Abstract
The problems associated with synthetic chemical pesticides include resistance, residues, pest resurgence and the adverse effects on non-target organisms. Hence, many ecofriendly newer alternatives are being evaluated among which one of the most important is the use of herbal agents. Many of the plant extracts or its fractions are dissolved in polar or non polar solvents or detergents before tested for acaricidal activity. The solvent or detergent used for dissolving the herbal extract should have little or no acaricidal effects. In the present study, laboratory tests were carried out on engorged female Rhipicephalus (Boophilus) annulatus ticks to detect the toxicity of common diluents such as dimethyl sulfoxide (DMSO), Tween 20 and Triton X 100 at 1% concentration. The result of the study revealed that least acaricidal activity was with Triton X 100 while the other two inhibited the hatching of eggs laid by treated ticks.
Keywords: DMSO, Triton X 100, Tween 20, Rhipicephalus (Boophilus) annulatus
Introduction
Ticks are obligate haematophagous ectoparasites of domestic animals. They are considered to be second to mosquitoes as vectors of human diseases (Parola and Raoult 2001) and the most important vectors affecting the cattle industry worldwide (Peter et al. 2005).
Despite efforts to control tick infestation, these ectoparasites remain a serious problem for human and animal health (de la Fuente and Kocan 2006; Willadsen 2006). In India, the cost of tick and tick-borne disease (TTBD) control in animals was estimated as US $498.7 million per annum (Minjauw and Mc Leod 2003). Rhipicephalus (Boophilus) annulatus was recorded as the commonest tick species in southern India (Jagannath et al. 1979; Koshy et al. 1982; Rajamohanan 1982). The problem of development of resistance to chemical acaricides and environmental pollution due to the residues necessitates the search for a possible ecofriendly alternative for the control of these acarine parasites. Development of herbal acaricides is one such method (Ghosh et al. 2007; FAO 2004). The herbal extracts have to be dissolved in a polar or non-polar solvent/detergents before testing their acaricidal activity. These agents should be non-toxic to the host and ideally with minimum acaricidal effects. Hence, the present investigation focuses on the effects of three common diluents viz., dimethyl sulfoxide (DMSO), Tween 20 and Triton X 100 at 1% concentration against the one host tick R. (B.) annulatus.
Materials and methods
Chemical reagents
Analytical reagent grade chemicals of DMSO, Tween 20 and Triton X 100 were used at 1% concentration for the experiment.
Ticks
Fully engorged adult R. (B.) annulatus female ticks were collected from infested animals, washed with water and dried using tissue paper. They were used for adult immersion test (AIT) based on Drummond et al. (1973).
Adult immersion test (AIT)
Four replicates of six ticks were used for testing one diluent. Each replicate was immersed in the solution (10 ml) at room temperature for 2 min in a 50 ml beaker with gentle agitation. Water was used as the control. Ticks were recovered from the solutions, dried and placed in plastic specimen tube (25 × 50 mm). They were incubated at 28°C and 80% relative humidity in a BOD incubator.
Assessment of the effect on ticks
The effect of DMSO, Triton X 100 and Tween 20 on ticks was assessed based on the mortality, inhibition of fecundity and hatching. Adult tick mortality was observed on day 15 after immersion. The eggs laid by the female ticks of each replicate were collected and weighed. Percentage inhibition of fecundity (FAO 2004) was calculated as follows:
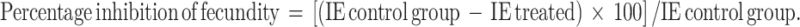 |
The index of fecundity was calculated using the formula,
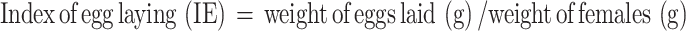 |
The eggs were incubated for hatching and approximate hatching percentage was assessed visually.
Results and discussion
The results of AIT using different agents are depicted in the Table 1.
Table 1.
Effects of DMSO, Tween 20 and Triton X 100 against R. (B.) annulatus
| Sl. no | Diluents (1%) | Mean ticks weight per replicate ± SEM | Mean % adult mortality within 15 days ± SEM | Mean eggs mass per replicate ± SEM | Index of fecundity ± SEM | Percentage inhibition of fecundity | Hatching % (visual) |
|---|---|---|---|---|---|---|---|
| 1 | Tween 20 | 0.8580 ± 0.037a | 8.33 ± 4.809b | 0.3625 ± 0.0228a | 0.4239 ± 0.0293a | 11.02 | 25 |
| 2 | Triton X 100 | 0.9330 ± 0.0479a | 0 ± 0a | 0.4323 ± 0.0272b | 0.4647 ± 0.0278a | 2.46 | 25 |
| 3 | DMSO | 0.9615 ± 0.0267a | 0 ± 0a | 0.4273 ± 0.0155b | 0.4445 ± 0.012a | 6.7 | 0 |
| 4 | Control (H2O) | 0.8928 ± 0.0257a | 0 ± 0a | 0.4238 ± 0.0089b | 0.4764 ± 0.0219a | 0 | 100 |
Values are mean ± SEM, n = 4 in each group
Values in a column with different superscripts differ (P < 0.05)
At 1% concentration both DMSO and Triton X 100 did not result in any mortality of adult ticks within 15 days of treatment. Inhibition of fecundity was lowest for Triton X 100. All agents resulted in significant reduction in hatching. DMSO completely inhibited the hatching of eggs laid by the treated ticks. Triton X 100 at 1% concentration can be considered as an agent with least acaricidal action and hence can be used for dissolving the herbal extracts for testing their acaricidal effects against R. (B.) annulatus.
DMSO can cause severe cell damage (Brayton 1986; Penninckx et al. 1983). Triton X 100 and Tween 20 are non-ionic polyoxyethylene detergents. DMSO [(H3C)2 S=O] (Stammati et al. 1996), Triton X 100 and Tween 20 are partly soluble in both aqueous and organic media. Due to the lipophilicity of these compounds, they can remove the epicuticular waxy layer of the ticks, delivering the active ingredients into the integument and thereby resulting in these effects. In the present study, the toxic effects produced by all these agents against adult ticks were comparatively lesser but resulted in inhibitory effect on hatching of eggs laid by them.
A previous study by Goncalves et al. (2007) recommended all these agents for use in bioassays involving R. (B.) microplus, especially DMSO. But the present study recommends Triton X 100 (1%) for use in bioassays involving R. (B.) annulatus. However, the solvent Tween 20 producing higher percentage adult mortality and inhibition of fecundity can be a good candidate as a solvent for topical formulation for in vivo application as its synergistic action with the herbal extracts can reduce the dose of the final product yielding better acaricidal effect.
Acknowledgment
Financial support from Indian Council of Agricultural Research through World Bank funded National Agricultural Innovation Project No. C2066 is thankfully acknowledged.
References
- Brayton CF. Dimethyl sulfoxide (DMSO): a review. Cornell Vet. 1986;76:61–90. [PubMed] [Google Scholar]
- la Fuente J, Kocan KM. Strategies for development of vaccines for control of Ixodid tick species. Parasite Immunol. 2006;28:275–283. doi: 10.1111/j.1365-3024.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- Drummond RO, Ernst SE, Trevino JL, Gladney WJ, Graham OH. Boophilus annulatus and Boophilus microplus: laboratory tests for insecticides. J Econ Entomol. 1973;66:130–133. doi: 10.1093/jee/66.1.130. [DOI] [PubMed] [Google Scholar]
- Ticks: acaricide resistance: diagnosis management and prevention in: guidelines resistance management and integrated parasite control in ruminants. Rome: FAO Animal Production and Health Division; 2004. [Google Scholar]
- Ghosh S, Azhahianambi P, Yadav MP. Upcoming and future strategies of tick control: a review. J Vector Borne Dis. 2007;44:79–89. [PubMed] [Google Scholar]
- Goncalves K, Toigo E, Ascoli B, Poser G, Ribeiro VS. Effects of solvents and surfactant agents on the female and larvae of cattle tick Boophilus microplus. Parasitol Res. 2007;100:1267–1270. doi: 10.1007/s00436-006-0418-2. [DOI] [PubMed] [Google Scholar]
- Jagannath MS, Muraleedharan K, Hiregoudar LS. Prevalence of Ixodid ticks of cattle at Bangalore. Indian J Anim Sci. 1979;49:890–894. [Google Scholar]
- Koshy TJ, Rajavelu G, Lalitha CM. Ecology and bionomics of boophilids of Tamil Nadu. Cheiron. 1982;11:25–30. [Google Scholar]
- Minjauw B, Mc Leod A (2003) Tick—borne diseases and poverty: the impact of ticks and tick-borne diseases on the livelihood of small scale and marginal livestock owners in India and eastern and southern Africa. Research report, DFID Animal Health Programme. Centre for Tropical Veterinary Medicine, University of Edinburgh, UK
- Parola P, Raoult D. Tick borne bacterial disease emerging in Europe. Clin Microbiol Infect. 2001;7:80–83. doi: 10.1046/j.1469-0691.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- Penninckx F, Cheng N, Kerremans R, Damme B, Loecker W. The effects of different concentrations of glycerol and dimethyl sulfoxide on the metabolic activities of kidney slices. Cryobiology. 1983;20:51–60. doi: 10.1016/0011-2240(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Peter RJ, Bossche P, Penzhorn BL, Sharp B. Tick, fly, and mosquito control—lessons from the past, solutions for the future. Vet Parasitol. 2005;132:205–215. doi: 10.1016/j.vetpar.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Rajamohanan K (1982) Identification of vector for babesiosis of cattle in Kerala. Proceedings of all India symposium of vectors and vector born diseases, Trivandrum, Kerala, pp 125–128
- Stammati A, Zampaglioni F, Zucco F. Furaltadone cytotoxicity on three cell lines in the presence or absence of DMSO: comparison with furazolidone. Cell Biol Toxicol. 1996;13:125–130. doi: 10.1023/B:CBTO.0000010397.26120.4d. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Tick control: thoughts on a research agenda. Vet Parasitol. 2006;138:161–168. doi: 10.1016/j.vetpar.2006.01.050. [DOI] [PubMed] [Google Scholar]


