Abstract
Epilepsy is the third most common brain disorder and affects millions of people. Epilepsy is characterized by the occurrence of spontaneous seizures, i.e., bursts of synchronous firing of large populations of neurons. These are believed to result from abnormal regulation of neuronal excitability that favors hypersynchrony. Among the intrinsic conductances that govern neuronal excitability, the hyperpolarization-activated current (Ih) plays complex and important roles in the fine-tuning of both cellular and network activity. Not surprisingly, dysregulation of Ih and/or of its conducting ion-channels (HCN) has been strongly implicated in various experimental models of epilepsy, as well as in human epilepsy. Here we provide an overview of recent findings on the distinct physiological roles played by Ih in specific contexts, and the cellular mechanisms that underlie these functions, including the subunit make-up of the channels. We further discuss current knowledge of dysregulation of Ih and HCN channels in epilepsy in light of the multifaceted functions of Ih in the brain.
The h-current (Ih): a versatile regulator of cellular and network excitability
Among the many ionic conductances that shape neuronal excitability, the hyperpolarization-activated current Ih has long been a subject of interest and debate among researchers. Unlike other voltage-gated currents, Ih is activated upon relative hyper-polarization of the cell membrane. Conducted by a mixed cationic current with reversal potential values around -30 mV, Ih activation counteracts the hyperpolarization that triggered it [1,2]. Because Ih is partially activated at physiological, “resting” conditions, it provides constant depolarization of the membrane potential [3-5]. Thus, Ih is thought to function as a stabilizing negative-feedback loop that responds to alterations in membrane potential [1,2]. Alongside its depolarizing role, Ih exerts a shunting effect on excitable cells: being open at subthreshold potentials, Ih reduces the input resistance of the membrane (Rin), thus dampening the ability of incoming inputs to alter membrane voltage.
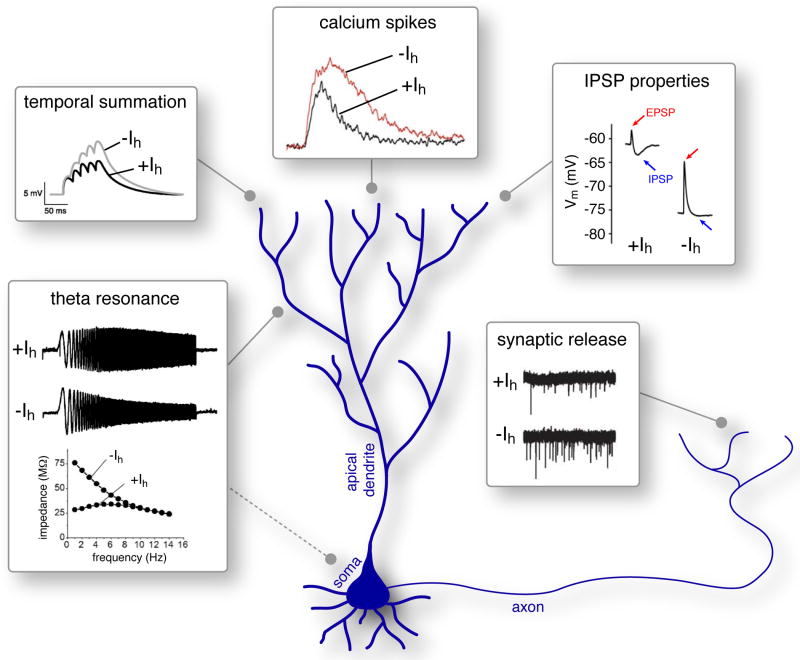
The net effect of the depolarizing and shunting properties of Ih on excitability is combinatorial and depends on many factors (Figure 1), as illustrated by the roles of Ih in CA1 hippocampal pyramidal neurons. In these neurons, the density of Ih along apical dendrites increases with the distance from the soma [6]. A physiological consequence of this heterogeneous distribution (mediated by the effect of Ih on the input resistance and membrane time constant) is that EPSP time course is increasingly shortened with the distance from the soma [6]. As a result, temporal summation of synaptic inputs at the soma is similar regardless of whether these inputs occur at proximal or distal sites [6] (but see [7]). Another physiological action of Ih, mediated by its tonic depolarizing effect, is to increase steady-state inactivation of low-threshold, voltage-gated calcium channels [8], and thus restrict the genesis of dendritic calcium spikes. The effects of Ih on excitability involve interactions with other intrinsic conductances: whereas Ih increases the peak voltage amplitude of weak EPSPs, it inhibits the peak amplitude of responses to strong stimuli, and the net effect on EPSPs depends upon an intricate interaction between the Ih–mediated tonic depolarization and the K+ -mediated conductance, IM [9]. Regulation of synaptic signaling by Ih is not limited to excitatory inputs. Ih suppresses inhibitory (GABAA-mediated) post-synaptic potentials (IPSPs) through interactions with other active conductances and cellular passive electric properties [10,11]. The relative depolarization generated by Ih in distal dendrites can alter GABAA signaling from shunting to hyperpolarizing, and thus shortens the time-window for coincidence-detection [12].
Figure 1. The multiple functions of Ih on neuronal activity are context-and location driven.
A schematized neuron depicting principal functions of Ih in distinct subcellular domains. In dendrites, Ih reduces dendritic summation (inset from [1], with permission); the current also represses dendritic calcium spikes (inset from [8], with permission), and converts the effect of IPSPs on the membrane potential from shunting to hyperpolarizing (inset modified from [12], with permission). In axon terminals, Ih reduces synaptic release through interaction with calcium channels (inset from [17], with permission). In both soma and dendrites, Ih augments theta-frequency resoancne (inset modified from [19], with permission). In neurons that possess increased dendritic Ih density, the effects on theta-frequency resonance are more pronounced in dendrites compared to the soma.
From the above, it is clear that Ih has multiple effects in regulating dendritic excitability, including through interactions with other intrinsic conductances. Yet how do these different effects of Ih on inhibitory, excitatory and intrinsic signals integrate in a physiological context? This question was recently addressed in subthalamic nucleus (STN) neurons [13]. In these neurons (which express different HCN channel subunits compared with CA1 pyramidal neurons), dendritic Ih was activated only upon strong hyperpolarizing input onto the dendrite, and thus served a homeostatic role in counteracting GABAA-mediated signals. In an analogous manner to that described in CA1 pyramidal neurons [8], activation of Ih by inhibitory input facilitated steady-state inactivation of low-threshold voltage-gated calcium channels, leading to inhibition of dendritic calcium spikes. Whereas theoretically Ih could suppress also the temporal summation of excitatory inputs, the authors demonstrated that the same inhibitory input that activated Ih shunted the effects of Ih on EPSP summation, and concluded that, in STN neuronal dendrites, Ih selectively regulated inhibitory signaling [13].
Ih is not limited to somato-dendritic subcellular distribution, and presynaptic Ih has been reported in various classes of neurons [14-17]. The presynaptic functions of Ih in mammalian brain have remained elusive [1, 16], but, in axon terminals of layer 3 entorhinal cortex neurons, Ih-mediated depolarization was recently found to restrict the activity of T-type Ca2+ channels, leading to reduced calcium influx and inhibition of synaptic release [17]. It remains to be seen whether similar mechanisms exist in presynaptic terminals of interneurons [14,15] or whether Ih serves an opposite, facilitatory role in these neuronal populations [14].
In addition to its roles in dendritic integration, membrane potential stabilization and regulation of synaptic transmission, Ih is an important modulator of oscillatory activity at both cellular and network levels. At cellular levels, Ih is critical for theta resonance (the preferential response of a neuron to oscillating inputs at specific frequencies). The slow kinetics and sub-threshold activation of Ih enable this current to filter out inputs at low frequencies (<3 Hz). This high-pass filtering property, in combination with low-pass filtering properties provided by the membrane capacitance, render cells with a strong Ih particularly responsive to inputs in the theta range (3-12 Hz) [18,19], a property also found in specific types of interneurons [20]. In cell-types with high Ih density at distal dendrites, dendritic Ih-dependent resonance is more pronounced, and preferentially filters signals that propagate from the soma to dendrites [19]. The filtering properties of Ih may contribute to regulating in vivo theta rhythms [4], and other network oscillations (e.g., delta and gamma) and rhythmic firing (reviewed by [1]).
Ih diversity originates from exquisite control of HCN channels at multiple levels and enables physiological plasticity
The functional diversity and multiple roles of Ih in different physiological settings (discussed above) require tight regulation of the pore-forming proteins that conduct Ih, namely, HCN channels. HCN channel regulation involves many processes that take place at three levels: (1) regulation of the biophysical properties of the channels (such as voltage-activation profile and kinetics); (2) regulation of the number of channels expressed on the plasma membrane; and (3) localization of the channels to distinct subcellular compartments.
A critical determinant of the biophysical properties of HCN channels is their molecular make-up. Four different channel isoforms exist (HCN1-4) that can assemble in different combinations to yield homo- or hetero-tetrameric complexes with different properties [1,2,21]. Indeed, variability in Ih properties among cell populations and during different developmental stages is often associated with distinct expression profiles of specific HCN channel isoforms [22-25]. The conductive properties of HCN channels are further diversified by discrete interactions with other molecules, notably cyclic AMP (cAMP): cAMP binds a sequence on the C terminus of the channel, and influences HCN channel function by accelerating its kinetics and shifting its voltage-activation curve to more depolarized values [1,2]. The sensitivity of HCN channels to cAMP is isoform-specific: HCN4> HCN2 ≫ HCN1 [1,2]. Thus, isoform-specific interaction of HCN channels with regulating molecules provides another dimension to the diversity of Ih. HCN channel function is modified by other ‘small’ molecules such as PIP(2) [26,27], as well as by interacting/auxiliary proteins (reviewed in [28]). Finally, phosphorylation of different HCN channel isoforms at multiple sites influences their biophysical properties (e.g. [29-31]).
The number of surface-expressed HCN channels influences the magnitude and properties of Ih (via changes in relative abundance of the constituents of the channel). Mechanisms controlling the number of surface HCN channels act at different time-scales. At a relatively long time scale (hours to days), transcriptional regulation determines the total amount of HCN channel protein in the cell [1]. This regulation is isoform-specific, acts via specific transcription factors [32], and depends on network activity [33]. Post-translational mechanisms also modulate HCN channels. For example, these channels are heavily glycosylated in the mammalian brain [34,35] and glycosylation influences both the total number of HCN channels in the membrane and their heteromerization (and hence properties) in a subunit-specific manner [35,36]. Modulation of HCN channel surface expression at shorter time-scales can occur via local regulation of channel membrane insertion, internalization and recycling [37,38]. Importantly, the dynamics of HCN channel trafficking and surface expression are activity-dependent [38], with implications to neuroplasticity and disease. Auxiliary proteins interact with HCN channels to regulate their surface expression: a family of splice variants of TRIP8b (an auxiliary protein that interacts directly with HCN channels) can either up- or down-regulate HCN channel surface expression [39,40]. Other candidate auxiliary proteins have been implicated in surface regulation of HCN channels (reviewed in [28]).
The targeting of HCN channels to distinct sub-cellular domains influences their location-dependent roles in regulation of excitability. Distinct distribution patterns of HCN channels exist in somata, dendrites and axons of specific cell types and brain regions, as well as during development [16,17,23,41,42]. Whereas ample information exists on the distribution of HCN channels, little is known of the molecular mechanisms that underlie their targeting to sub-cellular domains. Time-lapse live imaging in hippocampal neurons indicates that vesicular trafficking dynamics of HCN channels are isoform-specific [38], and neuronal activity influences both trafficking and long-term subcellular distribution [38,43]. Because the HCN-interacting protein TRIP8b colocalizes with HCN1 channels and the distribution of the protein is disrupted in HCN1 knockout mice, TRIP8b is a candidate for dendritic targeting of HCN channels [44,45]. In addition to a heterogeneous distribution in the different cell compartments, HCN channels appear to be differently distributed within the same brain region. Thus, Ih decreases along the dorsal-ventral axis in entorhinal cortex stellate cells, providing these neurons with different integrative properties [46,47].
Together, the diverse regulatory mechanisms described above enable exquisite functional plasticity of Ih, such as seen during development [16, 22] or when synaptic plasticity is triggered [18,38]. When the mechanisms of HCN channel plasticity are disrupted, the resulting dysregulation of Ih might contribute to neurological disorders including epilepsy.
(3) HCN channel dysregulation in epilepsy: the wrong amount in the wrong place, at the wrong time
Since the original implication of HCN channels in epilepsy in 2001 [48], many studies have linked these channels to the epileptogenic process. In resected hippocampi from patients with temporal-lobe (limbic) epilepsy, enhanced levels of HCN1 channel expression and dendritic localization were found in granule cells of the dentate gyrus [49], and recent work has identified a mutation in the HCN2 gene and augmentation of Ih in patients with genetic epilepsy with febrile seizures plus (GEFS+) [50]. Deletion of the HCN1 gene in mice results in increased excitability and seizure susceptibility [51,52], and reduction or deletion of the HCN2 isoform leads to spontaneous ‘absence’ seizures [53,54].
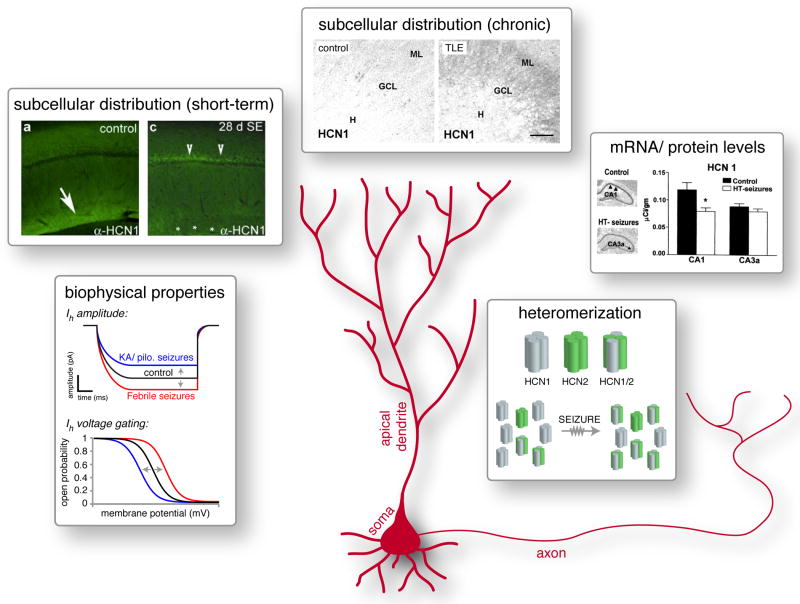
In accord with the diverse regulatory mechanisms and versatile functions of Ih in the normal brain, the dysregulation of Ih and HCN channels in epilepsy is dynamic and intricate (Figure 2). HCN channel abnormalities in the epileptic brain can manifest as altered mRNA and protein expression [55-60], sub-cellular distribution [44,61] or biophysical properties [48,58,62]. The causal relationship between aberrant HCN channel regulation and the epileptic process is further complicated by the fact that alterations in HCN channel expression, localization and function vary across animal models of epilepsy. Both early and late changes affecting diverse isoforms in distinct spatial patterns have been reported. For example, in the pilocarpine model, progressive reduction in HCN1 and HCN2 protein levels results in diminished Ih amplitude in dendrites of CA1 pyramidal neurons, leading to the disruption of theta resonance [58,63]. In contrast, following hyperthermia-induced seizures, the same population of cells exhibit enhanced dendritic Ih accompanied by altered gating properties [48,62], likely mediated by isoform-specific transcriptional regulation and increased HCN1/HCN2 heteromerization [56]. These seemingly conflicting results from different animal models demonstrate that augmented Ih can be associated with both increased and decreased excitability, depending on the physiological context [64], and potential interaction with other conductances.
Figure 2. Abnormal HCN channel regualtion and function in epilepsy.
Dysregulation of HCN channels/ Ih in epilepsy occurs at multiple levels. Seizure-induced alterations of the biophysical properties of Ih include either up- or down- regulation of current amplitude and modification of gating. The sub-cellular distribution of HCN channels along the somato-dendritic axis is altered both short-term, e.g., in hippocampal CA1 neurons in the pilocarpine model (insert modified, with permission, from [44]), and in dentate gyrus granule cells of human epileptic patients (insert from [49], with permission). Seizure-induced alterations in HCN channel mRNA and protein levels occur in many epilepsy models, and are both region- and channel isoform- specific (insert from [55], with permission). Altered synthesis of specific HCN channel isoforms may drive, at least in part, the increased, seizure-induced HCN1/2 heteromerization in hippocampus [55,56].
The temporal patterns of HCN dysregulation in the epileptic brain are complex, and both transient and long-lasting alterations in hippocampal HCN/ Ih have been reported in pilocarpine, KA and febrile seizures models [55,56,58,60,63]. Interestingly, alterations in Ih gating properties in CA1 pyramidal neurons were reported within hours following induction of seizure-like-activity in vitro, attributable to activation of the phosphatase calcineurin and inhibition of p38 MAPK [65].
Spatial selectivity of HCN-channel pathology further contributes to the complex involvement of Ih in epilepsy. In acquired hippocampal epilepsy, HCN channel expression levels vary across different hippocampal regions in isoform-specific and region-specific fashions [55,57]. Region-specific alterations have been found in the WAG/Rij rat model of genetic absence epilepsy, where reduced HCN1 channel expression in layer 5 cortical neurons was reported to increase excitability via enhanced calcium electrogenesis [66], while increased HCN1 levels in thalamocortical neurons impaired their firing pattern via reducing the responsiveness of Ih to cAMP [59]. Reduced response of Ih to cAMP (triggered by imbalance of HCN subunit expression) was found also in the GAERS rat model of absence epilepsy [25].
Epilepsy may also involve abnormal subcellular distribution of HCN channels. Dendritic HCN1 localization was augmented in hippocampi resected from patients with epilepsy [49]. In animal models, transient upregulation of HCN1 surface expression occurred in CA1 pyramidal neurons 1-2 days after an epilepsy-provoking insult, followed by a downregulation 4 weeks later with mislocalization of the channels from distal dendritic domains to somata [44]. Recent work in freeze-lesion models of cortical dysplasia-provoked epilepsy found reduced HCN1 channel presence in distal dendrites of layer 5 cortical neurons [61].
In summary, multiple mechanisms, including transcriptional control, trafficking and channel modification act at different temporal and spatial scales to modulate Ih in the epileptic brain. In general, such changes might contribute to epileptogenesis, i.e., ‘cause’ epilepsy, or be a result of the epilepsy. The occurrence of several of these HCN changes early after the insult that triggers epilepsy, and/ or prior to the onset of spontaneous seizures [32,55,66] suggests a causal role. To fully grasp the contribution of these alterations to the epileptogenic process, physiological, molecular and cellular approaches should be integrated (Box 1). Recent discoveries have greatly advanced our understanding of the protean roles that Ih plays in the normal brain in different contexts, and their underlying cellular mechanisms. These, and future studies, will ultimately lead to an integrated view on the role of HCN channel pathology in epilepsy, at levels of analysis spanning single molecules to cellular and network domains. This understanding, in turn, will promote the design of new strategies for better treatment of the epilepsies.
BOX 1. Factors that influence the potential contribution of HCN channel dysregulation to hyperexcitability and epilepsy.
Brain region and neuronal population: In what cell populations are Ih /HCN channels altered? (Principal cells? Interneurons? Which brain region?); What is the specific role of Ih in this population and how does it influence network excitability?
Sub-cellular distribution: Do changes in Ih /HCN channels take place in dendrites, soma, axon terminals?
Quantitative properties: Is Ih upregulated or reduced?
Qualitative properties: How is the molecular makeup of the channels altered? How are biophysical properties changed (voltage-activation, kinetics, cAMP gating)?
Temporal pattern: Are changes in Ih dynamic or static? Activity dependent? Are they a result of other cellular and network changes?
Interplay with active and passive properties: Are Ih /HCN changes accompanied by changes in other intrinsic conductances, synaptic inputs or the passive properties of the cell? Do changes in Ih lead to alterations of other channels?
Highlights.
The hyperpolarization-activated current Ih plays complex and important roles in the fine-tuning of both cellular and network activity
The distinct physiological roles of Ih depend on factors including HCN subunit composition, interacting proteins and molecules, subcellular localization and the context of synaptic activity
Ih dysregulation in epilepsy occurs at multiple regulatory levels and time frames, with complex effects on neuronal excitability
A full understanding of the physiological and pathological roles of Ih will clarify the role of HCN channels as molecular targets in epilepsy therapy
Acknowledgments
We thank Barbara Cartwright for editorial assistance. The authors are supported by NIH grants R37 NS35439 (TZB); T32 NS04550 (YN); INSERM, ANR ANTARES and MINOS grants (CB), and a grant from the Dutch Epilepsy Fund (NEF 08.01, YN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- *1.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. A comprehensive review of HCN channel structure, function and regulation. [DOI] [PubMed] [Google Scholar]
- 2.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annual Review of Physiology. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 3.Lupica CR, Bell JA, Hoffman AF, Watson PL. Contribution of the hyperpolarization-activated current (I(h)) to membrane potential and GABA release in hippocampal interneurons. J Neurophysiol. 2001;86:261–268. doi: 10.1152/jn.2001.86.1.261. [DOI] [PubMed] [Google Scholar]
- 4.Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Meuth SG, Kanyshkova T, Meuth P, Landgraf P, Munsch T, Ludwig A, Hofmann F, Pape HC, Budde T. Membrane resting potential of thalamocortical relay neurons is shaped by the interaction among TASK3 and HCN2 channels. J of Neurophysiol. 2006;96:1517–1529. doi: 10.1152/jn.01212.2005. [DOI] [PubMed] [Google Scholar]
- 6.Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nature Neuroscience. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 7.Angelo K, London M, Christensen SR, Hausser M. Local and global effects of I(h) distribution in dendrites of mammalian neurons. J Neurosci. 2007;27:8643–8653. doi: 10.1523/JNEUROSCI.5284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56:1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.George MS, Abbott LF, Siegelbaum SA. HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat Neurosci. 2009;12:577–584. doi: 10.1038/nn.2307. Using experimental and modeling approaches, the authors assess the relative contribution of Ih-mediated depolarization and shunting to dendritic excitability, and the important interplay between HCN channels and other dendritic channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie JB, Pearce RA. Active and passive membrane properties and intrinsic kinetics shape synaptic inhibition in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:8559–8569. doi: 10.1523/JNEUROSCI.0547-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams SR, Stuart GJ. Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci. 2003;23:7358–7367. doi: 10.1523/JNEUROSCI.23-19-07358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlov I, Scimemi A, Savtchenko L, Kullmann DM, Walker MC. I(h)-mediated depolarization enhances the temporal precision of neuronal integration. Nat Commun. 2011;2:199. doi: 10.1038/ncomms1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Atherton JF, Kitano K, Baufreton J, Fan K, Wokosin D, Tkatch T, Shigemoto R, Surmeier DJ, Bevan MD. Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus. J Neurosci. 2010;30:16025–16040. doi: 10.1523/JNEUROSCI.3898-10.2010. A comprehensive analysis of the role of HCN channel in regulating dendritic excitability in subthalamic nucleus neurons, showing complex, context-dependent roles mediated by interaction of Ih with GABA(A) receptors and low-threshold calcium channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aponte Y, Lien CC, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol. 2006;574:229–243. doi: 10.1113/jphysiol.2005.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lujan R, Albasanz JL, Shigemoto R, Juiz JM. Preferential localization of the hyperpolarization-activated cyclic nucleotide-gated cation channel subunit HCN1 in basket cell terminals of the rat cerebellum. Eur J Neurosci. 2005;21:2073–2082. doi: 10.1111/j.1460-9568.2005.04043.x. [DOI] [PubMed] [Google Scholar]
- 16.Bender RA, Kirschstein T, Kretz O, Brewster AL, Richichi C, Ruschenschmidt C, Shigemoto R, Beck H, Frotscher M, Baram TZ. Localization of HCN1 channels to presynaptic compartments: novel plasticity that may contribute to hippocampal maturation. J Neurosci. 2007;27:4697–4706. doi: 10.1523/JNEUROSCI.4699-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, Shah MM. Presynaptic HCN1 channels regulate Ca(V)3.2 activity and neurotransmission at select cortical synapses. Nat Neurosci. 2011;14:478–486. doi: 10.1038/nn.2757. A pioneering study of the role of axonal HCN channels in synaptic transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayanan R, Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron. 2007;56:1061–1075. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Vervaeke K, Graham LJ, Storm JF. Complementary theta resonance filtering by two spatially segregated mechanisms in CA1 hippocampal pyramidal neurons. J Neurosci. 2009;29:14472–14483. doi: 10.1523/JNEUROSCI.0187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemankovics R, Kali S, Paulsen O, Freund TF, Hajos N. Differences in subthreshold resonance of hippocampal pyramidal cells and interneurons: the role of h-current and passive membrane characteristics. J Physiol. 2010;588:2109–2132. doi: 10.1113/jphysiol.2009.185975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoro B, Baram TZ. The multiple personalities of h-channels. Trends Neurosci. 2003;26:550–554. doi: 10.1016/j.tins.2003.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender RA, Baram TZ. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog Neurobiol. 2008;86:129–140. doi: 10.1016/j.pneurobio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex. 2007;17:702–712. doi: 10.1093/cercor/bhk021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci. 2009;29:8847–8857. doi: 10.1523/JNEUROSCI.0689-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuisle M, Wanaverbecq N, Brewster AL, Frere SG, Pinault D, Baram TZ, Luthi A. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J Physiol. 2006;575:83–100. doi: 10.1113/jphysiol.2006.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–1036. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Pian P, Bucchi A, Decostanzo A, Robinson RB, Siegelbaum SA. Modulation of cyclic nucleotide-regulated HCN channels by PIP(2) and receptors coupled to phospholipase C. Pflugers Arch. 2007;455:125–45. doi: 10.1007/s00424-007-0295-2. [DOI] [PubMed] [Google Scholar]
- 28.Lewis AS, Estep CM, Chetkovich DM. The fast and slow ups and downs of HCN channel regulation. Channels (Austin) 2010;4 doi: 10.4161/chan.4.3.11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li CH, Zhang Q, Teng B, Mustafa SJ, Huang JY, Yu HG. Src tyrosine kinase alters gating of hyperpolarization-activated HCN4 pacemaker channel through Tyr531. Am J Physiol Cell Physiol. 2008;294:C355–362. doi: 10.1152/ajpcell.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Z, Lockhead D, Larson ED, Proenza C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J Gen Physiol. 2010;136:247–258. doi: 10.1085/jgp.201010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammelmann V, Zong X, Hofmann F, Michalakis S, Biel M. The cGMP-dependent protein kinase II Is an inhibitory modulator of the hyperpolarization-activated HCN2 channel. PLoS One. 2011;6:e17078. doi: 10.1371/journal.pone.0017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClelland S, Flynn C, Dubé C, Richichi C, Zha QQ, Ghestem A, Esclapez M, Bernard C. NRSF-mediated HCN channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011 doi: 10.1002/ana.22479. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richichi C, Brewster AL, Bender RA, Simeone TA, Zha Q, Yin HZ, Weiss JH, Baram TZ. Mechanisms of seizure-induced ‘transcriptional channelopathy’ of hyperpolarization-activated cyclic nucleotide gated (HCN) channels. Neurobiol Dis. 2008;29:297–305. doi: 10.1016/j.nbd.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- 35.Zha Q, Brewster AL, Richichi C, Bender RA, Baram TZ. Activity-dependent heteromerization of the hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels: role of N-linked glycosylation. J Neurochem. 2008;105:68–77. doi: 10.1111/j.1471-4159.2007.05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegle AP, Nazzari H, Roth A, Angoli D, Accili EA. Evolutionary emergence of N-glycosylation as a variable promoter of HCN channel surface expression. Am J Physiol Cell Physiol. 2010;298:C1066–1076. doi: 10.1152/ajpcell.00389.2009. [DOI] [PubMed] [Google Scholar]
- 37.Hardel N, Harmel N, Zolles G, Fakler B, Klocker N. Recycling endosomes supply cardiac pacemaker channels for regulated surface expression. Cardiovasc Res. 2008;79:52–60. doi: 10.1093/cvr/cvn062. [DOI] [PubMed] [Google Scholar]
- *38.Noam Y, Zha Q, Phan L, Wu RL, Chetkovich DM, Wadman WJ, Baram TZ. Trafficking and surface expression of hyperpolarization-activated cyclic nucleotide-gated channels in hippocampal neurons. J Biol Chem. 2010;285:14724–14736. doi: 10.1074/jbc.M109.070391. The original characterization of HCN channel trafficking dynamics in live neurons, using time-lapse imaging techniques. Activity-dependent channel trafficking and membrane expression point out roles in plasticity and pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci. 2009;29:6250–6265. doi: 10.1523/JNEUROSCI.0856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santoro B, Piskorowski RA, Pian P, Hu L, Liu H, Siegelbaum SA. TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron. 2009;62:802–813. doi: 10.1016/j.neuron.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- 42.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- 43.Shin M, Chetkovich DM. Activity-dependent regulation of h channel distribution in hippocampal CA1 pyramidal neurons. J Biol Chem. 2007;282:33168–33180. doi: 10.1074/jbc.M703736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiol Dis. 2008;32:26–36. doi: 10.1016/j.nbd.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science. 2007;315:1719–1722. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolan MF, Dudman JT, Dodson PD, Santoro B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J Neurosci. 2007;27:12440–12451. doi: 10.1523/JNEUROSCI.2358-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;23:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Dibbens LM, Reid CA, Hodgson B, Thomas EA, Phillips AM, Gazina E, Cromer BA, Clarke AL, Baram TZ, Scheffer IE, et al. Augmented currents of an HCN2 variant in patients with febrile seizure syndromes. Ann Neurol. 2010;67:542–546. doi: 10.1002/ana.21909. This study provides evidence for the possible involvement of HCN channel mutations in familial forms of human epilepsy. A triple amino acid deletion HCN2 gene was found in a subset of human subjects with febrile seizures-related syndromes and was associated with augmented Ih. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Z, Walker MC, Shah MM. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J Neurosci. 2009;29:10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santoro B, Lee JY, Englot DJ, Gildersleeve S, Piskorowski RA, Siegelbaum SA, Winawer MR, Blumenfeld H. Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia. 2010;51:1624–1627. doi: 10.1111/j.1528-1167.2010.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung WK, Shin M, Jaramillo TC, Leibel RL, LeDuc CA, Fischer SG, Tzilianos E, Gheith AA, Lewis AS, Chetkovich DM. Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2. Neurobiol Dis. 2009;33:499–508. doi: 10.1016/j.nbd.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powell KL, Ng C, O’Brien TJ, Xu SH, Williams DA, Foote SJ, Reid CA. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia. 2008;49:1686–1695. doi: 10.1111/j.1528-1167.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 58.Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Budde T, Caputi L, Kanyshkova T, Staak R, Abrahamczik C, Munsch T, Pape HC. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci. 2005;25:9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hablitz JJ, Yang J. Abnormal pyramidal cell morphology and HCN channel expression in cortical dysplasia. Epilepsia. 2010;51(Suppl 3):52–55. doi: 10.1111/j.1528-1167.2010.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dyhrfjeld-Johnsen J, Morgan RJ, Foldy C, Soltesz I. Upregulated H-current in hyperexcitable CA1 dendrites after febrile seizures. Front Cell Neurosci. 2008;2:2. doi: 10.3389/neuro.03.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Marcelin B, Chauviere L, Becker A, Migliore M, Esclapez M, Bernard C. H Channel-Dependent Deficit of Theta Oscillation Resonance and Phase Shift in Temporal Lobe Epilepsy. Neurobiol Dis. 2009;33:436–447. doi: 10.1016/j.nbd.2008.11.019. This study links Ih dysregulation and network excitability in epilepsy by demonstrating reduced hippocampal theta resonance due to down-regulation of HCN channel expression and Ih. [DOI] [PubMed] [Google Scholar]
- **64.Dyhrfjeld-Johnsen J, Morgan RJ, Soltesz I. Double Trouble? Potential for Hyperexcitability Following Both Channelopathic up- and Downregulation of I(h) in Epilepsy. Front Neurosci. 2009;3:25–33. doi: 10.3389/neuro.01.005.2009. A treatise on the possible implications of up- and down-regulation of Ih in epilepsy. The authors challenge unilateral views of Ih roles in epilepsy, and suggest a complex paradigm whereby either of these changes can result in hyperexcitability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung S, Bullis JB, Lau IH, Jones TD, Warner LN, Poolos NP. Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signaling. J Neurosci. 2010;30:6678–6688. doi: 10.1523/JNEUROSCI.1290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kole MH, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578:507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]