairaumari Pathar recorded the highest number of vector anophelines (42.5%) while Nigam recorded the lowest number (27.5%). An. philippinensis/nivipes (36.8%) and An. annularis (25.6%) were the most abundant among the vector anophelines whereas An. fluviatilis s.l. (3.8%), An. dirus s.l. (3.6%) and An. varuna (3.1%) were the least abundant. An. minimuss.l. and An. culicifacies s.l. constituted 15.8 and 11.2% of the adult collections, respectively.
Table 1.
Percent composition of known Indian malaria vectors in light trap collections from three forest fringe villages in Sonitpur district of Assam (India) during 2004–2005
| Vector species | Bengenajuli | Sapairaumari Pathar | Nigam | Total | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| An. annularis | 667 | 28.13 | 761 | 22.72 | 590 | 27.21 | 2018 | 25.58 |
| An. culicifacies s.l. | 218 | 9.19 | 429 | 12.81 | 240 | 11.07 | 887 | 11.24 |
| An. dirus s.l. | 213 | 9.00 | 0 | 0 | 72 | 3.32 | 285 | 3.61 |
| An. fluviatilis s.l. | 88 | 3.71 | 116 | 3.46 | 100 | 4.61 | 304 | 3.85 |
| An. minimus s.l. | 320 | 13.50 | 545 | 16.27 | 383 | 17.67 | 1248 | 15.82 |
| An. philippinensis/nivipes | 773 | 32.60 | 1455 | 43.45 | 674 | 31.09 | 2902 | 36.80 |
| An. varuna | 92 | 3.88 | 43 | 1.28 | 109 | 5.03 | 244 | 3.09 |
| Total (% of total collection) | 2371 (30.06) | 3349 (42.46) | 2168 (27.48) | 7888 (100) | ||||
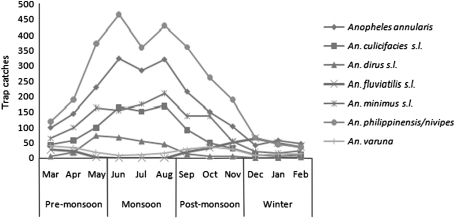
Season-wise analysis of light trap collections indicated that the highest density of malaria vectors was observed during monsoon (F = 12.16; P = 0.002) while the lowest during winter (Table 2). The pre-monsoon and post-monsoon densities did not show any significant difference (P > 0.05). The densities of An. philippinensis/nivipes, An. annularis, An. minimus s.l., An. dirus s.l. and An. culicifacies s.l. started to increase during pre-monsoon period, peaked in monsoon and declined during post-monsoon period (Fig. 1). The populations of these species were sparse during winter season. However, An. fluviatilis s.l. increased during the drier period of the year from post-monsoon to pre-monsoon period and was not recorded during monsoon months in any of the study areas. The highest number of An. varuna was recorded during post-monsoon and pre-monsoon periods and the lowest during monsoon and winter months. Comparisons between the light trap collections made from HD and CS indicated that higher number adults were trapped from CS (71.4%) than HD (28.6%). The preference for animal hosts was significant (P < 0.05) for An. annularis, An. fluviatilis s.l., and An. varuna. However, more number of An. dirus s.l. (91.6%) was collected from HD than from CS indicating anthropophagic nature of the species (Table 3).
Table 2.
Seasonal prevalence of known Indian malaria vectors in light trap collections from three forest fringe villages in Sonitpur district of Assam (India) during 2004–2005
| Species | Mosquitoes collected* | P# | |||
|---|---|---|---|---|---|
| Pre-monsoon (Mar–May) | Monsoon (Jun–Aug) | Post-monsoon (Sep–Nov) | Winter (Dec–Feb) | ||
| An annularis | 158 ± 20.53b | 309 ± 29.82c | 156.67 ± 18.76b | 49 ± 9a | 0.000 |
| An culicifacies s.l. | 66.67 ± 1.45ab | 162.33 ± 56.10b | 58 ± 9.54ab | 8.67 ± 1.76a | 0.029 |
| An dirus s.l. | 31.67 ± 24.11a | 55.33 ± 32.34a | 7.33 ± 6.36a | 0.67 ± 0.67a | 0.290 |
| An fluviatilis s.l. | 17 ± 3.21ab | 0a | 34.67 ± 7.42bc | 49.67 ± 8.41c | 0.002 |
| An minimus s.l. | 108 ± 14.84a | 179.33 ± 31.15a | 109.33 ± 17.14a | 19.33 ± 3.93 | 0.003 |
| An philippinensis/nivipes | 227.67 ± 67.22ab | 419.67 ± 84.13c | 271 ± 95.77ab | 49 ± 4.73a | 0.040 |
| An varuna | 30 ± 9.87a | 10 ± 9.02a | 30.67 ± 3.48a | 10.67 ± 2.4a | 0.115 |
| Total | 639 ± 64.14ab | 1135.67 ± 161.48b | 667.67 ± 135.95ab | 187 ± 21.93a | 0.002 |
Values in rows followed by the same letters are not significantly different (P > 0.05)
* Mean ± SEmean for collections from three villages during each season
#ANOVA followed by Tukey HSD
Fig. 1.
Seasonal densities of known Indian malaria vectors in light trap collections from three forest fringe villages in Sonitpur district of Assam (India) during 2004–2005
Table 3.
Habitat-wise comparison of light trap collections of known Indian malaria vectors from three forest fringe villages in Sonitpur district of Assam (India) during 2004–2005
| Species | Mosquitoes collecteda | pb | |
|---|---|---|---|
| HD | CS | ||
| An. annularis | 175 ± 10.02 | 497.67 ± 47.81 | 0.022 |
| An. culicifacies s.l. | 70.67 ± 9.91 | 225 ± 57.56 | 0.086 |
| An. dirus s.l. | 87 ± 57.64 | 8 ± 4.93 | 0.273 |
| An. fluviatilis s.l. | 28.33 ± 0.88 | 73 ± 8.66 | 0.040 |
| An. minimus s.l. | 132 ± 14.15 | 284 ± 53.58 | 0.064 |
| An. philippinensis/nivipes | 237.67 ± 27.72 | 730 ± 218.16 | 0.123 |
| An. varuna | 22.33 ± 6.89 | 59 ± 12.9 | 0.026 |
| Total | 753 ± 33.96 | 1876.67 ± 364.03 | 0.092 |
HD human dwelling, CS cattle sheds
aMean ± SEmean for collections from three villages for each habitat
bStudent t test (paired samples)
Examination of blood smears from Bengenajuli (n = 1,742), Sapairaumari Pathar (n = 1,379) and Nigam (n = 1,179) revealed 26.1, 28.5 and 24% SPR, respectively, in the three villages. The overall SPR was 26.1%, which ranged from 10.3 to 45.4% with the predominance (79.8%) of P. falciparum. The difference in prevalence of malaria among the four seasons (Table 4) was significant (χ2 = 147.46; P < 0.0001). The highest SPR was recorded during monsoon season (36%) followed by post-monsoon (28.1%), pre-monsoon (17.1%) and the lowest during winter months (12.5%) (Table 4).
Table 4.
Seasonal incidence of malaria in forest fringe villages in Sonitpur district of Assam (India) during 2004–2005
| Season | ABER | SPRa | Relative proportion of malaria incidence (%) | SfR | %Pf |
|---|---|---|---|---|---|
| Pre-monsoon | 10.49 | 16.44 | 15.95 | 12.30 | 74.86 |
| Monsoon | 15.26 | 36.15 | 51.07 | 29.65 | 82.02 |
| Post-monsoon | 9.92 | 28.93 | 26.56 | 23.40 | 83.39 |
| Winter | 5.74 | 12.08 | 6.42 | 8.68 | 69.44 |
| Total | 41.41 | 26.09 | 100 | 20.81 | 79.77 |
ABER annual blood examination rate, SPR slide positivity rate, Sfr slide falciparum Rate; %PfPlasmodium falciparum percentage
aχ2 = 147.46; P < 0.0001
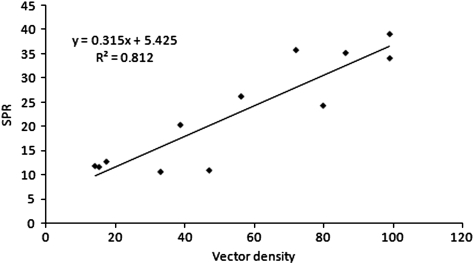
The seasonal incidence of malaria and VD followed the same pattern with two peaks during the year. SPR was high during June–July and September while the vector densities were high during June and August. SPR was significantly (P < 0.001) correlated with VD (r = 0.902; 95% CI 0.779–0.974) and the relationship between the two can be expressed as, SPR = 5.425 + 0.315 VD (R2 = 0.812) (Fig. 2). The density of malaria vectors was found to be correlated with mean minimum temperature (Tmin) and RH. Stepwise linear regression yielded the relationship between VD and meteorological parameters as, VD = 4.201 Tmin + 3.619 RH − 333.889 (R2 = 0.931).
Fig. 2.
Relationship between SPR and known Indian malaria VD (r = 0.902; P < 0.001) in three forest fringe villages in Sonitpur district of Assam (India) during 2004–2005
Discussion
The main vectors of malaria in India are six species of anopheline mosquitoes namely, An. culicifacies s.l., An. dirus s.l., An. fluviatilis s.l., An. minimus s.l., An. sundaicus and An. stephensi whereas, An. philippinensis/nivipes, An. varuna, An. annularis and An. jeyporiensis are considered to be vectors of local importance (Dash et al. 2007). Malaria transmission is perennial in the state of Assam in the northeastern region of India, aided by An. minimus s.l., An. dirus s.l. and An. fluviatilis s.l. (Dev et al. 2004). In the present study, the seasonal densities of these three species and the other known vectors were estimated using light trap collections. It was observed that the population of Indian malaria vector anophelines in the study area increased with the onset of pre-monsoon rain (March), peaked in June and remained high till August and thereafter decreased with the decline in rainfall. However, An. fluviatilis s.l. showed high densities in the winter months (December–February) probably due to the abundance of larval habitats after the rains. An. fluviatilis s.l. is an efficient vector of malaria during post-monsoon season in Assam (Nandi et al. 1993).
Anopheles dirus s.l. was recorded from the foothill villages with high population densities during March–August. The pattern of high density of An. diruss.l. in monsoon and post-monsoon months and low density in cool dry months was also reported from Assam (Dutta et al. 1991) and Arunachal Pradesh (Sen et al. 1973) in India. An. diruss.l. is responsible for hyper-endemic malaria (Rosenberg and Maheswari 1982) and its importance as an efficient malaria vector in the northeastern states of India was well documented (Sen et al. 1973; Dutta et al. 1989). Prakash et al. (1997) reported that deep forest areas in northeastern India offer favourable breeding grounds for this species and that the abundance is determined by the pattern of rainfall. Hence, the villages near the forest, which are under the influence of An. dirus s.l., are more prone to malaria incidence than the other villages (Dutta et al. 1991).
Anopheles minimus s.l. is the principal vector of malaria in the northeastern states of India (Nagpal and Kalra 1997) and its role in malaria transmission is well established (Dutta and Baruah 1987; Dutta and Mahanta 1995; Prakash et al. 1996). High densities of An. minimus s.l. were recorded during July–October in the present study, which was also observed by Baruah et al. (2007) in Assam and Chareonviriyaphap et al. (2003) in Thailand. The peak population of An. culicifacies s.l. was observed during June–August in Nigam and Sapairaumari Pathar and during May–August in Bengenajuli. Bimodal peaks of malaria vector populations were also observed by Sharma and Prasad (1991) in Shahjahanpur, Uttar Pradesh, Das et al. (1990) in Orissa, Kulkarni (1990) in Madhya Pradesh and Shukla et al. (2007) in Uttaranchal in India. An. culicifacies s.l. was incriminated as a vector during an outbreak of malaria in Sonitpur district of Assam (Bhuyan et al. 1997).
The seasonal prevalence of An. varuna did not show a uniform pattern in the study villages possibly due to the variations in the ecological factors. An. varuna and An. annularis were recorded in high density during winter (January–February) and early summer (March–April) in Koraput district of Orissa (Das et al. 1990). However, Tiwari et al. (1997) reported the prevalence of An. varuna only during winter from Allahabad district of Uttar Pradesh. In the present study, An. philippinensis/nivipes and An. annularis were found prevalent through out the year with varying densities in all the study areas with peak during June–August, while peak population during May–June was reported by Rao (1984) from Bengal and southern India. An. philippinensis/nivipes was incriminated as a malaria vector in Assam (Anderson and Viswanathan 1941) and Meghalaya (Rajagopal 1976) long ago. More recently, based on ELISA results, this species was suggested to play a probable role in malaria transmission in northeastern India (Prakash et al. 2005).
Malaria prevalence closely followed the abundance pattern of the known malaria vector species as evident by the high SPR recorded during June–September following the high densities of malaria vectors during May–August. Malaria transmission appeared to be, by and large, perennial though May–November was the period of high transmission. Prolonged seasonal malaria transmission in the forest fringe areas was attributed to the survival of vectors due to the co-existence of various breeding habitats in the two adjacent ecosystems (Kondrashin et al. 1991).
The management of forest malaria is complicated due to various socioeconomic, cultural and geographical factors. The occurrence of drug resistant P. falciparum malaria in India was reported for the first time from Karbi Anglong district of Assam, which is transmitted by An. dirus s.l. along with An. minimuss.l. in northeastern India (Srivastava et al. 2001). Therefore, the forest and forest fringe areas in northeastern India have the potential to serve as the foci of drug resistance. There is a need for the surveillance of the villages situated near the forests in order to monitor the densities of vector anophelines and to detect the occurrence of drug resistant malaria in the local population. The persons moving in and out of these highly malaria prone areas may be subjected to chemoprophylaxis. The use of insecticide impregnated bed nets and indoor residual spray of insecticides along with the organisation of malaria awareness programmes could help in reducing malaria incidence in the region.
Acknowledgment
The authors would like to thank Dr. Lokendra Singh, Director, DRL, Tezpur for his constant encouragement and support during the conduct of the study.
References
- Anderson LAP, Viswanathan DK. The Assam Medical Research Society, Shillong: a resume of its activities during 1931–1941. Calcutta: Thacker Spink & Co.; 1941. [Google Scholar]
- Baruah I, Das NG, Kalita J. Seasonal prevalence of malaria vectors in Sonitpur district of Assam. J Vector Borne Dis. 2007;44:149–153. [PubMed] [Google Scholar]
- Bhuyan M, Das NG, Chakraborty BC, Talukdar PK, Sarkar PK, Das SC, Santhanam K. Role of Anopheles culicifacies during an outbreak of malaria in Garubandha PHC, Assam. J Commun Dis. 1997;29:243–246. [PubMed] [Google Scholar]
- Chareonviriyaphap T, Prabaripai A, Bangs MJ, Aum-Aung B. Seasonal abundance and blood feeding activity of Anopheles minimus Theobald (Diptera: Culicidae) in Thailand. J Med Entomol. 2003;40:876–881. doi: 10.1603/0022-2585-40.6.876. [DOI] [PubMed] [Google Scholar]
- Das PK, Gunasekaran K, Sahu SS, Sadanandane C, Jambulingam P. Seasonal prevalence and resting behaviour malaria vectors in Koraput district, Orissa. Indian J Malariol. 1990;27:173–181. [PubMed] [Google Scholar]
- Das NG, Talukdar PK, Das SC. Epidemiological and entomological aspects of malaria in forest-fringed villages of Sonitpur district, Assam. J Vector Borne Dis. 2004;41:5–9. [PubMed] [Google Scholar]
- Dash AP, Adak T, Raghavendra K, Singh OP. The biology and control of malaria vectors in India. Curr Sci. 2007;92:1571–1578. [Google Scholar]
- Dev V, Bhattacharyya PC, Talukdar R. Transmission of malaria and its control in the northeastern region of India. J Assoc Physicians India. 2003;51:1073–1076. [PubMed] [Google Scholar]
- Dev V, Phookan S, Sharma VP, Anand SP. Physiographic and entomologic risk factors of malaria in Assam, India. Am J Trop Med Hyg. 2004;71:451–456. [PubMed] [Google Scholar]
- Dev V, Sharma VP, Hojai D. Malaria transmission and disease burden in Assam: challenges and opportunities. J Parasit Dis. 2009;33:13–22. doi: 10.1007/s12639-009-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P, Baruah BD. Incrimination of Anopheles minimus Theobald as a vector of malaria in Arunachal Pradesh. Indian J Malariol. 1987;24:159–162. [PubMed] [Google Scholar]
- Dutta P, Mahanta J. Incrimination of Anopheles minimus as a vector of malaria in Karbi Anglong District of Assam. Indian J Malariol. 1995;32:129–131. [PubMed] [Google Scholar]
- Dutta P, Bhattacharyya DR, Sharma CK, Dutta LP. The importance of Anopheles dirus (Anopheles balabacensis) as a vector of malaria in northeast India. Indian J Malariol. 1989;26:95–101. [PubMed] [Google Scholar]
- Dutta P, Bhattacharyya DR, Dutta LP. Epidemiological observation of malaria in some parts of Tengakhat PHC, Dibrugarh district, Assam. Indian J Malariol. 1991;28:121–128. [PubMed] [Google Scholar]
- Erhart A, Thang ND, Ky PV, Tinh TT, Overmeir CV, Speybroeck N, Obsomer V, Hung LX, Thuan LK, Coosemans M, D’alessandro U. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006;100:189–204. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashin AV. Malaria in the WHO southeast Asia region. Indian J Malariol. 1992;29:129–160. [PubMed] [Google Scholar]
- Kondrashin AV, Jung RK, Akiyama J (1991) Ecological aspects of forest malaria in southeast Asia. In: Sharma VP, Kondrashin AV (eds) Forest malaria in southeast Asia. Proceedings of an informal consultative meeting. WHO/MRC, 18–22 Feb 1991. World Health Organization, New Delhi, pp 1–28
- Kulkarni SM. Density pattern of anophelines and their relation to malaria in Bastar district, Madhya Pradesh. Indian J Malariol. 1990;27:187–194. [PubMed] [Google Scholar]
- Nagpal BN, Kalra NL. Malaria vectors in India. J Parasit Dis. 1997;21:105–112. [Google Scholar]
- Nagpal BN, Srivastava A, Saxena R, Ansari MA, Dash AP, Das SC. Pictorial identification key for Indian anophelines. Delhi: Malaria Research Centre (ICMR); 2005. [Google Scholar]
- Nandi J, Misra SP, Rajagopal R, Narasimham MVVL. Present perspectives of malaria transmission in Boko area of Assam. J Commun Dis. 1993;25:18–26. [PubMed] [Google Scholar]
- Prakash A, Mohapatra PK, Srivastava VK. Vector incrimination in Tamulpur Primary Health Centre, District Nalbari, lower Assam during malaria outbreak 1995. Indian J Med Res. 1996;103:146–149. [PubMed] [Google Scholar]
- Prakash A, Bhattacharyya PK, Mohapatra PK, Mahanta J. Seasonal prevalence of Anopheles dirus and malaria transmission in a forest fringed village of Assam, India. Indian J Malariol. 1997;34:117–125. [PubMed] [Google Scholar]
- Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Potential of Anopheles philippinensis-nivipes complex mosquitoes as malaria vector in north-east India. J Environ Biol. 2005;26:719–723. [PubMed] [Google Scholar]
- Rajagopal R. Studies on persistent transmission of malaria in Meghalaya. J Commun Dis. 1976;8:235–245. [Google Scholar]
- Rao TR. The anophelines of India. Delhi: Malaria Research Centre (ICMR); 1984. [Google Scholar]
- Rosenberg R, Maheswari NP. Forest malaria in Bangladesh, II. Transmission by Anopheles dirus. Am J Trop Med Hyg. 1982;31:183–191. doi: 10.4269/ajtmh.1982.31.183. [DOI] [PubMed] [Google Scholar]
- Sen AK, John VM, Krishnan KS, Rajagopal R. Studies on malaria transmission in Tirap district, Arunachal Pradesh (NEFA) J Commun Dis. 1973;5:98–110. [Google Scholar]
- Sharma SN, Prasad RN. Bionomics of Anopheles culicifacies Giles in reverine tract rural areas of district Shahjahanpur, Uttar Pradesh. Indian J Malariol. 1991;28:19–28. [PubMed] [Google Scholar]
- Shukla RP, Sharma SN, Dhiman RC. Seasonal prevalence of malaria vectors and its relationship with malaria transmission in three physiographic zones of Uttaranchal state, India. J Vector Borne Dis. 2007;44:75–77. [PubMed] [Google Scholar]
- Singh N, Mishra AK, Shukla MM, Chand SK. Forest malaria in Chhindwara, Madhya Pradesh, Central india: a case study in a tribal community. Am J Trop Med Hyg. 2003;68:602–607. doi: 10.4269/ajtmh.2003.68.602. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Nagpal BN, Saxena R, Subbarao SK. Predictive habitat modelling for forest malaria vector species An. dirus in India: a GIS-based approach. Curr Sci. 2001;80:1129–1134. [Google Scholar]
- Tiwari SN, Prakash A, Ghosh SK. Seasonality of indoor resting anophelines in stone quarry area of district Allahabad, UP. Indian J Malariol. 1997;34:132–139. [PubMed] [Google Scholar]
- Verdrager J. Localized permanent epidemics: the genesis of chloroquine resistance in Plasmodium falciparum. Southeast Asian J Trop Med Public Health. 1995;26:23–28. [PubMed] [Google Scholar]
- Wattal BL, Kalra NL. Regionwise pictorial keys to the female Indian Anopheles. Bull Natl Soc India Malar Other Mosquito-Borne Dis. 1961;9:85–138. [Google Scholar]