Abstract
The ability of the skeleton to adapt to mechanical stimuli diminishes with age in diaphyseal cortical bone, making bone formation difficult for adults. However, the effect of aging on adaptation in cancellous bone, tissue which is preferentially lost with age, is not well characterized. To develop a model for early post-menopausal women and determine the effect of aging on cancellous bone adaptation in the adult mouse skeleton, in vivo tibial compression was applied to adult (26 wk old) osteopenic female mice using loading parameters, peak applied load and peak diaphyseal strain magnitude, that were previously found to be osteogenic in young, growing (10 wk old) mice. A Load-Matched group received the same peak applied loads (corresponding to +2100 με at the medial diaphysis of the tibia) and a Strain-Matched group received the same peak diaphyseal strains (+1200 με, requiring half the load) as the young mice. The effects of mechanical loading on bone mass and architecture in adult mice were assessed using micro-computed tomography and in vivo structural stiffness measures. Adaptation occurred only in the Load-Matched group in both the metaphyseal and diaphyseal compartments. Cancellous bone mass increased 54% through trabecular thickening, and cortical area increased 41% through medullary contraction and periosteal expansion. Adult mice were able to respond to an anabolic stimulus and recover bone mass to levels seen in growing mice; however, the adaptive response was reduced relative to that in 10 wk old female mice for the same applied load. Using this osteogenic loading protocol, other factors affecting pathological bone loss can be addressed using an adult osteopenic mouse model.
Keywords: aging, bone adaptation, mechanical loading, cancellous bone, cortical bone
Introduction
The skeleton’s ability to adapt to its mechanical environment diminishes with age, hampering efforts to prevent or recover bone loss due to aging and osteoporosis, particularly at cancellous sites [1]. In premenopausal women, high-impact and high-velocity exercises increase bone mass at critical cancellous sites [2-5]. The results are more variable in postmenopausal women, but exercise can prevent bone loss [6-10] and even induce marginal gains in bone mass [11-13], indicating that mechanical stimuli may be a potential therapy for treating osteoporotic bone loss and preventing associated fractures. Therefore, developing in vivo loading models of osteopenic, early post-menopausal women is useful for determining osteogenic stimuli in an aging population with low bone mass.
Several in vivo loading studies have demonstrated that adult rodents can respond positively to increased mechanical stimuli at cortical mid-diaphyseal sites. Adult rodents increased cortical bone mass in limbs subjected to controlled, in vivo loading by tibial four-point [14, 15] and cantilever bending [16], ulnar compression [17, 18] and tibial compression [19-21]. As observed in humans, the adaptive response of cortical bone to loading was reduced with aging for the majority of these models [15, 22]. However, the effects of aging on the response of cortical bone to tibial compression have been mixed. Although the tibial cortex of mice demonstrated an osteogenic response to in vivo compression, growing female mice exhibited a reduced response at the mid-diaphysis relative to adult mice [20], and adult male mice exhibited a reduced endocortical response and a similar periosteal response relative to senescent mice [19]. Therefore, tibial compression may be an effective method to increase cortical bone mass in adult animals.
Less evidence is available for the effects of aging on the functional adaptation of cancellous bone, which is more susceptible to osteoporotic fracture [23]. Based on cortical bone behavior, cancellous bone likely also exhibits reduced adaptive capabilities with aging. But, because cancellous bone is distinct from cortical bone in structure and metabolic activity, distinguishing the effects of aging on mechanotransduction in this tissue is important to many clinical conditions, such as osteoporosis and orthopaedic implant performance. As in diaphyseal bone, changes in cancellous bone mass with tibial compression have been mixed. Tibial compression either increased [21] or did not alter [20] cancellous mass in adult female mice. Additionally, tibial compression led to a reduction in cancellous mass in adult male mice, but maintained cancellous mass in senescent male mice [19]. However, these studies employed different strains or sexes of mice, and each study applied distinct loading waveforms that differed from our own [24, 25]. These experimental differences likely contributed to varying results in cancellous adaptation to tibial compression. When comparing tibial compression studies using young, growing mice, our loading approach induced greater cancellous adaptation [20, 24], suggesting our particular mechanical parameters may similarly induce greater adaptation similarly in adult female mice than others have reported.
Therefore, we applied tibial compression to 26 wk old C57Bl/6 adult female mice using loading parameters based on our previous experiment in growing female mice [24, 26]. One of our objectives was to develop an osteogenic loading protocol in adult mice that modeled adaptation in early post-menopausal women that could be used in future studies to the influence of estrogen status and/or genetic manipulation on skeletal adaptation to applied load. 26 week old mice are fully grown and considered osteopenic due to reduced bone mass in the proximal tibia [27], but are not yet senescent [28]. As an additional objective, we wanted to assess the effects of aging on adaptation by comparing our current study to our earlier one using growing mice [24, 26]. Previously, we controlled two parameters of our loading waveform that were found to produce an osteogenic response to applied load in 10 wk old female mice: the peak applied load and the peak mid-diaphyseal strain magnitude. For growing mice, these parameters were −11.5 N and +1200 με at the tibial mid-diaphysis, respectively. To allow comparison to our earlier study, we maintained each one of these parameters in separate groups by applying the same peak compressive loads to one group of adult female mice (Load-Matched) and the same peak mid-diaphyseal strains to a second group of adult female mice (Strain-Matched). We hypothesized that tibial compression will induce cancellous bone formation in adult female mice, and that the adaptive response will be attenuated relative to growing mice.
Materials and Methods
Animals
Forty-one female C57Bl/6 mice (Jackson Labs) were received and acclimatized in our animal facility for one week. Mice were housed 3-5 per cage with ad libitum access to food and water. Body masses were recorded daily and used to monitor the health of the mice over the course the experiment. At 26 weeks of age, mice were divided into a basal strain calibration group (n=5) and two tibial loading groups: Load-Matched (LM, n=18) and Strain-Matched (SM, n=18). The loading groups were assigned based on a previous study by our group using 10 wk old female mice that received peak compressive loads of −11.5 N corresponding to +1200 με at the tibial medial mid-diaphysis [24]. The Load-Matched (LM) group received matching peak loads (−11.5 N) while the Strain-Matched (SM) group received loads corresponding to matching peak mid-diaphyseal strains (+1200 με). At the start of the experiment, mice weighed 23.1±1.9 g. All experimental procedures were approved by Cornell University’s Institutional Animal Care and Use Committee.
In vivo Load-Strain Calibration
To determine the diaphyseal strains engendered by a −11.5 N load for the LM group and the peak applied load that engendered +1200 με at the medial midshaft of the tibia for the SM group, the in vivo strain-load relationship was established for the tibiae of 26 wk old basal mice using methods previously described [29]. In brief, single element strain gauges (EA-06-015LA-120, Micromeasurements) were prepared and attached to the medial surface of the tibial midshaft aligned with the tibial long axis. While mice (n=5) were anesthetized, a range of dynamic compressive loads (peak loads ranging from −4 to −12 N) were applied, and strain measurements recorded simultaneously (Labview v8.2, National Instruments). Structural stiffness (N/με), a measure that characterizes the structural behavior of the tibial diaphysis specifically when the whole bone is subjected to in vivo tibial compression, was calculated as the change in load (ΔN) over the change in strain (Δμε) during the loading portion of the waveform and averaged across four consecutive load cycles [29]. Structural stiffnesses in the left and right tibiae were similar (left: −0.0050 N/με (95% CI: [−0.0062, −0.0040]); right: −0.0068 N/με (95% CI: [−0.0078, −0.0058]), p>0.05, paired t-test). Using the structural stiffness of the left tibia, a 6.0 N peak compressive load was required to engender +1200 με at the medial mid-diaphysis and an 11.5 N peak compressive load engendered +2100 με in 26 wk old mice (Table 1). Following 2 weeks of loading, post-adaptation structural stiffness was also measured in a subset of loaded animals (SM, n=6; LM, n=5). Three animals in the SM group and one animal in the LM were excluded from post-adaptation structural stiffness calculations because placement of the strain gauges was too far from the midshaft (>5% of bone’s length) [29], leaving 3 SM mice and 4 LM mice with valid post-adaptation load-strain data.
Table 1.
Peak applied compressive loads and resulting peak mid-diaphyseal strains on the medial tibia and peak proximal metaphyseal cancellous tissue strains.
| Experimental Group |
Applied Load (N) |
Mid-diaphyseal Strains (με)b |
Cancellous Tissue Strains (με)c |
|---|---|---|---|
| Growinga | −11.5±0.3 | +1200 | −2437±155 |
| Load-Matched | −11.3±0.5 | +2200 | −2130±273 |
| Strain-Matched | −5.9±0.5 | +1200 | −1178±109 |
10wk female mice undergoing 2wks in vivo loading [24]
Determined by in vivo strain gauging at the medial mid-diaphysis
Determined by finite element modeling of the proximal metaphysis in control limbs.
Data represented as mean ± SD where appropriate.
In vivo Mechanical Loading
The loading protocol for both loading groups consisted of 1200 compressive cycles administered to the left limb at 4 Hz for 5 days/week over 2 weeks (Days 1-5, 8-12) [24, 25]. The LM group received an average peak compressive load of 11.3±0.5 N, corresponding to +2100 με, at the tibial mid-shaft. The SM group received a mean peak compressive load of 5.9±0.5 N, corresponding to +1200 με. The average load during the rest phase between loading cycles for both groups was −1.5±0.6 N. The right tibia served as the non-loaded control limb. During all loading sessions, mice were maintained under general anesthesia (2% Isoflurane, 1.0 L/min, Webster). Normal cage activity was allowed between loading sessions. Mice were euthanized on Day 15 by CO2 inhalation. Tibiae were dissected free of soft tissue and fixed in 10% neutral buffered formalin for 48 hrs, then stored in 70% ethanol.
Micro-computed Tomography (microCT)
All pairs of tibiae were scanned in phosphate-buffered saline using micro-computed tomography with an isotropic voxel resolution of 15 μm (eXplore Locus SP MicroCT, GE Healthcare Systems; 80 kVp, 80μA, 500 views, 2 frames/view, 2000 ms exposure time) [25, 29]. A 0.020 in aluminum filter reduced the effects of beam hardening. Cancellous and cortical volumes of interest (VOIs) were defined manually in each tibia. The purely cancellous VOI began approximately 0.5 mm distal to the proximal growth plate, excluding the primary spongiosa and cortical shell, and extended 10% of the total tibial length. Cancellous mineralized tissue was segmented from water and soft tissue using a global threshold of 270 mg HA/cc. Cancellous outcomes included bone volume fraction (BV/TV), tissue mineral density (cnTMD, mg/cc), and trabecular thickness and separation (Tb.Th and Tb.Sp, mm). The cortical VOI was centered at the mid-diaphysis and spanned 2.5% of the total tibial length. Cortical mineralized tissue was segmented from water and soft tissue using a global threshold of 640 mg HA/cc. Cortical outcomes included tissue mineral density (ctTMD, mg/cc), cortical and medullary area (AC, AM, mm2), and principal moments of inertia (IMIN, IMAX, mm4). Total tibial length (LT, mm) and whole bone radii of curvature in the anteroposterior and mediolateral (CAP and CML, respectively, mm) directions were also measured [29].
Finite Element (FE) Analysis
Specimen-specific FE models were used to calculate strains in the cancellous tissue during loading and to determine the structural stiffness of the metaphysis in control and loaded tibiae. FE VOIs were defined in a subset of representative tibiae (n=5/per group) and used to generate corticocancellous and cortical models. The FE VOIs extended 5% of total tibial length and were centered length-wise within the cancellous microCT VOIs [24]. Corticocancellous VOIs included both the cancellous tissue and the cortical shell; cortical VOIs included only the cortical shell. MicroCT image data were converted to FE models using custom software (MATLAB v7.5, The Mathworks, Inc.). Following a convergence study, microCT scans were coarsened by combining 2×2×2 voxels to yield a single voxel with side length 0.03 mm. The coarsened voxels were converted into 8-noded linear brick elements (~49K elements per corticocancellous model) and assigned isotropic material properties with a uniform modulus of 20 GPa and a Poisson’s ratio of 0.3 (Abaqus v6.7, Dassault Systèmes Simulia Corp) [30]. Nodes at the distal surface were restricted to in-plane motion and prevented from rigid-body motion. Strains were determined at the element centroids.
Because bone mass is fairly constant in adult mice [27], we assumed that tissue strains in the control limbs with loading were representative of the strains induced at the onset of the experiment. The strains in the proximal metaphysis were characterized by applying a uniform displacement to the proximal surface of the corticocancellous models of the control (right) tibiae. Elements were identified within the models that coincided with the cancellous VOIs used for the microCT analysis. In these elements, the longitudinal strains (e33) were determined. For LM animals, a uniform 0.08% apparent strain applied at the proximal surface resulted in −11.3 N at the distal surface. The cancellous tissue strains ranged from an average peak strain of −2130±273 με to an average minimum strain of +338±118 με (mean strain −506±71 με) (Table 1). For SM animals, a uniform 0.05% apparent strain applied at the proximal surface resulted in −5.9 N at the distal surface. Similar to the measured in vivo diaphyseal strains, cancellous tissue strains engendered in the SM animals were approximately half those in the LM animals, ranging from −1178±109 με to +165±42 με (mean: −233±29 με) (Table 1).
To characterize the effects of adaptation on the apparent structural stiffness of the proximal metaphysis in both groups, the reaction force (N) was calculated for a uniform strain of 0.07% applied to the proximal surface of corticocancellous and cortical models for right and left limbs. The proportion of the load transmitted through the cancellous tissue was determined from the ratio of the cortical to corticocancellous reaction forces, an indicator of load sharing between the metaphyseal cortex and cancellous tissue [24].
Statistical Analysis
The effects of the two loading protocols on the adaptive response in the cancellous and cortical compartments, on tibial length, and on body mass were determined using a linear mixed-model with repeated-measures (JMP v8.0, SAS Institute Inc.). The between-subject factor was loading protocol (LM versus SM) and the within-subject factor was loading [loaded (L) versus control (C) tibia] for the microCT and FE measurements or time (Day 1 versus Day 15) for body mass. All results presented are significant unless otherwise stated. If no significant interaction was present, only main effects are reported. For the main effect of loading, the percentage difference was based on the combined LM and SM data and calculated as [(LM + SM Loaded limbs) – (LM + SM Control limbs)]*100/(LM + SM Control limbs). If the interaction term was significant, a post-hoc means comparison was conducted with a Bonferroni correction. Statistical significance was set at p<0.05. All values are represented as mean ± SD.
Results
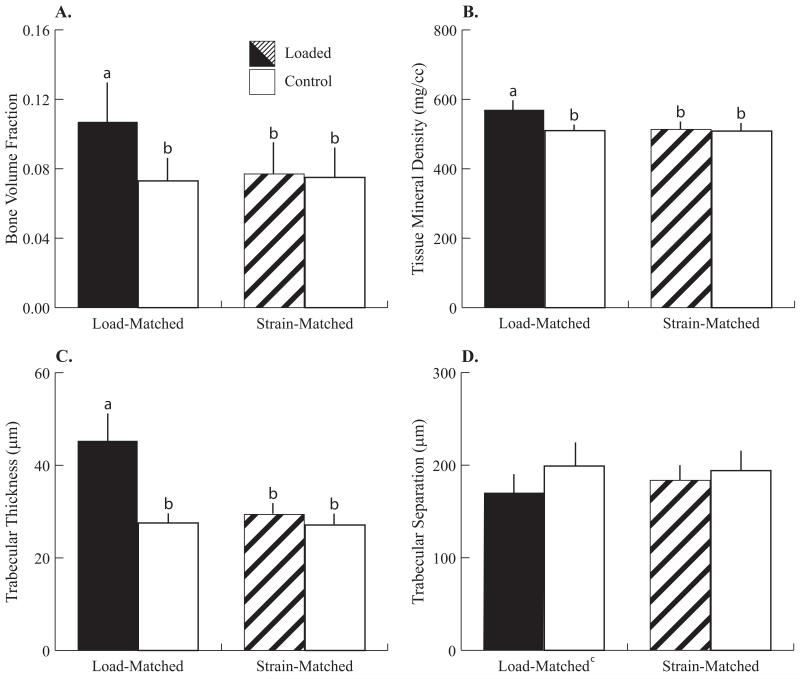
Only the LM group had greater cancellous bone mass and altered trabecular architecture after 2 weeks of in vivo tibial compression (Fig. 1). BV/TV in the loaded tibiae of the LM group was +49% larger than control tibiae (Fig. 2). Cancellous mass was greater primarily due to trabecular thickening, where Tb.Th in the loaded limbs of the LM group was +64% greater than control limbs. cnTMD was also greater in the loaded limbs of the LM group by +12%. Tb.Sp in loaded limbs was 14% lower than in control limbs [(LM + SM loaded) vs. (LM + SM control)]. Control limbs did not differ for any cancellous measure between the LM and SM groups.
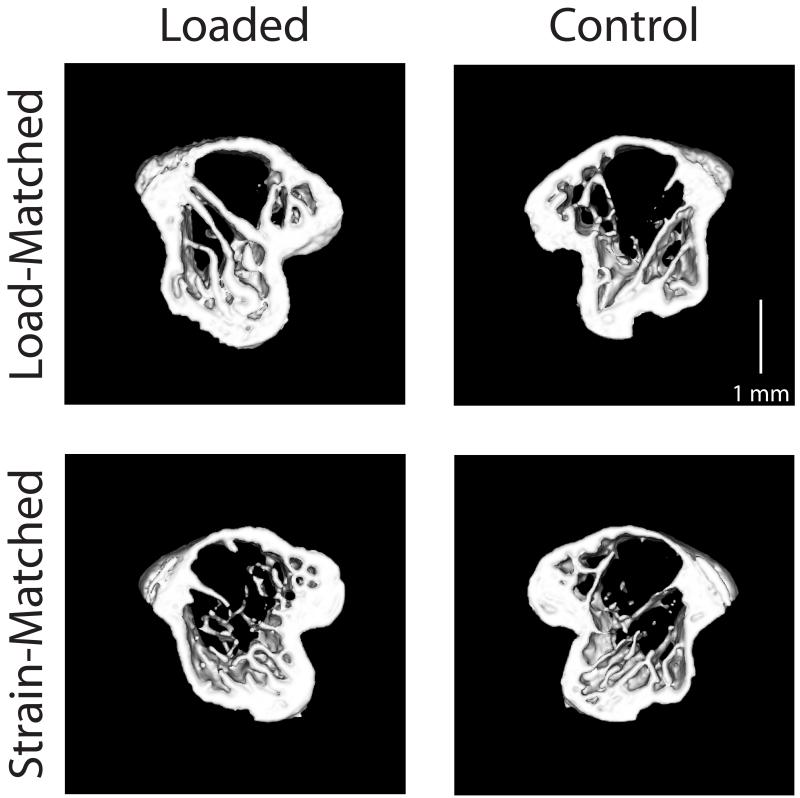
Figure 1.
Transverse sections (0.4 mm thick, 0.5 mm distal to the growth plate) through the proximal metaphysis from representative microCT scans. Representative loaded (left) and control (right) tibiae are shown from both Load-Matched (top) and Strain-Matched (bottom) groups.
Figure 2.
Cancellous parameters after 2 weeks of loading, analyzed by microCT from a purely cancellous VOI in the proximal metaphysis. Significant effects of loading were detected only in the Load-Matched group. (A) Bone volume fraction increased 49%, (B) Tissue mineral density (mg/cc) increased 12%, and (C) Trabecular thickness (mm) increased 62%, in loaded limbs of the LM group relative to control limbs. (D) Trabecular separation (mm) decreased 14% due to loading ([LM + SM loaded limbs] vs [LM + SM control limbs]). Data are represented as mean + SD.
a-bgroups with different letters are significantly different p<0.05 by linear-mixed model with repeated measures followed by means comparisons with Bonferroni correction. cp<0.05 main effect of loading.
Metaphyseal structural stiffness for the LM group was also greater after in vivo loading. Compressive reaction forces were nearly doubled in the corticocancellous FE models in the loaded tibiae relative to the control tibiae (L: 18.3±1.6 N vs. C: 9.5±0.7 N) whereas the reaction forces of the SM group did not change after loading (SM pooled: 9.5±0.7 N). However, the proportion of load carried by cancellous tissue relative to the cortical shell was not altered in either loading group. The proportion of load borne by the cortical relative to corticocancellous bone was 0.75±0.03 (LM and SM pooled) in both loaded and control limbs.
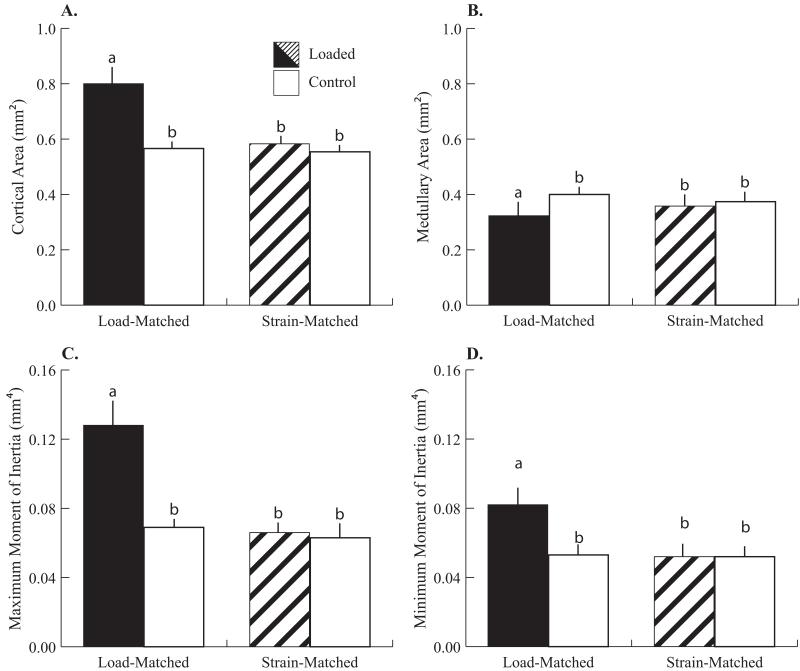
As in the metaphysis, when cortical adaptation at the mid-diaphysis was examined, only the cortices of the LM group changed after 2 weeks of tibial compression (Fig. 3). Cortical area was +41% larger through a 19% reduction in AM in the loaded limbs of the LM group relative to control limbs (Fig. 4). Loading-induced medullary contraction (0.071mm2) was responsible for one third of the total difference in cortical area between the loaded and control limbs (0.216mm2). Thus, periosteal deposition was responsible for the majority of the increase in cortical bone mass with load. Consequently, the principal moments of inertia in the loaded limbs of the LM group were larger relative to control limbs. IMAX and IMIN in the loaded tibiae were +88% and +54% greater than in control tibiae, respectively. ctTMD was 4% smaller after loading in the LM group (L: 1124±21 mg HA/cc vs. C: 1166±18 mg HA/cc) and did not change in the SM group (L + C: 1172±13 mg HA/cc). CAP and CML were not affected by loading. Control limbs did not differ in any microCT-derived parameter between LM and SM groups. Post-adaptation diaphyseal structural stiffness was −0.0052 N/με (95% CI: [−0.0063, −0.0042], all groups pooled together) and was not altered by loading in either group. LT was 16.7±0.42 mm and was not different between the loaded and control limbs.
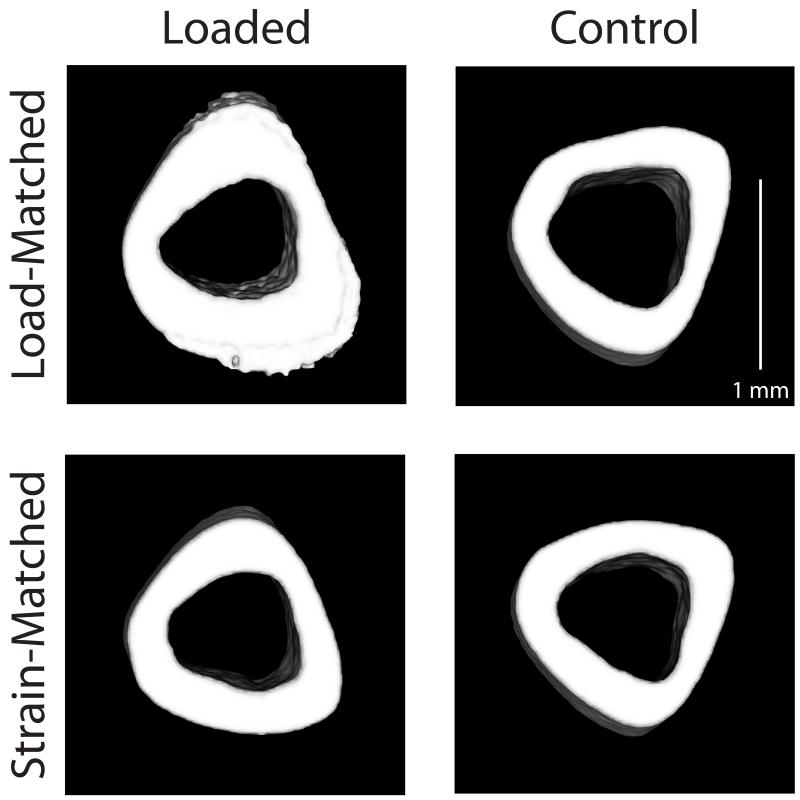
Figure 3.
Transverse sections (0.5 mm thick) centered at the tibial mid-diaphysis from representative microCT scans. Representative loaded (left) and control (right) tibiae are shown from both Load-Matched (top) and Strain-Matched (bottom) groups.
Figure 4.
Cortical parameters after 2 weeks of loading, analyzed by microCT from a VOI centered at the mid-diaphysis. Significant effects of loading were detected only in the Load-Matched group. (A) Cortical area (mm2) increased 41%, (B) Medually area (mm2) decreased 19%, (C) Maximum moment of inertia (mm4) increased 88%, and (D) Minimum moment of inertia (mm4) increased 54% in loaded limbs relative to control limbs. Mean + SD.
a-bgroups with different letters are significantly different p<0.05 by linear-mixed model with repeated measures followed by means comparisons with Bonferroni correction.
After 2 weeks, body mass was not altered by loading (22.4±1.5 g, all mice). Two mice were lost during the loading experiment. One mouse in the SM group died due to complications with anesthesia, and one mouse in the LM group was euthanized due to a fracture during loading, leaving 12 LM mice and 11 SM mice.
Discussion
In vivo tibial compression successfully elicited a robust adaptive response in adult, osteopenic female mice (LM group) in both cancellous and cortical compartments when similar peak compressive forces were applied relative to those used in growing mice. In adult mice, similar compressive forces induced similar cancellous strains and doubled cortical strains relative to growing mice. Cancellous bone mass increased primarily through trabecular thickening, resulting in stiffer metaphyseal tissue. Mid-diaphyseal cortical bone mass increased through medullary contraction and periosteal expansion, resulting in greater principal moments of inertia. The adaptive response in both the cortical and cancellous tissue of adult mice, however, was attenuated when compared to that in growing mice. Our data indicate that mechanical stimuli are a potential therapy for increasing cancellous bone mass in an adult osteopenic population despite reduced responsiveness.
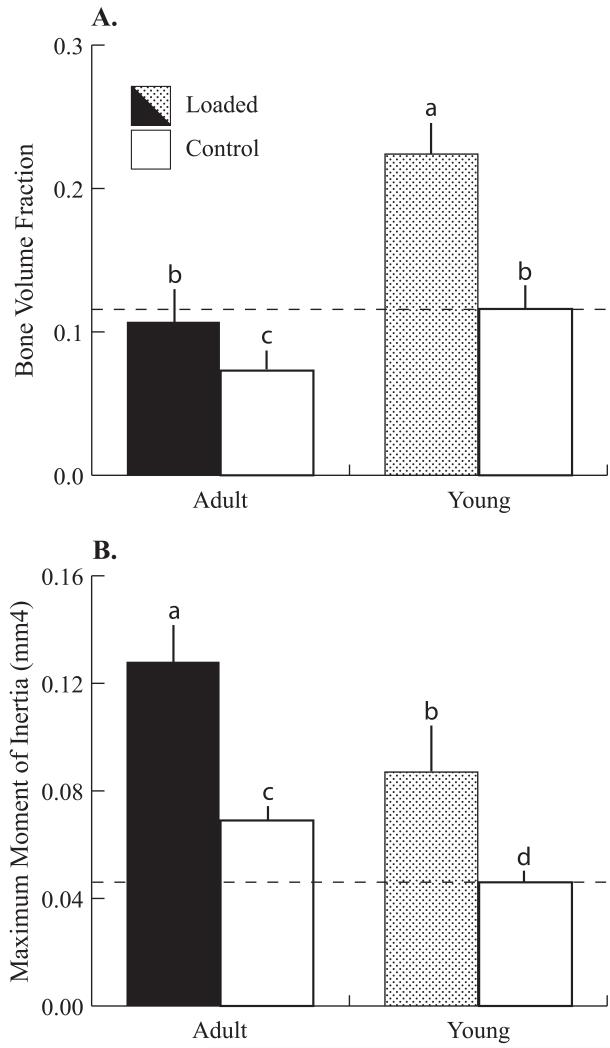
In adult female LM mice, the adaptive response of cancellous bone was reduced relative to that in 10 wk old growing animals, although the peak tissue strains were similar (Table S1). Despite this reduced response, in vivo tibial compression recovered age-related bone loss to levels equivalent to the control limbs of the growing mice [24] (Fig. 5). In addition to a decline in cellular population and activity with aging [31-35], bone formation in the cancellous compartment in adults may also be substrate-limited by the smaller available surface area for bone deposition (Fig. 5). The absolute BV in control limbs of adult mice was lower than that in growing animals (adult: 0.10±0.02; young 0.16±0.02) and the control limbs in adults had thicker, more widely separated trabeculae, leading to an overall reduced bone mass [24, 27]. Other studies using tibial compression also reported reduced cancellous responses to loading in adult mice. Loading increased bone mass in growing mice while middle-aged adult mice lost bone mass with loading [19, 20]. However, our results demonstrate that mechanical loading can be used to recover cancellous bone loss due to aging with potential applications to other causes of pathological bone loss, such as estrogen withdrawal. Although comparison of current results with previously published results from our group for young mice is a limitation, these studies were conducted and analyzed by the same personnel, using the same equipment and in the same animal facility as the previous study, justifying these comparisons.
Figure 5.
Comparison between the Load-Matched group (‘Adult’) and 10 wk old female mice (‘Young’), both loaded for 2 weeks. (A) Bone volume fraction was reduced 38% in adult mice (control limbs). Adult mice demonstrated a reduced response to loading relative to Young mice (+49% versus +95%, loaded versus control). Loading increased bone mass in Adult mice to control levels in Young mice (Adult loaded limbs versus Young control limbs). (B) Maximum moment of inertia increased 48% in Adult mice (control limbs) due to expanding cortices. Adult mice achieved greater gains with loading than Young mice (+90% versus +87%, loaded versus control). Dashed lines represent mean values from control limbs in Young female mice loaded for 2 weeks. Data for 10 wk old mice are from (24). Mean + SD.
a-dgroups with different letters are significantly different p<0.05 by linear-mixed model with repeated measures followed by means comparisons with Bonferroni correction. Within-subject factor: loading; between-subject factor: age.
Contrary to cancellous adaptation, the diaphysis of the LM group exhibited a mixed response to mechanical loading relative to young mice. In adult mice, medullary contraction was enhanced and periosteal expansion was reduced relative to growing animals (Table S2) [19, 20, 26]. Under similar loads (higher peak diaphyseal strains), medullary area decreased more in adults (−19.3%) than in growing animals (−9.2%). Conversely, periosteal expansion accounted for 85% of the increase in cortical area in 10 wk old mice and only 67% of the increased cortical area in 26 wk old mice. Adult animals had greater cortical area, thus available substrate for cortical bone formation was not a limiting factor [36, 37]. Modest bone formation at the periosteum of a larger cortex would have a greater impact on bending resistance (IMAX, IMIN) than in a smaller cortex. Therefore, adult mice do not require as much periosteal bone deposition to increase bending resistance as young mice do. Indeed, maximum moment of inertia was greater in adults, as was the magnitude of the increase with loading, than in growing animals. However, these load-induced differences were similar when normalized to control limb values (+88% vs +90% respectively, adult versus young) (Fig. 5).
Under tibial compression, peak applied forces that induced diaphyseal strains of +1200 με were osteogenic in growing mice [26], but were insufficient to elicit a cortical or cancellous response in adult mice. In previous studies, cortical bone formation occurred when applied forces corresponded to tibial mid-diaphyseal strains of +1200 με using cantilever bending in senescent mice [22] and four-point bending in adult rats [15]. However, tibial compression represents a more physiological loading condition than four-point or cantilever bending; therefore, the introduction of a novel strain distribution may account for the osteogenic responses in other loading models [38]. In the metaphysis, the peak cancellous tissue strains in the SM group were half of those observed in growing mice and in the LM group (Table 1) and were likely not great enough to elicit a response in an adult mouse [15, 39]. Other factors could contribute to the lack of response in addition to peak applied load and tissue strains. For example, the applied strain rate in the SM group was half of that applied to the LM group. Osteogenesis may have occurred if the strain rate was increased while the other parameters were maintained [40].
In previous studies, the cortical response to in vivo loading was reduced with aging, as characterized via histomorphometry [15, 22, 41]. When peak induced strains were matched across ages, the skeletons of older animals either did not respond to loading [41] or exhibited a reduced osteogenic response that did not result in meaningful structural adaptation [15, 22]. When peak strains were doubled to +2400 με in senescent mice undergoing tibial cantilever bending, the boney response was not enhanced [22]. However, senescent mice may be less responsive than the adult mice examined here [42]. In our study, doubling the diaphyseal strains enhanced cortical adaptation, as would be expected [18, 40]. In contrast to these previous studies, the outcome measures we focused on (post-experimental structure and density measures) did not capture dynamic indices of bone cell activity. In contrast to cantilever bending, senescent male mice exhibited enhanced endocortical bone formation and similar periosteal bone formation under tibial compression when compared to adults [19]. This study characterized the effects of aging at a different stage of a mouse’s life than we did, and the strains induced by tibial compression may be increased in senescent mice with further reduced bone mass. Additionally, these data may conflict due to the use of a different strain of mouse (Balb/c) and a different compressive loading waveform. Further studies are needed to interpret how these factors affect the adaptive response to tibial compression.
Structural stiffness decreased with aging, as measured by in vivo strains on the diaphysis during tibial compression. The applied load required to induce +1200 με at the mid-shaft in adult mice was half that used in young mice [24, 26], indicating adult tibiae are less stiff under in vivo compression. In contrast, data from whole bone mechanical tests indicate structural rigidity increased with age under torsion and four-point bending of the diaphysis [36]. Structural stiffness reported here is specific to our particular functional loading configuration, tibial compression, and decreased stiffness likely reflects changes in longitudinal curvature. Though cortical cross-sectional geometries were greater in adult mice, tibial curvature also increased with aging in both planes (CAP: +28%, CML: +200%, adult vs. young), thereby increasing bending moments in the adult tibiae generated during tibial compression. Therefore, changes in curvature counteracted age-related increases in cortical cross-sectional geometry, resulting in decreased structural stiffness. Similarly, age-related increases in tibial curvature resulted in similar structural stiffnesses between 6 wk and 16 wk old female mice, despite larger cortical geometries in the older mice [29]. Thus, inducing similar diaphyseal deformations in growing and adult mice required lower peak applied loads in adults. Post-adaptation stiffness was not altered after the loading period, which is counterintuitive given the changes in cross-sectional geometry. Based on a post-hoc power analysis, over 500 animals would be required to detect a statistically significant effect of loading with 90% confidence due to the high variance in stiffness values, which is likely an unavoidable limitation of this measurement technique.
In contrast to the load-induced cortical strains, cancellous tissue strains during tibial compression in the LM group were similar to those observed in growing mice despite adults having lower adult BV and BV/TV. This similarity is likely due to the larger metaphyseal cortical shell in adult mice. Diaphyseal cortical cross-sectional area and shell thickness are larger in adults [36, 37], and the proximal cortical shell expands with tibial compression [21, 43]. Based on FE results, the metaphyseal cortical shell in adults transmitted a greater proportion of applied load than the metaphyseal shell in young mice (cortical:corticocancellous load ratio 0.76 in adult vs 0.61 in young mice), and maintained this proportion even after loading-induced adaptation, likely contributing to similar cancellous tissue strains in adult LM mice despite decreased cancellous bone mass.
In vivo loading of the mouse tibia allows functional adaptation to be studied simultaneously at cortical and cancellous sites within the same bone, a strength of our experimental approach. Furthermore, the applied loading combines both compression and bending loads within the tibia as experienced in vivo [44, 45]. Despite controlling the applied stimulus, coupling between the loading parameters was unavoidable. The increased load in the LM group also increased the rate of load application and tissue strain rate, a parameter also known to influence skeletal adaptation [40]. However, matching the strain rates would have increased the pause between load cycles in the SM group, and introduced another variable known to enhance the skeletal response under cantilever bending [22], but not under tibial compression [19, 20]. Despite these potential ambiguities, our data demonstrate definitively different outcomes between the two loading protocols and confirm that bone mass in adults with low bone mass can be restored to young control levels with appropriately chosen loading parameters despite a reduction in the adaptive response. Whether this response could be improved upon further can be established with experiments varying additional parameters. Additionally, comparing the loading response in young, growing and adult mice to young, fully grown mice would further characterize how the response to mechanical loading is altered with aging.
In summary, in vivo tibial compression enhanced bone mass and architecture in adult osteopenic mice. Though the cancellous adaptive response diminished with age, mechanical loading increased metaphyseal stiffness and recovered bone mass to that of young growing mice. Principal moments of inertia were greater in the adult diaphysis, but changed similarly with loading because a greater proportion of the newly formed bone in adult mice was formed endocortically than in young mice. The anabolic response seen in both cancellous and cortical compartments was not present when the loads were halved to match cortical strains induced in young mice. Our data show that cancellous bone in the adult skeleton remains responsive to mechanical loading, but requires greater osteogenic stimuli than a younger skeleton. Our osteogenic protocol for adult mice can be used to investigate how other factors, such as reduced estrogen levels, interact with age to modulate cancellous bone adaptation. Results from such studies will help direct the use of mechanical loading to induce architectural changes that will increase bone strength and reduce fracture risk in middle-aged individuals.
Supplementary Material
*Research Highlights.
Adult mice underwent tibial compression to test the effects of age on adaptation.
2 groups received matching peak loads/strain based on that used in growing mice.
Only the Load-Matched group increased cortical and cancellous bone mass.
The adaptive response was reduced with aging in adults.
Tibial compression recovered age-related bone mass to levels in growing mice.
Acknowledgements
We thank Sarah Yagerman for her assistance during the loading experiments and the Cornell Center for Animal Resources and Education staff for their assistance with animal care. Partial support was obtained from the Clark and Kirby Foundations. Funding for this study was provided by: NIH R01-AG028664-01, NIH P30-AR46121, S10-RR024547, F32-AR054676, NSF GRFP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have no conflicts of interest.
References
- 1.Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res. 1998;13:1805–13. doi: 10.1359/jbmr.1998.13.12.1805. [DOI] [PubMed] [Google Scholar]
- 2.Heinonen A, Kannus P, Sievanen H, Oja P, Pasanen M, Rinne M, Uusi-Rasi K, Vuori I. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet. 1996;348:1343–7. doi: 10.1016/S0140-6736(96)04214-6. [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Terashima T, Yamashita T, Hatanaka Y, Honda A, Umemura Y. Effect of low-repetition jump training on bone mineral density in young women. J Appl Physiol. 2006;100:839–43. doi: 10.1152/japplphysiol.00666.2005. [DOI] [PubMed] [Google Scholar]
- 4.Vainionpaa A, Korpelainen R, Leppaluoto J, Jamsa T. Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos Int. 2005;16:191–7. doi: 10.1007/s00198-004-1659-5. [DOI] [PubMed] [Google Scholar]
- 5.Vainionpaa A, Korpelainen R, Vihriala E, Rinta-Paavola A, Leppaluoto J, Jamsa T. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int. 2006;17:455–63. doi: 10.1007/s00198-005-0005-x. [DOI] [PubMed] [Google Scholar]
- 6.von Stengel S, Kemmler W, Kalender WA, Engelke K, Lauber D. Differential effects of strength versus power training on bone mineral density in postmenopausal women: a 2-year longitudinal study. Br J Sports Med. 2007;41:649–55. doi: 10.1136/bjsm.2006.033480. discussion 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurzenski MK, Briffa NK, Price RI, Khoo BC, Devine A, Beck TJ, Prince RL. Geometric indices of bone strength are associated with physical activity and dietary calcium intake in healthy older women. J Bone Miner Res. 2007;22:416–24. doi: 10.1359/jbmr.061115. [DOI] [PubMed] [Google Scholar]
- 8.Korpelainen R, Keinanen-Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J. Effect of impact exercise on bone mineral density in elderly women with low BMD: a population-based randomized controlled 30-month intervention. Osteoporos Int. 2006;17:109–18. doi: 10.1007/s00198-005-1924-2. [DOI] [PubMed] [Google Scholar]
- 9.Stengel SV, Kemmler W, Pintag R, Beeskow C, Weineck J, Lauber D, Kalender WA, Engelke K. Power training is more effective than strength training for maintaining bone mineral density in postmenopausal women. J Appl Physiol. 2005;99:181–8. doi: 10.1152/japplphysiol.01260.2004. [DOI] [PubMed] [Google Scholar]
- 10.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000;55:M489–91. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 11.Kohrt WM, Ehsani AA, Birge SJ., Jr. Effects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older women. J Bone Miner Res. 1997;12:1253–61. doi: 10.1359/jbmr.1997.12.8.1253. [DOI] [PubMed] [Google Scholar]
- 12.Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res. 1996;11:218–25. doi: 10.1002/jbmr.5650110211. [DOI] [PubMed] [Google Scholar]
- 13.Kohrt WM, Snead DB, Slatopolsky E, Birge SJ. Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res. 1995;10:1303–1311. doi: 10.1002/jbmr.5650100906. [DOI] [PubMed] [Google Scholar]
- 14.Raab-Cullen DM, Akhter MP, Kimmel DB, Recker RR. Bone response to alternate-day mechanical loading of the rat tibia. J Bone Miner Res. 1994;9:203–11. doi: 10.1002/jbmr.5650090209. [DOI] [PubMed] [Google Scholar]
- 15.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10:1544–9. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- 16.LaMothe JM, Hamilton NH, Zernicke RF. Strain rate influences periosteal adaptation in mature bone. Med Eng Phys. 2005;27:277–84. doi: 10.1016/j.medengphy.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36:454–64. doi: 10.1016/j.bone.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Mosley JR, March BM, Lynch J, Lanyon LE. Strain magnitude related changes in whole bone architecture in growing rats. Bone. 1997;20:191–8. doi: 10.1016/s8756-3282(96)00385-7. [DOI] [PubMed] [Google Scholar]
- 19.Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared to young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25:2006–2015. doi: 10.1002/jbmr.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone. 2008;43:238–48. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33:946–55. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Melton LJ, 3rd, Gabriel SE, Crowson CS, Tosteson AN, Johnell O, Kanis JA. Cost-equivalence of different osteoporotic fractures. Osteoporos Int. 2003;14:383–8. doi: 10.1007/s00198-003-1385-4. [DOI] [PubMed] [Google Scholar]
- 24.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, van der Meulen MC. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–91. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Main RP, Lynch ME, Van der Meulen MC. Tibial Strains Decrease Following In Vivo Loading in Female Not Male Mice. Trans Orthop Res Soc. 2008;33 [Google Scholar]
- 27.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 28.Flurkey K, Currer J, Harrison D, editors. The mouse in biomedical research. Academic Press; 2007. Mouse models in aging research. [Google Scholar]
- 29.Main RP, Lynch ME, van der Meulen MC. In vivo tibial stiffness is maintained by whole bone morphology and cross-sectional geometry in growing female mice. J Biomech. 2010;43:2689–94. doi: 10.1016/j.jbiomech.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Meulen MC, Yang X, Morgan TG, Bostrom MP. The effects of loading on cancellous bone in the rabbit. Clin Orthop Relat Res. 2009;467:2000–6. doi: 10.1007/s11999-009-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;281:C1635–41. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- 32.Fedarko NS, Vetter UK, Weinstein S, Robey PG. Age-related changes in hyaluronan, proteoglycan, collagen, and osteonectin synthesis by human bone cells. J Cell Physiol. 1992;151:215–27. doi: 10.1002/jcp.1041510202. [DOI] [PubMed] [Google Scholar]
- 33.Tonna EA. Skeletal cell aging and its effects on the osteogenetic potential. Clin Orthop Relat Res. 1965;40:57–81. [PubMed] [Google Scholar]
- 34.Tonna EA. Electron microscopy of aging skeletal cells. III. The periosteum. Lab Invest. 1974;31:609–32. [PubMed] [Google Scholar]
- 35.Tonna EA. Electron microscopic study of bone surface changes during aging. The loss of cellular control and biofeedback. J Gerontol. 1978;33:163–77. doi: 10.1093/geronj/33.2.163. [DOI] [PubMed] [Google Scholar]
- 36.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14:2159–66. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 37.Price C, Herman BC, Lufkin T, Goldman HM, Jepsen KJ. Genetic variation in bone growth patterns defines adult mouse bone fragility. J Bone Miner Res. 2005;20:1983–91. doi: 10.1359/JBMR.050707. [DOI] [PubMed] [Google Scholar]
- 38.Rubin CT, Lanyon LE. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res. 1987;5:300–10. doi: 10.1002/jor.1100050217. [DOI] [PubMed] [Google Scholar]
- 39.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 40.Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am J Physiol. 1995;269:E438–42. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- 41.Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50:306–13. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- 42.Priemel M, Schilling AF, Haberland M, Pogoda P, Rueger JM, Amling M. Osteopenic mice: animal models of the aging skeleton. J Musculoskelet Neuronal Interact. 2002;2:212–8. [PubMed] [Google Scholar]
- 43.Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone. 2009 doi: 10.1016/j.bone.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanyon LE. Functional strain in bone tissue as an objective, and controlling stimulus for adaptive bone remodelling. J. Biomech. 1987;20:1083–1093. doi: 10.1016/0021-9290(87)90026-1. [DOI] [PubMed] [Google Scholar]
- 45.Biewener AA. Musculoskeletal design in relation to body size. J Biomech. 1991;24(Suppl 1):19–29. doi: 10.1016/0021-9290(91)90374-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.