Abstract
The leaves of Houttuynia cordata Thunb. (Saururaceae) are considered to have anthelmintic properties in the traditional medicine of Naga tribes in Northeast India and, therefore, are used by the natives to treat the intestinal worm infections. In the present study, the anticestodal activity of H. cordata leaf extract was investigated against Hymenolepis diminuta, a zoonotic cestode, in experimentally infected albino rats. For the assessment of anticestodal efficacy, the eggs per gram (EPG) of faeces counts and worm loads of animals were monitored following treatment with 200, 400 and 800 mg/kg p.o. doses of leaf extract to different groups of rats harbouring larval, immature and mature H. diminuta infections. The efficacy of the extract was found to be dose-dependent (P < 0.05). Further, the extract showed its maximum efficacy against the mature Hymenolepis worms. In this case, the 800 mg/kg dose of extract significantly reduced (P < 0.001) the EPG counts of animals by 57.09% and worm load by 75.00%, at post-treatment. In comparison, the reference drug praziquantel at 5 mg/kg showed a reduction in the EPG counts and worm load of experimental animals by 80.37 and 87.50%, respectively. These findings indicate that leaves of H. cordata possess significant anticestodal property and provide a rationale for their use in traditional medicine as an anthelmintic.
Keywords: Anthelmintic, Gastro-intestinal parasites, Houttuynia cordata, Neglected tropical diseases, Saururaceae, Tapeworms, Traditional medicine
Introduction
Intestinal helminths are one of the most common causes of infections in humans, especially in tropical and subtropical countries. Current estimates suggest that over half’s of the world population is infected with intestinal helminths (Ascaris, hookworms, Trichuris, Enterobius, Strongyloides and tapeworms), and that most of these infected people live in remote rural areas in the developing countries (Horton 2003). These diseases which are currently referred to as Neglected Diseases of Neglected Populations, cause enormous hazards to the health of people, particularly of children, by contributing to malnutrition, anaemia and retarded growth (Hotez et al. 2007). Although there are several drugs effective against intestinal helminths, the fact remains that about one-third of the world’s population still lacks regular access to essentials drugs, with the figure rising to over 50% in many poor countries (WHO 2002). In this connection, traditional medicines, based largely on herbs and trees, offer a major and accessible source of health care to people living in developing countries (WHO 2002). Medicinal plants have been used as a major source of therapeutic agents by human being for thousand of years and continue to be so in the recent times (Handa 2004; Tandon et al. 2011).
Houttuynia cordata Thunb. (Saururaceae) is a perennial herbaceous plant that grows wild in moist and shady places across many Asian countries, including India. This plant has been widely used in China, Japan and other Asian countries as a folk medicine with antiviral, antibacterial, immunostimulant, diuretic, anti-cancer and anti-inflammatory effects (Yoshino et al. 2005; Lu et al. 2006). In the traditional medicines of Naga tribes of Northeast India, the leaves of H. cordata are considered to have anthelmintic properties and hence used by the natives as a popular cure against intestinal helminthic infections. In our previous study, on in vitro screening of indigenous anthelmintic plants used in this area, we validated the presence of significant anticestodal property in this plant (Temjenmongla and Yadav 2005). Although in vitro testing of antiparasitic properties of plants extracts provide first-hand information about activity of plant extracts, the conclusions drawn through in vitro studies often remain questionable until verified further through in vivo experimentation (Athanasiadou et al. 2007). It was, therefore, considered worthwhile to further substantiate the alleged efficacy of plant through in vivo experimentation. The paper reports the anticestodal efficacy of H. cordata leaf extract against Hymenolepis diminuta in experimentally infected albino rats.
Materials and methods
Preparation of plant extract
Fresh leaves of H. cordata were collected by Dr. Temjenmongla from forest areas near Mokukchung, Nagaland (India) and identified by Dr. P. B. Gurung, Curator, Department of Botany, North-Eastern Hill University (NEHU), Shillong. A voucher specimen (No. AKY/003) of the collected material has been retained at the Department of Zoology, NEHU, Shillong. The shade-dried leaves were powdered and extracted with methanol at 40°C by Soxhlet extraction method. The extract was reduced to dryness using a rotary evaporator and stored at +8°C for further experimentation.
Animal stock
Healthy male and female Wistar strain rats (90–120 g) were used for the study. They were kept at standard environmental conditions and fed with rodent diet (Pranov Agro Industries Ltd., Delhi, India) and water ad libitum. Prior to inclusion in experiments, the faecal samples of animals were examined to confirm that they are not infected with intestinal parasites. All the experiments were performed according to the rules laid down by the Institutional Animal Care and Use Committee (IACUC) of NEHU, Shillong, India.
Maintenance of Hymenolepis diminuta infection
H. diminuta infection was maintained in the laboratory by cyclic passage through Wistar rats and flour beetle, Tribolium confusum Jacquelin du Val (Tenebrionidae) as described by Dixon and Arai (1991). Cysticercoids were dissected from the haemocoel of beetles and administered, by gavage in 1.0 ml of saline, to naive Wistar rats. In 16–18 days time, H. diminuta eggs were detected in the faeces of rats, which constituted the source of infection to intermediate host (flour beetle).
Anticestodal activity
The efficacy of extract was evaluated against the larval, immature and mature H. diminuta infections in rats. For each experiment, animals were divided randomly into five groups comprising of six animals each. Each animal was infected by oral inoculation with four cysticercoids and maintained in a separate cage. Plant extract and praziquantel (PZQ, the reference drug) solutions were prepared fresh in 0.9% phosphate buffer saline. Group 1 of animals served as untreated control and received only the vehicle. While, groups 2–4 of animals were treated with 200, 400 and 800 mg/kg doses of plant extract, respectively. Group 5 of animals were treated with the reference drug praziquantel at 5 mg/kg to compare the efficacy of plant extract.
In order to determine the efficacy against the larval stage, animals were administered with extract and PZQ from day 2 to 6 post inoculation (p.i.) of cysticercoids. From day 18 post infection, fresh faecal samples of rats were collected from cages and eggs per gram (EPG) counts were determined for each animal (Anonymous 1977) for 3 days (days 18–20). All animals were then sacrificed under chloroform anesthesia on day 31 and their intestines were examined under a binocular microscope for the presence of worms. The efficacy of extract was adjudged on the basis of reduction in EPG counts and worm load of animals after treatment, as described by Rim et al. (1980):
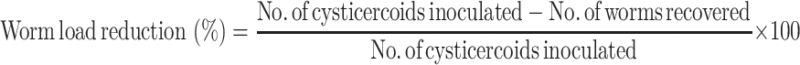 |
In case of efficacy against immature stages, animals were treated from day 8 to 12 p.i. of cysticercoids, and their EPG counts were determined on days 18–20. They were autopsied on day 31, and the number of worms in their intestines was counted to determine the reduction in worm load. To evaluate the efficacy of extract against mature stages, animals were treated from day 21 to 25 p.i. of cysticercoids. The EPG counts were performed on days 18–20 (pre-treatment period) and days 26–28 (post-treatment period) and faecal egg count reduction (FECR) was calculated as described by Iqbal et al. (2004). All animals were sacrificed at day 39 p.i. and reduction in their worm load was calculated as described previously.
 |
Statistical analysis
Results are reported as mean ± SEM for each group. Student’s t-test was used to determine the level of significance of results. The values were considered significant when P < 0.05.
Results and discussion
The Northeast region of India is home to a number of aboriginal tribes (Boro, Naga, Khasi, Mizo etc.) who use several traditional medicinal plants to cure intestinal parasitic infections. However, there exists only a limited scientific evidence regarding the alleged efficacy of these plants, as claimed by local people, so as to justify their logical use in traditional medicine (Temjenmongla and Yadav 2005; Yadav and Tangpu 2008). We have previously reported the in vitro anticestodal activity of one such plant, namely H. cordata, using poultry cestode as a test parasite (Temjenmongla and Yadav 2005). In the present study, we were interested to further verify the alleged anthelmintic efficacy of H. cordata using an appropriate in vivo experimental model. This study was therefore carried out using H. diminuta-rat experimental model, which has been recognized as a suitable model for screening anticestodal drugs (Andreassen 1991).
In this study, the efficacy of extract was investigated against the larval, immature, and mature stages of parasite. However, most previous studies document the anthelmintic effects of plant extracts on mature parasites and there is paucity of information on effects of plant extracts on larval or immature parasites (Galal et al. 1991; Ghosh et al. 1996). The present study revealed that H. cordata extract possesses a dose-dependent, and moderate to moderately high level of efficacy (P < 0.05) against various stages of parasite. Except for the adult stage, where the efficacy of extract was found to be maximum, the extract did not showed a comparable level of efficacy with that of the reference drug, PZQ against the rest two stages of parasite (Tables 1, 2, 3). To determine the efficacy of extract against the larval stage, animals were treated from day 2 to 6 post inoculation of cysticercoids. The extract showed a dose-dependent, moderate level of efficacy (P < 0.001) against this stage. The autopsied animals in the 800 mg/kg extract-treated group exhibited only 50.00% reduction in their worm load, whereas the reference drug PZQ (5 mg/kg) exhibited comparatively better efficacy and revealed 66.50% reduction in worm load. Almost similar trends were noticeable in terms of EPG counts of animals. The animals treated with 800 mg/kg dose of extract revealed an EPG count of 8111 ± 263, compared to 3900 ± 243 recorded in the PZQ-treated group (Table 1). A moderate level of extract efficacy during this phase of infection suggests that the larval stages of parasite are relatively less susceptible to extract treatment. With regard to the effects of extract on immature stages, the results were almost of similar nature as observed in the case of larval stages. The reduction in worm load by 800 mg/kg dose of extract was observed to be 66.50% compared to 81.25% by PZQ. In a similar manner, the EPG counts of animals treated with the same dose of extract was recorded to be 8555 ± 58 compared to 4200 ± 114 in observed in the PZQ-treated group (Table 2).
Table 1.
Anticestodal effects of H. cordata leaf extract on larval H. diminuta infections in rats
| Treatmenta | EPG count (mean ± SEM) (days 18–20) | No. of worms at necropsy (mean ± SEM) | Percent reduction in worm load at necropsy |
|---|---|---|---|
| Control (vehicle) | 18888 ± 379 | 3.84 ± 0.17 | 4.00 |
| Plant extract | |||
| 200 mg/kg | 16333 ± 358* | 3.00 ± 0.26* | 25.00 |
| 400 mg/kg | 14000 ± 379* | 2.34 ± 0.21* | 41.50 |
| 800 mg/kg | 8111 ± 263* | 2.00 ± 0.26* | 50.00 |
| Praziquantel | |||
| 5 mg/kg | 3900 ± 243* | 1.34 ± 0.21* | 66.50 |
No. of animals in each group, n = 6
* P < 0.001 vs. control value
aTreatment from day 2 to 6 after inoculation of 4 cysticercoids per rat
Table 2.
Anticestodal effects of H. cordata leaf extract on immature H. diminuta infections in rats
| Treatmenta | EPG count (mean ± SEM) (days 18–20) | No. of worms at necropsy (mean ± SEM) | Percent reduction in worm load at necropsy |
|---|---|---|---|
| Control (Vehicle) | 17988 ± 425 | 3.84 ± 0.17 | 4.00 |
| Plant extract | |||
| 200 mg/kg | 13800 ± 217* | 2.50 ± 0.34** | 37.50 |
| 400 mg/kg | 12377 ± 81* | 1.50 ± 0.22* | 62.50 |
| 800 mg/kg | 8555 ± 58* | 1.34 ± 0.21* | 66.50 |
| Praziquantel | |||
| 5 mg/kg | 4200 ± 114* | 0.75 ± 0.25* | 81.25 |
No. of animals in each group, n = 6
* P < 0.001 and ** P < 0.01 vs. control value
aTreatment from day 8 to 12 after inoculation with 4 cysticercoids per rat
Table 3.
Anticestodal effects of H. cordata leaf extract on adult H. diminuta infections in rats
| Treatmenta | EPG count (mean ± SEM) | Percentage reduction in EPG counts at post-treatment | No. of worms at necropsy (mean ± SEM) | Percent reduction in worm load at necropsy | |
|---|---|---|---|---|---|
| Pre-treatment (days 18–20) | Post-treatment (days 26–28) | ||||
| Control (vehicle) | 17777 ± 313 | 17711 ± 281 | 0.37 | 3.67 ± 0.21 | 8.25 |
| Plant extract | |||||
| 200 mg/kg | 15155 ± 557 | 10955 ± 848** | 27.71 | 2.50 ± 0.22**** | 37.50 |
| 400 mg/kg | 17600 ± 290 | 11311 ± 241* | 35.73 | 1.34 ± 0.21*** | 66.50 |
| 800 mg/kg | 17400 ± 517 | 7466 ± 385* | 57.09 | 1.00 ± 0.37*** | 75.00 |
| Praziquantel | |||||
| 5 mg/kg | 18111 ± 316 | 3555 ± 243* | 80.37 | 0.50 ± 0.22*** | 87.50 |
No. of animals in each group, n = 6
* P < 0.001 and ** P < 0.02 vs. pre-treatment value. *** P < 0.001 and **** P < 0.01 vs. control value
aTreatment from day 21 to 25 after inoculation with 4 cysticercoids per rat
In contrast to the preceding two stages, the extract showed its maximum efficacy (in terms of reduction in EPG count and worm load) against the mature H. diminuta in rats. The animals treated with 800 mg/kg dose of extract exhibited 75.00% reduction in the worm load as compared to 87.50% by 5 mg/kg dose of PZQ. In a similar manner, the animals treated with the same dose of extract showed 57.09% FECR at post-treatment period, compared to 80.37% by PZQ. The animals in the control group, however, neither revealed any noticeable reduction in worm load or EPG counts at post-treatment (Table 3). It is, therefore, evident from these observations that comparatively more numbers of worms are cleared from host if the extract is administered during this phase of infection. This high level of efficacy against mature parasites could be due to destrobilation and subsequent expulsion of adult tapeworms under the influence of plant extract. It has been reported that the process of destrobilation and expulsion in adult cestodes may initiate if they encounter a hostile physiological environment, such as the presence of anthelmintic compound in the intestine (Hopkins et al. 1973).
The findings of present study are in agreement with other related studies on anthelmintic effects of anticestode drugs or plant extracts on Hymenolepis infections in rodents. For example, Maki and Yanagisawa (1985) in a study on comparative anthelmintic effects of four anticestode drugs on immature and mature Hymenolepis nana in mice noted that these drugs have rather low level of efficacy against immature than mature worms. Similarly, Marshall (1982) reported a 100% reduction in adult worms using PZQ in mice, however, the efficacy of drug was noted be quite low against the larval stage of parasite. In another study, the alcoholic extract of a shrub Diospyros mollis, known as Ma-klua in Thailand was found to be quite effective in elimination of adults but not the larvae of tapeworm in mice (Maki et al. 1983). It is worth mentioning here that alkaloids, flavonoids and essential oils constitute the major chemical constituents of the aerial parts of H. cordata (Pröbstle and Bauer 1992; Hu and Du 1997; Xu et al. 2006). There are several published reports which demonstrate the presence of anthelmintic activity in these plant constituents (Akhtar et al. 2000; Athanasiadou et al. 2007). Due to wide applicability of H. cordata extract to cure a whole variety of illness and ailments, the plant extract has also been subjected to a detailed oral toxicity study in experimental rats. In general, the extract was found to be safe, and no treatment related adverse effects on body weight, food and water consumption, or on ophthalmology, haematology and urinalysis were noted in the experimental animals (Yoshino et al. 2005).
In conclusion, the findings of the present study indicate that leaf extract of H. cordata possesses potential anticestodal activity. This activity thus lends support to the suggested use of the plant as anthelmintic in the folk medicine of Naga tribes in Northeast India.
Acknowledgments
This study was supported by a grant under the DSA-SAP Programme of UGC, New Delhi to the Department of Zoology, NEHU, Shillong. Temjenmongla was recipient of a Senior Research Fellowship by CSIR, New Delhi.
References
- Akhtar MS, Iqbal Z, Khan MN, Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Rumin Res. 2000;38:99–107. doi: 10.1016/S0921-4488(00)00163-2. [DOI] [Google Scholar]
- Andreassen J. Immunity to adult cestodes: basic knowledge and vaccination problems: a review. Parassitologia. 1991;33:45–53. [PubMed] [Google Scholar]
- Anonymous (1977) Manual of veterinary parasitological laboratory techniques. Technical Bulletin No. 18. Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationery Office, London, pp 1–57
- Athanasiadou S, Githiori J, Kyriazakis I. Medicinal plants for helminth parasite control: facts and fiction. Animal. 2007;1:1392–1400. doi: 10.1017/S1751731107000730. [DOI] [PubMed] [Google Scholar]
- Dixon BR, Arai HP. Anthelmintic-induced destrobilation and its influence on calculated drug efficacy in Hymenolepis diminuta infections in rats. J Parasitol. 1991;77:769–774. doi: 10.2307/3282714. [DOI] [PubMed] [Google Scholar]
- Galal M, Bashir AK, Salih AM, Adam SEI. Activity of water extracts of Albizzia anthelmintica and A. lebbek barks against experimental Hymenolepis diminuta infections in rats. J Ethnopharmacol. 1991;31:333–337. doi: 10.1016/0378-8741(91)90019-A. [DOI] [PubMed] [Google Scholar]
- Ghosh NK, Sinha Babu SP, Sukul NC, Ito A. Cestocidal activity of Acacia auriculiformis. J Helminthol. 1996;70:171–172. doi: 10.1017/S0022149X00015340. [DOI] [PubMed] [Google Scholar]
- Handa H. Handbook on herbal drugs and plant sources. New Delhi: National Institute of Industrial Research; 2004. [Google Scholar]
- Hopkins CA, Grant PM, Stallard H. The effect of oxyclozanide on Hymenolepis microstoma and H. diminuta. Parasitology. 1973;66:355–365. doi: 10.1017/S0031182000045285. [DOI] [PubMed] [Google Scholar]
- Horton J. Human gastrointestinal helminth infections: are they now neglected diseases? Trends Parasitol. 2003;19:527–531. doi: 10.1016/j.pt.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. New Eng J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Hu SH, Du AF. Treatment of bovine mastitis with houttuyin sodium bisulphate. J Vet Med B. 1997;44:365–370. doi: 10.1111/j.1439-0450.1997.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Lateef M, Ashraf M, Jabbar A. Anthelmintic activity of Artemisia brevifolia in sheep. J Ethnopharmacol. 2004;93:265–268. doi: 10.1016/j.jep.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Lu H-M, Liang Y-Z, Wu X-J, Qiu P. Tentative fingerprint-efficacy study of Houttuynia cordata injection in quality control of traditional Chinese traditional medicine. Chem Pharm Bull. 2006;54:725–730. doi: 10.1248/cpb.54.725. [DOI] [PubMed] [Google Scholar]
- Maki J, Yanagisawa T. Anthelmintic effects of bithionol, paromomysin sulphate, flubendazole and mebendazole on mature and immature Hymenolepis nana in mice. J Helminthol. 1985;59:211–216. doi: 10.1017/S0022149X00007963. [DOI] [PubMed] [Google Scholar]
- Maki J, Kondo A, Yanagisawa T. Effects from alcoholic extracts from Ma-Klua (Diospyros mollis) on adults and larvae of dwarf tapeworm Hymenolepis nana in mice and on the infectivity of the eggs. Parasitology. 1983;87:103–111. doi: 10.1017/S0031182000052458. [DOI] [PubMed] [Google Scholar]
- Marshall I. The effects of sustained-release praziquantel on the survival of Hymenolepis nana in laboratory mice. Ann Trop Med Parasitol. 1982;75:115–116. doi: 10.1080/00034983.1982.11687514. [DOI] [PubMed] [Google Scholar]
- Pröbstle A, Bauer R. Aristolactams and a 4,5-dioxoaporphine derivative from Houttuynia cordata. Planta Med. 1992;58:568–569. doi: 10.1055/s-2006-961554. [DOI] [PubMed] [Google Scholar]
- Rim HJ, Ha JH, Kim SJ. Experimental study on the therapeutic effects of praziquantel (Embay 8440) in rats experimentally infected with Clonorchis sinensis. Kisaengchunghak Chapchi. 1980;18:65–80. doi: 10.3347/kjp.1980.18.1.65. [DOI] [PubMed] [Google Scholar]
- Tandon V, Yadav AK, Roy B, Das B. Phytochemicals as cure of worm infections in traditional medicine systems. In: Srivastava UC, Kumar S, editors. Emerging trends in zoology. New Delhi: Narendra Publishing House; 2011. pp. 351–378. [Google Scholar]
- Temjenmongla, Yadav AK. Anticestodal efficacy of folklore medicinal plants of Naga tribes in north-east India. Afr J Trad CAM. 2005;2:129–133. [Google Scholar]
- WHO (2002) WHO traditional medicine Strategy 2002–2005. WHO/EDM/TRM/2002.1
- Xu X, Xe X, Wang W, Yu L, Chen G. Determination of flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by capillary electrophoresis with electrochemical detection. Talanta. 2006;68:759–764. doi: 10.1016/j.talanta.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Tangpu V. Anticestodal activity of Adhatoda vasica extract against experimental Hymenolepis diminuta infections in rats. J Ethnopharmacol. 2008;119:322–324. doi: 10.1016/j.jep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Yoshino H, Imai N, Nabae K, Doi Y, Tamano S, Ogawa K, Shirai T. Thirteen-week oral toxicity study of Dokudami extract (Houttuynia cordata Thunb.) in F344/DuCrj rats. J Toxicol Pathol. 2005;18:175–182. doi: 10.1293/tox.18.175. [DOI] [Google Scholar]


