Abstract
The therapeutic efficacy of antimalarial drugs and their effect on various organs in the form of surface morphological deformations can be analyzed using scanning electron microscopy (SEM). Present study has been undertaken on Plasmodium berghei (NK-65), a lethal rodent malaria parasite, to monitor the morphological changes in blood cells induced by the treatment with combination of artesunate and homeopathic medicine . Combination therapy of artesunate (100 mg/kg) and China ϕ was found to be highly effective in clearing the blood stage infection of Plasmodium berghei and it also enhanced the mean survival time (28 ± 0 days) of mice. Not much morphological changes were induced on WBCs and RBCs of mice treated with combination therapy but in treated groups the number of live PMN cells was more as observed in AO/EB staining. In normal mice the mononuclear cells were both smooth surfaced and layered surfaced, whereas, polymorphonuclear cells were having finger like projections. The combination of artesunate and China was found to be very effective and did not cause any alteration on the surface of blood cells as observed in SEM.
Keywords: Scanning electron microscopy, Plasmodium berghei, Artesunate, Homeopathy, China
Introduction
WHO has banned the monotherapy of artemisinin (IASG 2004; Jambou et al. 2005) because of its poor bioavailability and the possibility of emergence of artemisinin resistant Plasmodium falciparum strains due to the problem of recrudescence. WHO recommends in particular the use of artemisinin based combination therapy (WHO 2006). Due to increasing resistance to classical antimalarials there is a pressing need for discovering and developing novel chemotherapeutic agents which can be better-tolerated and combined with artemisinin and its derivatives.
Homeopathy is a nontoxic form of alternative medicine which can be combined with classical treatment methods for avoiding the risk of adverse effects (WHO 2002). Homeopathic drugs have been claimed for their efficacy, no side effects and low cost. Thus they can be evaluated for their suitable candidature along with artemisinin derivatives. China (Cinchona, Quinine) produced from bark of Cinchona officinalis was the first homeopathic drug discovered by Hahnemann justifying the principle of homeopathy i.e. “Like Cures Like”. The Cinchona bark is the source of a variety of alkaloids which include cinchonine, cinchonidene, quinine, quininidine, dihydroquinidine and dihydroquinine all used as anti-fever agents and also for treating malaria (Yarnell and Abascal 2004). China also cures low forms of fever, remittent or intermittent or malaria and additionally kills parasites (Saxena 2006). The combination of artesunate (100 mg/kg) and another homeopathic medicine eucalyptus mother tincture (ϕ) has been reported to provide a mutual protection against the Plasmodium berghei infection in Balb/c mice (Bagai et al. 2008). In another study, it was confirmed that 7 days oral administration of homeopathic medicine China (ϕ) or China 30 potency in monotherapy, leads to inhibition of parasite load in P. berghei infected Balb/c mice up to a large extent with 50% survival rate of mice till 28th day. Both showed high efficacy when given in combination with artesunate 100 mg/kg (Rajan and Bagai 2009). In the present study Plasmodium berghei (NK-65), a lethal rodent malaria parasite has been used to check the efficacy of artesunate in combination with homeopathic drug China with the objective of checking their effect on the morphological organization of blood cells.
Blood cells were among the first biological specimens to be studied using SEM. Numerous ultra structural studies of RBC’s infected with P. berghei have been made by Bodammer and Bahr (1973) who reported two types of alterations on surface of red cells. The infected red cells had been shown to have randomly located depressions in scanning electron micrographs. White cell populations are generally considered to comprise of granulocytes, monocytes and lymphocytes (Cline 1975). The murine mononuclear population consists of spreading cells with pseudopodia, surface ruffles, ridges and micro processes, as well as a population of spherical generally non-spreading cells with variable number of small surface villi (Wetzel 1976). Lymphocytes have been defined as smooth, villous or relatively smooth (Schneider et al. 1978; Firket and Schaaf-Lafantaine 1976).
It is necessary to combine SEM studies of mixed white cell populations with an independent method of identifying individual cells. SEM has been combined with light microscopy to identify individual cells (Wetzel et al. 1973). Present study has been undertaken to evaluate the effect of artesunate in combination with homeopathic medicine China on morphological organization of blood cells in P. berghei (NK-65) infected Balb/c mice.
Materials and methods
Mouse and parasite strain
White swiss mice Mus musculus of Balb/c strain (weighing 22–35 g and 4–6 weeks old) of either sex, obtained from the Central Animal House, Panjab University, Chandigarh were used as experimental models. They were maintained on a standard pellet diet and water ad libitum. P. berghei (NK-65) was maintained by intraperitoneal inoculation of 1×106 infected RBCs to naïve mice (Santiyanont 1985).
Ethical clearance
The treatment of mice was according to the guidelines of committee for the purpose of control and supervision of experiments on animals (Reg No. 45/1999/CPCSEA), Panjab University, Chandigarh, India.
Experimental design
Eight groups having ten mice each (same sex and age) were used for the present study. Groups were designated as G-1 to G-8 (Table 1). All groups except G-1 were injected with 1×106P. berghei parasitized red blood cells on day 0 (D0). Dose of various antimalarials/vehicles (0.2 ml/mouse/day) was administered orally to mice of different groups for 7 days (D0–D6).
Table 1.
Table showing percent parasitaemia on various days of study and mean survival time (days) in experimental groups
| Group | Day 3 | Day 5 | Day 7 | Day 14 | Day 21 | Day 28 | Mean survival time (days) |
|---|---|---|---|---|---|---|---|
| G-1 (DW) | 0 | 0 | 0 | 0 | 0 | 0 | 28 ± 0 |
| G-2 (DW) | 4 ± 2.3 | 36.25 ± 5.2 | 49.5 ± 3.53 | 7 ± 0 | |||
| G-3 (China ϕ) | 0.93 ± 0.7 | 5.38 ± 4.43** | 4.9 ± 0.92** | 10.3 ± 2.2 | 8.9 ± 4.52 | 2.5 ± 1.44 | 18.3 ± 10.4 |
| G-4 (nascent alcohol) | 3.72 ± 2.37 | 25.1 ± 0 | 5 ± 0 | ||||
| G-5 (AS 100 mg/kg) | 2.1 ± 0.9 | 1.78 ± 0.05** | Clear** | Clear | 2.07 ± 0.64 | 3.99 ± 0.99 | 28 ± 0 |
| G-6 (5%NaHCO3 + 0.9%NaCl) | 3.76 ± 1.05 | 12.26 ± 0.11* | 31.19 ± 1.77 | 6.5 ± 0.5 | |||
| G-7 (AS 100 mg/kg + China ϕ) | 1.56 ± 0.3 | 0.68 ± 0.1** | 0.12 ± 0** | Clear | Clear | Clear | 28 ± 0 |
| G-8 (AS + SP) | 0.9 ± 0.02 | 0.5 ± 0.02** | 0.16 ± 0** | Clear | Clear | Clear | 21 ± 6.27 |
AS is administered BD on day 0 and OD on D1–D6, China is given 1 h after the oral administration of artesunate and standard dose of 4 mg AS + 1.2 mg/kg SP is given to G-8. All the groups except G-1 was injected with 1 × 106 infected RBC on day 0. Data is given as mean ± SD
P value in comparison to infected control (G-2) is shown as # statistically significant (P < 0.05), * very statistically significant (P < 0.005), ** extremely statistically significant (P < 0.0005)
Drugs used
Falcigo tablet containing 50 mg artesunate base (Cadila Pharmaceutical Company, India) was used. The tablet was suspended in 1 ml of (5%) Na2HCO3 and 5 ml (0.9%) NaCl. Artesunate was given (100 mg/kg) bi daily (BD) on first day and once in a day (OD) for 6 days. China mother tincture (ϕ) (Dr. Willmar Schwabe India Pvt. Ltd., Noida, India) was used. China ϕ, which consisted of one part of drug in nine parts of nascent alcohol was diluted in distilled water in ratio of 1:2 and administered OD for 7 days (0.2 ml/mice/day), 1–2 h after the administration of AS. AS + SP was kept as positive control and standard recommended dose of AS (4 mg/kg) + SP (1.2 mg/kg; Laridox tab. containing 500 mg Sulphadoxine and 25 mg Pyremethamine) was administered.
Mice from groups G-1, G-2, G-3, G-5, G-7 and G-8 were sacrificed on 7th day post inoculation. Blood was aspirated out in citrate saline after jugular vein incision. For SEM studies blood was subjected to double density gradient centrifugation for isolation of mononuclear (MN) and polymorphonuclear (PMN) cells. SEM was combined with light fluorescent microscopy (Acridine Orange/Ethidium Bromide staining, AO/EB) to calculate the percentage of live and dead cells.
Separation of WBC’s by double density gradient centrifugation
MN and PMN cells were separated through double density gradient centrifugation using histopaque-1119 and 1077 (Czuprynski and Brown 1998). The percentage of live/dead/apoptotic cells was calculated by fluorescent staining of cells using AO/EB (Kasibhatla 1998). Five hundred cells were counted in each group to calculate percentage of live and dead cells MN and PMN cells. Then the cells were subjected to SEM for detailed morphological studies.
SEM studies of WBC’s and RBC’s
One drop of cell suspension (RBC/MN/PMN) was fixed in 2.5% (v/v) gluteraldehyde in PBS (pH 7.2) for 20 min at room temperature. After fixation and washing, stubbs were prepared. Stubbs were sputtered for 30 min. in a sputterer and viewed in SEM at Central Instrumentation Laboratory, Panjab University Chandigarh, under different magnifications.
Results
Course of infection
Maximum infection of 49.5 ± 3.53% was observed in infected control (G-2) by 7th day post inoculation after which all the mice died due to heavy infection whereas mice treated with vehicle (G-4) died by day 5 (25.1%) confirming the non involvement of nascent alcohol in clearing P. berghei infection. In G-3, the infection was observed to decline continuously and 4.9 ± 0.92% infection (P < 0.0005) was observed on day 7 post inoculation (Table 1). During the follow up period of one month, 2.5 ± 1.44% infection was recorded on 28th day with 50% survival of mice. In AS monotherapy (G-5) the infection was reduced to 1.7 ± 0.66% (P < 0.0005) by day 5 but many free merozoites outside the RBCs and dead parasites were observed in blood smears. Recrudescence was observed on day 21 (2.07 ± 0.64%) which increased up to 3.99 ± 0.07% by day 28. In the mice treated with combination of artesunate and China ϕ, the infection was completely cleared with 90% survival of mice. In G-8 (AS + SP) also, the infection was reduced to 0.16 ± 0.2% on day 7 after which it was completely cleared but only 30% of mice survived.
Mortality of WBC
WBCs isolated by double density gradient centrifugation from various groups on day 7 were subjected to AO/EB staining to calculate the percentage of live/dead/apoptotic MN and PMN cells. The cells which fluoresced green were differentiated as live where as orange and yellow cells were characterized as dead and apoptotic cells respectively.
Maximum number of live MN cells (97.9%) was observed in G-1 followed by G-8 (95.3%), G-2 (93.6%), G-7 (93.57%), G-3 (74.04%) and G-5 (62.92%). No apoptotic cells were seen in G-7 and G-8. The percentage of live PMN cells was maximum in G-7 (98.77%) followed by G-8 (98.02%), G-5 (96.11%), G-3 (93.96%), G-2 (91.97%) and G-1 (87.1%).
Scanning electron microscopy
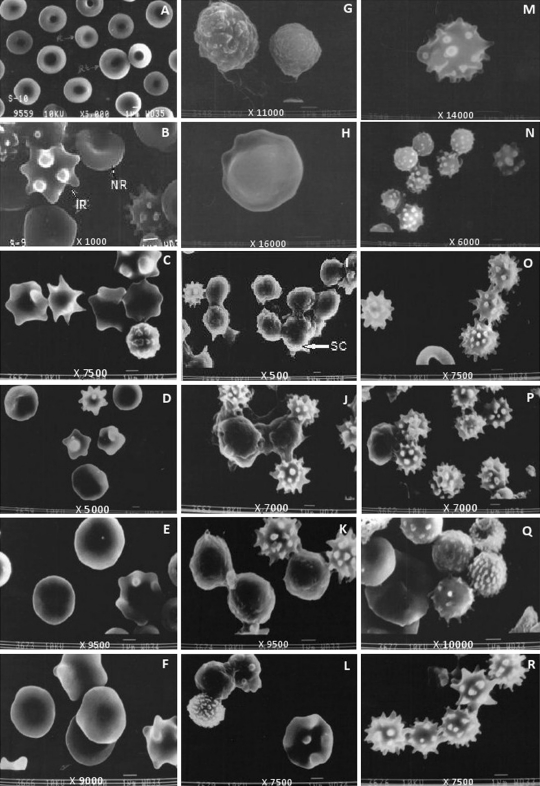
Red blood cells of normal mice (G-1) appeared biconcave and elliptical in shape. Normal reticulocytes have been observed to be larger (3 μm) than normal red blood cells (2.5–2.8 μm) in SEM (Fig. 1a). Plasmodium berghei infected red blood cells (G-2) were enlarged (4 μm) with distorted shape. Bulgings and protuberances (knobs) were present in membrane due to presence of parasites inside (Fig. 1b). In G-3 large numbers of RBCs were distorted (Fig. 1c). Among the treated groups maximum size of RBC was observed in G-5 (4.2 μm) (Fig. 1d) whereas, in G-7 (Fig. 1e) and G-8 (Fig. 1f) RBCs (2–3.0 μm) were comparable to normal RBC.
Fig. 1.
Scanning electron micrographs showing red blood cells a G-1, b G-2, c G-3, d G-5, e G-7, f G-8; MN cells g G-1, h G-2, i G-3, j G-5, k G-7, l G-8 and PMN cells m G-1, n G-2, o G-3, p G-5, q G-7, r G-8 obtained after separation of blood cells using double density gradient centrifugation on day 7. PMN polymorphonuclear cells, M microvilli, SC smooth surface cells, LC layered surfaced cells, PMN polymorphonuclear cells, M microvilli, NR normal RBC, IR infected RBC, P platelet
MN cells of G-1 exhibited different types of surfaces as visible in SEM (Fig. 1g). Various types of depressions over the surface of cells were also visible in some cells. MN cells of G-2 (3.5–4.0 μm) showed smoother surfaces as compared to normal cells (Fig. 1h). In G-3, most of the MN cells were smooth surfaced having a size of 3.0–3.5 μm and few cells with microvilli like structures were also observed (Fig. 1i). MN cells of G-5 (Fig. 1j), G-7 (Fig. 1k) and G-8 (Fig. 1l) were having both smooth and rough surfaced cells.
PMN cells of G-1 (3.5–3.8 μm) displayed a variety of shapes. Cells were having blebs and finger like projections on them (Fig. 1m). In G-2, PMN cells were smaller in size (2–2.5 μm) and exhibited distorted shapes with depressions and finger like projections (Fig. 1n). In all the treated groups cells were having large finger like projections, blebs and microvilli like structures [G-7 (Fig. 1q); G-8(Fig. 1r)]. The smallest cells were observed in G-3 (Fig. 1o) (1–2 μm) and largest in G-5 (Fig. 1p) (3 μm).
Discussion
Plasmodium berghei (NK-65) has again been confirmed to be lethal to white swiss mice of Balb/c strain. All the mice, injected with 1×106P. berghei infected RBCs, died by day 7 post inoculation, because of heavy infection except those in which parasite migrated to reticulocytes. The phenomenon of reticulocytosis in P. berghei has been reported earlier too (Das Combe et al. 1995).
Parasite grows and multiplies with in host red blood cells where it modifies the membrane permeability and cytosolic composition of host cells. Erythrocyte serves as novel determinant of drug susceptibility in rodent malaria (Fitch et al. 1978). SEM studies provide information about the morphological variation in surface of cells under the influence of pathogen. Plasmodium berghei infection leads to distortion in shape of RBCs due to the presence of parasite in them. During the asexual life cycle, the parasite remodels the host RBC which includes generation of clefts and protrusions on the infected RBC surface termed as knobs (Cooke et al. 2004). In the present study RBCs of infected mice were distorted in shape with knobs on them. The formation of such knobs might be related to the release of merozoites from infected RBC’s (Sherman 1988). Gruenberg et al. (1983) reported that P. falciparum infected cells bearing trophozoite stage parasite (24–36 h) have small protrusions (knobs) with diameters varying from 110–160 nm and when parasites were fully mature (schizonts, 40–44 h), the knob size decreased (100–170 nm). The size and density of the knobs varied inversely suggesting that knob production occurred throughout intra erythrocytic parasite development from trophozoite to schizont and was related to dynamic changes of the erythrocyte membrane. Knobs are believed to be involved in the occlusive vascular pathology of malaria (Langreth et al. 1978). These knobs are reported to regulate adhesive properties of infected red cells (An and Mohandas 2010). Most of the RBCs in G-3, G-5, G-7 and G-8 were normal with no knob like structures.
Morphological changes in surface of MN cells have also been observed. The number of smooth surfaced cells were more in P. berghei infected (G-2) and various drug treated groups. Albrecht et al. (1978) also reported that the peripheral blood granulocyte population appears by SEM to be comprised of several cell populations or a single cell population of considerable morphologic diversity. In G-3 and G-5, MN cells having smooth surface have observed to stick to one another. This may be because of the knob like processes which extend outward to engulf the parasitic cells. PMN cells have been observed to have long finger like projections on the surface. The number of PMN cells has been observed to increase in drug treated mice. This may be because of their activation for engulfing P. berghei parasitized red cells. In G-7 some PMN cells had microvilli like structures. These cells were bigger in size as compared to other PMN cells. This might be due to enhanced phagocytosis of infected cells.
The effect of drugs on white blood cells was also evident by change in number of live and dead cells. Among the treated groups, maximum percentage of live mononuclear cells has been found in G-7 (93.57%) followed by G-3 (74.04%) and G-5 (62.92%). However, maximum percentage of live PMN cells has been again observed in G-7 (98.7%) followed by G-5 (96.11%) and G-3 (93.96%).
Present study confirms that the combination of artesunate + China does not harm the morphological and functional organization of blood cells.
Acknowledgments
The authors hereby declare that experiments comply with the current laws in India. The authors are grateful to chairperson and UGC-CAS program of the department of Zoology, Panjab University, Chandigarh for continued support and University Grants commission, New Delhi, India for funding the present work through Rajiv Gandhi National fellowship to Aswathy Rajan.
References
- Albrecht RM, Jordan C, Hong R. Identification of monocytes, granulocytes and lymphocytes: correlation of histological, histochemical and functional properties with surface structure as viewed by SEM. Scan Elec Micros. 1978;2:521–522. [Google Scholar]
- An X, Mohandas N. Red cell membrane and malaria. Transfus Clin Biol. 2010;17:197–199. doi: 10.1016/j.tracli.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Bagai U, Rajan A, Chandel S (2008) Efficacy of eucalyptus (a homeopathic medicine) in combination with artesunate to clear Plasmodium berghei infection in Balb/c mice. Current trends in parasitology. In: Proceedings of 20th National Congress of Parasitology, NEHU Shillong, India, 3–5 Nov 2008 pp 203–211
- Bodammer JE, Bahr GF (1973) The initiation of a metabolic window in the surface of host erythrocytes by P. berghei Nyu-2. Lab Invest 28:708–718 [PubMed]
- Cline MJ (1975) The white cell. Harvard University Press, Cambridge, MA, USA, pp 39
- Cooke BM, Mohandas N, Coppel RL. Malaria and the red blood cell membrane. Semin Hematol. 2004;41:173–188. doi: 10.1053/j.seminhematol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Czuprynski CJ, Brown JF (1998) In vitro analysis: isolation and preparation of lymphocytes from injected animals. In: Kaufmann SHE, Kabelitz D (eds) Immunology of infection, vol 25. Acdemic Press, New York. pp 189–194
- Das Combe MJ, Ahmed KW, Oyewo EA, Sidara JY (1995) In: Nagasaka T, Milton AS (eds) Body temperature and metabolism. IPEC, Tokyo. pp 61–66
- Firket H, Schaaf-Lafantaine N (1976) Etude au microscope electronique a bolayage de la surface des lymphocytes isoles du sang ou des organs lymphoid. J Microsc Biol Cell 25:223–226
- Fitch CD, Ng RC, Chevli R. Erythrocytic surface serves as novel determinant of drug susceptibility in rodent malaria. Antimicrob Agent Chemother. 1978;14(2):185–193. doi: 10.1128/aac.14.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Allred DR, Shermen IW. Scanning electron microscope-analysis of protrusions (Knobs) present on the surface of Plasmodium falciparum infected-erythrocytes. J cell boil. 1983;97:795–802. doi: 10.1083/jcb.97.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Artemisinin Study Group Artesunate combination for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/S0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- Jambou RE, Legrand M, Niang N, Khim P, Lim B, Volney MT, Ekala C, Boucheir P, Esterre T, Fandeur O, Mercereau-Puijalon Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutation of the SERCA-type pfATPase 6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S (1998) Apoptotic assays. In: Jansen K (ed) Cell— a laboratory manual, vol 1. Cold Spring Harbour Press, New York, 1:15: pp 1–15
- Langreth SG, Jensen JB, Reese RT. Fine structure of human malaria in vitro. J Protozool. 1978;25:443–452. doi: 10.1111/j.1550-7408.1978.tb04167.x. [DOI] [PubMed] [Google Scholar]
- Rajan A, Bagai U (2009) In vivo efficacy of combination therapy of Artesunate + S/P and Artesunate + China against P. berghei infection. In: Proceedings of 21st national congress of parasitology. Panjab University, Chandigarh, India. 14–16 Nov 2009 p 60
- Santiyanont R (1985) Parasite identification, counting and staining: Application of genetic engineering techniques in tropical diseases, pathogens with special reference to plasmodia. A laboratory manual of selected techniques. In: UNDP/World Bank/WHO special program for research and techniques in tropical diseases, Bangkok Thailand 8.7, pp 413–448
- Saxena R (2006) Role of homeopathy in infectious diseases (philosophy and therapeutics). ISBN 81-8056-701-X, B. Jain publishers (P) Ltd, New Delhi. pp 1–181
- Schneider GB, Pockwinse SM, Billings-Gagliardi (1978) Morphological changes in isolated lymphocytes during preparation foe SEM; a comparative TEM/SEM study of freeze drying and critical point drying. SEM sympo II, pp 77–84 [DOI] [PubMed]
- Sherman IW. Mechanisms of molecular trafficking in malaria. Parasitol. 1988;96:557–581. doi: 10.1017/s003118200008598x. [DOI] [PubMed] [Google Scholar]
- Wetzel B (1976) Cell kinetics: an interpretive review of the significance of cell surface form. Proc 9th SEM Symp 1976 (Part V), pp 135–142, Illinois Institute of Technology, Chicago
- Wetzel B, Erickson BW, Levis WR (1973) The need for positive identification of leucocytes examined by S.E.M. Proc 6th SEM Symp 1973, pp 535–542, Illinois Institute of Technology, Chicago
- WHO (2002) WHO traditional medicine strategy 2002–2005. Geneva, Switzerland Report No. WHO/EDM/TRM/2002.1
- WHO (2006) Guidelines for the treatment of malaria. Roll back malaria, World Health Organisation and United Nations Children Funds (UNICEF) 2005 Report No. WHO/HTM/MAL/2005.11.02
- Yarnell E, Abascal K (2004) Botanical treatment and prevention of malaria. Part 2-selected botanical. Alt Compl Therapies 10(5):277–284