Abstract
The cloning and characterization of a gene (MsHSP23) coding for a heat shock protein in alfalfa in a prokaryotic and model plant system is described. MsHSP23 contains a 633 bp ORF encoding a polypeptide of 213 amino acids and exhibits greater sequence similarity to mitochondrial sHSPs from dicotyledons than to those from monocotyledons. When expressed in bacteria, recombinant MsHSP23 conferred tolerance to salinity and arsenic stress. Furthermore, MsHSP23 was cloned in a plant expressing vector and transformed into tobacco, a eukaryotic model organism. The transgenic plants exhibited enhanced tolerance to salinity and arsenic stress under ex vitro conditions. In comparison to wild type plants, the transgenic plants exhibited significantly lower electrolyte leakage. Moreover, the transgenic plants had superior germination rates when placed on medium containing arsenic. Taken together, these overexpression results imply that MsHSP23 plays an important role in salinity and arsenic stress tolerance in transgenic tobacco. This approach could be useful to develop stress tolerant crops including forage crops.
Electronic supplementary material
The online version of this article (doi:10.1007/s10529-011-0750-1) contains supplementary material, which is available to authorized users.
Keywords: Abiotic stress, Arsenic, E. coli, Heat shock proteins, Medicago sativa, MsHSP23, Salinity stress
Introduction
The role of heat shock proteins (HSPs) in protecting cells against damage associated with environmental stresses has been well documented in a wide range of organisms, ranging from humans to bacteria. This protection stems from the stress induced transcriptional activation of HSPs (Lindquist 1986). Plant small HSP genes (sHSPs) are encoded in the nuclear genome and localized to different cellular compartments. These include chloroplasts, mitochondria, the endoplasmic reticulum, and the cytosol (Vierling 1991). sHSPs are induced by various stress conditions including heat (Heckathorn et al. 2002; Lee et al. 1999; Prändl et al. 1998 ), chilling (Neta-Sharir et al. 2005; Sabehat et al. 1996; Soto et al. 1999), toxic metals (Wollgiehn and Newmann 1995), and oxidative injury (Banzet Banzet et al. 1998), as well as developmental stimuli. The correlation between sHSP synthesis and the stress response led to the hypothesis that sHSPs protect cells from the detrimental effects of stress. Plants contain diverse sHSPs, probably reflecting molecular adaptations to plant-specific stress conditions.
As an important organelle in plant cells, mitochondria perform many essential functions. Mitochondrial structural breakdown and abnormal metabolic activity affects cell viability at high temperatures. Therefore, the capacity of mitochondria to tolerate high temperatures greatly impacts the activity and survival of the entire cell. Mitochondrial sHSPs (msHSPs) are synthesized in the cytoplasm as precursors and then transported into the mitochondria. In addition to the carboxyl-terminal heat shock domain, msHSPs, contain a common, conserved α-crystallin domain. This domain is often represented as a consensus region I and consensus region II (Chen and Vierling 1991). Plant and mammalian msHSPs, as well as α-crystallins, form 200–800 kDa multimeric complexes that exhibit molecular chaperone activity. This prevents the thermal aggregation of proteins and facilitates the refolding of denatured proteins (Vierling 1991). Although sHSP overexpression studies have resulted in enhanced stress tolerance, plant sHSP functional studies are limited. In the present study, full-length alfalfa HSP23 (MsHSP23) cDNA was cloned and characterized in a prokaryotic and model plant system.
Materials and methods
Isolation of HSP23 from alfalfa
A 863 bp MsHSP23 gene was cloned using the GeneFishing DEG (Lee et al. 2009) kit (Seegene, Seoul, South Korea). The PCR product was purified, cloned into the pTOP TA V2 vectors, and sequenced.
Bioinformatics analysis
The nucleotide sequence, deduced amino acid sequence, and open reading frame (ORF) were analyzed using DNAMAN software (Lynnon Biosoft, Vaudreuil-Dorion, Quebec, Canada). The sequence comparison was conducted using BLAST. MsHSP23 and other sHSPs retrieved from GenBank were aligned using ClustalW. A phylogenetic tree was constructed using algorithms from ClustalW [version 2, EMBL-EBI website (http://www.ebi.ac.uk)]. The transmembrane domain was analyzed by TMHMM 2.0, and homology-based structural modeling was achieved using Swiss-Model (http://www.expasy.org/).
Expression and purification of recombinant protein
The MsHSP23 PCR product was digested with BamHI and HindIII, and ligated into the pET-28a plasmid. The resulting plasmid (pET-MsHSP23) was transformed into E. coli BL21, and proper insertion was confirmed by enzyme digestion and sequencing. A single selected clone was cultured overnight at 37°C. The overnight culture was diluted with LB medium (1:100) and cultured for additional 2–3 h to produce log-phase (OD600: 0.6–0.8). To induce protein expression, IPTG was added to the log-phase bacterial culture at 1 mM.
E. coli was harvested by centrifugation. Cells were disrupted via sonication in sample buffer (0.5 M Tris–HCl, 1% 2-mercaptoethanol, 10% v/v glycerol, 1% SDS and crystals of bromophenol blue). The protein concentration was determined by the Lowry method using a Bio-Rad protein assay kit. For SDS-PAGE, equal amounts (20 μg) of protein were separated on a 12.5% polyacrylamide gel using a constant 20 mA current. The separated polypeptides were visualized by staining with Coomassie brilliant blue (CBB). To assist in estimating the size of the separated polypeptides, a protein molecular weight marker (SM 0431; Fermentas Inc., Hanover, MD) was included.
Survival assay of transgenic E. coli under salinity and arsenic stresses
Tolerance to arsenic (Na2HAsO4·7H2O) was determined by an agar dilution method (Washington and Sutter 1981). Plates containing 20 ml media supplemented with various arsenic concentrations (0.01–10 mM) were inoculated with E. coli cultures and incubated at 37°C for 2 days. For analysis of salinity tolerance, an E. coli suspension was supplemented with 5 mM NaCl, and the growth rate was determined in terms of OD600 for 24 h at hourly intervals.
Construction of the MsHSP23 binary expression vector for plant transformation
To provide constitutive expression, the MsHSP23 cDNA fragment was ligated to the cauliflower mosaic virus promoter (35s). The resulting chimeric gene cassette was then inserted into the KpnI/HindIII site of pCAMBIA1300. Recombinant pCAMMsHSP23 (Supplementary figure 1A) was introduced into Agrobacterium tumefaciens strain EHA105, which was then used for plant transformation. The transformation of wild type tobacco (Nictiana tabacum L. cv. SR-1) leaf discs was performed as described by Horsch et al. (1985). Discs infected with A. tumefaciens were incubated on medium to induce shoot growth. After a few weeks, the regenerated shoots were transferred to medium to induce root growth. The integration of the T-DNA into the putative transgenic plant genome was confirmed by PCR and Southern blot analyses (Supplementary figure 1). Based on the Southern blot analysis, each transgenic line appeared to represent an independent integration event (Supplementary figure 1C).
Salinity and arsenic tolerance assay
Relative electrolyte leakage analysis was performed as described by Lee et al. (2007). Leaf disks were harvested from the fully expanded leaves of plants grown in a greenhouse for 1 month. The disks were floated onto a solution containing salt (0, 100, 200 and 300 mM) and arsenic (0, 100, 300 and 500 μM) for 5 days. Thereafter, the loss of cytoplasmic solutes was determined by measuring the electrical conductance of the solution using an ion conductivity meter, after salt and arsenic treatment. Finally, to examine the germination rate under arsenic stress, hygromycin-selected 2 week old T3 transgenic seeds were planted on MS medium containing arsenic (100, 300 or 500 μM). The seeds were incubated in the growth room for 10 days, and the germination rate was calculated.
Results and discussion
Functional prediction and homology model
Using the GeneFishing DEG kit (Seegene, Seoul, South Korea), MsHSP23 was isolated using an ACP-based PCR method as described by Lee et al. (2009). To determine the sequence homology with other plant mitochondrial-localized sHSPs, an amino acid sequence alignment of MsHSP23 was generated using ClustalW and GeneDoc for multi-alignment analysis. MsHSP23 had high amino acid sequence similarity to pea HSP22 (PsHSP22, 82%) and shared sequence similarity with Arabidopsis HSP23.6 (AtHSP23.6, 60%), soybean HSP23.9 (GmHSP23.9, 59%), chilli pepper HSP (CaHSP, 55%), tomato HSP (SlHSP, 53%), wheat HSP23.5 (TaHSP23.5, 42%) and corn HSP22 (ZmHSP22, 38%). As shown in Fig. 1a, MsHSP23 in alfalfa, a dicotyledon, displayed a higher sequence similarity with other dicotyledons (pea, Arabidopsis and soybean) MsHSPs than with other monocotyledons (wheat and corn) MsHSPs. This result was consistent with the phylogenetic analysis (Fig. 1b). The typically conserved region, the alpha-crystallin domain, was well conserved in the C-terminus of MsHSP23 (Fig. 1a).
Fig. 1.
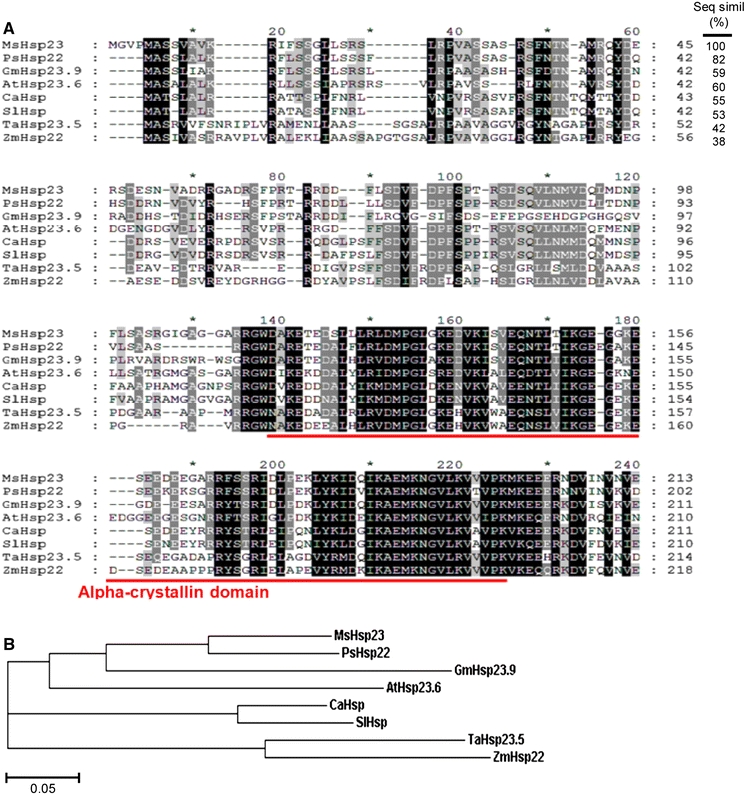
Multiple sequence alignment and phylogenetic analysis of MsHSP23. a Alignment of MsHSP23 with msHSPs homologs from pea (PsHSP22, P46254), soybean (GmHSP23.9, AAB03096), Arabidopsis (AtHSP23.6, NP_194250), chilli pepper (CaHSP, ADJ57588), tomato (SlHSP, BAA32547), wheat (TaHSP23.5, AAD03604) and corn (ZmHSP22, AAV32521) using the ClustalW and GeneDoc programs. The numbers at the right indicate the amino acid residue position. Fully identical residues are shaded in black, six identities in gray, and five identities in light gray. The conserved alpha-crystallin domain is indicated by a line. b The phylogenetic relationships between MsHSP23 and other msHSPs homologs. The phylogenetic tree shows a graphical representation of the evolutionary relationships and was constructed using the EBI-ClustealW2 algorithm
Expression and analysis of recombinant MsHSP23
Under the control of the T7 promoter, recombinant MsHSP23 was rapidly and largely induced in E. coli after the addition of various IPTG concentrations and at various incubation times. SDS-PAGE analysis revealed that the recombinant MsHSP23 was expressed as a protein of approximately 28 kD (Fig. 2), which is similar to the predicted value. Optimal expression was obtained with 1 mM IPTG at 37°C and after 5 h incubation.
Fig. 2.
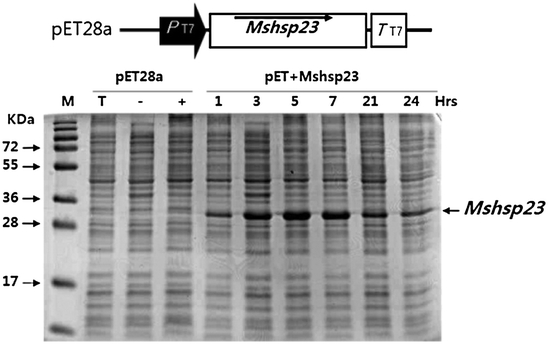
Overexpression of MsHSP23 in E. coli. SDS-PAGE analysis of the recombinant protein expressed in E. coli. Lane 1 BL21 total protein, lane 2 pET28a total protein (−IPTG), lane 3 pET28a total protein (+IPTG), lane 4 MsHSP23 soluble protein Induction 1 h (+IPTG), lane 5 MsHSP23 soluble protein Induction 3 h (+IPTG), lane 6 MsHSP23 soluble protein Induction 5 h (+IPTG), lane 7 MsHSP23 soluble protein Induction 7 h (+IPTG), lane 8 MsHsp23 soluble protein Induction 21 h (+IPTG), lane 9 MsHSP23 soluble protein Induction 24 h (+IPTG)
Stress tolerance of E. coli carrying MsHSP23
To evaluate whether MsHSP23 expression enhanced the salinity or arsenic stress tolerance of recombinant microorganisms, the effects of MsHSP23 heterologous expression on the salinity tolerance of E. coli cells was determined. As shown in Fig. 3a, the MsHSP23-expressing strain exhibited increased survival following treatment with 5 mM NaCl in comparison to the vector control. Similarly, the transformed cells were also examined to determine survival under arsenic stress. As shown in Fig. 3b, the MsHSP23-expressing strain showed little tolerance at low concentrations of arsenic (0.01–0.1 mM). However, at higher arsenic concentrations (1–10 mM), the survival was nearly twofold to threefold higher than that of the vector control. All of these results reveal that the MsHSP23 recombinant protein conferred the host cells with tolerance to salinity and arsenic stress. There is an abundance of sHSPs that function as either molecular chaperones (ex. DnaK), proteases (ex. ClpB), and Lon. sHSPs are temporarily overexpressed when cells are exposed to high temperature or to other various environmental stresses, including high salinity and drought stress. Under these conditions, the sHSPs protect the host cells from damage caused by protein denaturation (Leshem 1992). As shown by these results, the MsHSP23-expressing E. coli had enhanced abiotic stress tolerance.
Fig. 3.
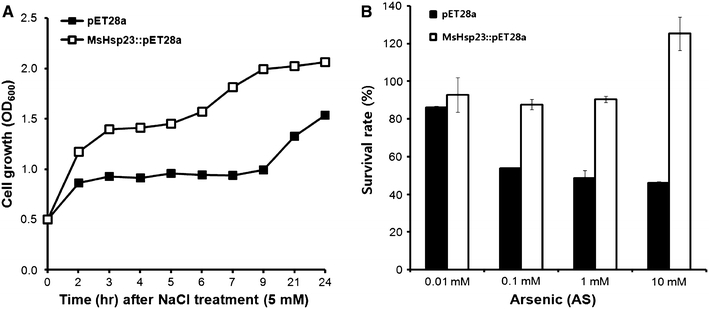
The effect of MsHSP23 expression on the growth of E. coil under salt and arsenic stress conditions. The height of each bar represents the average of three individual, identical experiments (±SE). The mean value of each transgenic line represents a statistically significant difference with respect to the control, as determined by the LSD t test (P < 0.05)
Overexpression of MsHSP23 reduces electrolyte leakage under stress conditions
Stress induced changes are frequently related to an increase in membrane permeability, resulting in disrupted membrane integrity and cell compartmentalization under stress conditions (Temsamani et al. 1995). Increased solute and electrolyte leakage occurs in a variety of stresses, including heat, salt, heavy-metal and methyl viologen stress (Sugimoto et al. 2003; Temsamani et al. 1995). Here, the amount of electrolyte leakage was quantified and used as an indicator of cellular damage following salinity and arsenic stresses. Under control conditions, no marked differences were observed between the control and the transgenic plants. However, the transgenic plants showed significantly lower electrolyte leakage in response to salinity and arsenic treatments (Fig. 4). This result suggests that the transgenic plants were better at maintaining membrane stability under stress conditions. Furthermore, overexpression of MsHSP23 in transgenic plants may enhance the scavenging of ROS in mitochondria, leading to better stress tolerance.
Fig. 4.
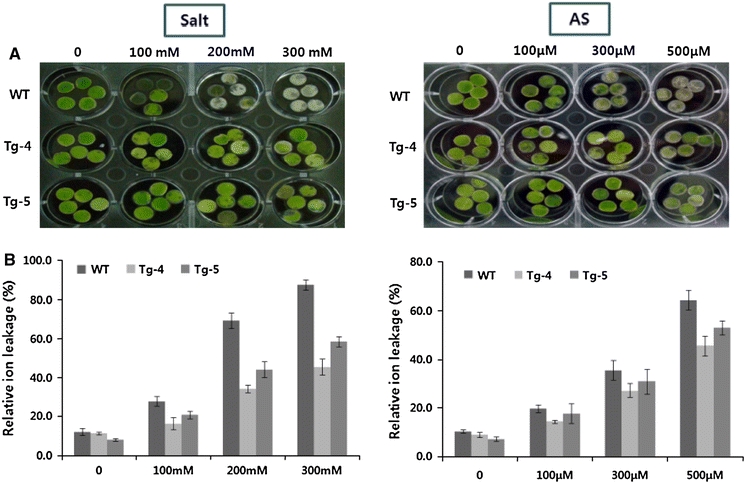
Visible damage in the detached leaves of control and transgenic plants exposed to salt and arsenic for 48 h (a), and the effects of salt and arsenic treatments on the relative ion leakage in the leaves of control and transgenic plants (b). The data represent the means and standard deviation (SD) of three independent measurements. The height of each bar represents the average of three individual identical experiments (±SE). The mean value of each transgenic line represents a statistically significant difference compared to the control, as determined by the LSD t test (P < 0.05)
Germination performance of transgenic tobacco seeds under arsenic stress conditions
The effect of arsenic on seed germination was tested to detect the heavy-metal tolerance of transgenic tobacco. The results showed that there was no obvious difference in the seed germination rate of control and transgenic plants when they were grown on typical MS medium containing 100 μM arsenic. However, when germinated on medium containing 300 μM or more of arsenic, the germination rate of the transgenic seeds was noticeably higher than that of the control seeds (Fig. 5). These results demonstrate that MsHSP23 enhances the germination of transgenic tobacco seeds under conditions of arsenic stress.
Fig. 5.
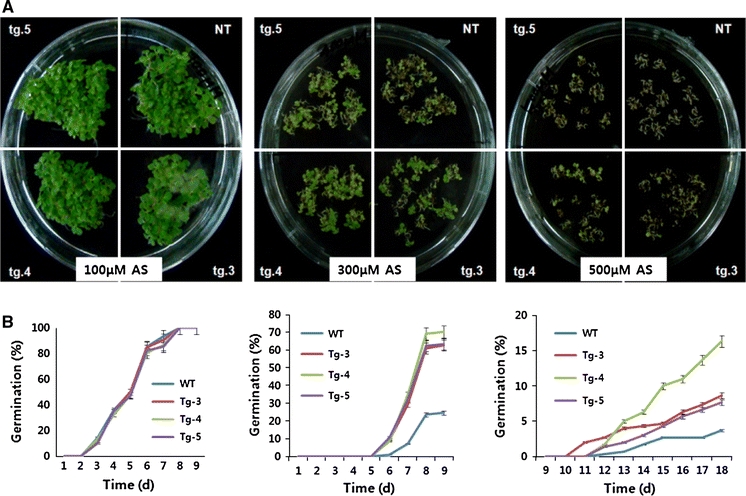
Seed germination assay of transgenic tobacco plants grown under various arsenic concentrations. The height of each bar represents the average of three individual and identical experiments (±SE). The mean value of each transgenic line represents a statistically significant difference compared to the control, as determined by the LSD t test (P < 0.05)
In conclusion the results of the present study show that overexpression of alfalfa mitochondrial MtHSP23 in both eukaryotic and prokaryotic model systems confers enhanced tolerance to salt and arsenic stress. This indicates that MtHSP23 could be used potentially for the development of stress tolerant transgenic crops, such as forages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Banzet N, Richaud C, Deveaux Y, Kazmaier M, Gagnon J, Triantaphylides C. Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant J. 1998;13:519–527. doi: 10.1046/j.1365-313X.1998.00056.x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vierling E. Analysis of conserved domains identifies a unique structural feature of chloroplast heat shock proteins. Mol Gen Genet. 1991;226:425–431. doi: 10.1007/BF00260655. [DOI] [PubMed] [Google Scholar]
- Heckathorn SA, Ryan SL, Baylis JA, Wang DF, Hamilton EW, Cundiff L, Luthe DS. In vivo evidence from an Agrostis stolonifera selection genotype that chloroplast small heat-shock proteins can protect photosystem during heat stress. Funct Plant Biol. 2002;29:933–944. doi: 10.1071/PP01191. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim KY, You SH, Kwon SY, Kwak SS. Molecular characterization and expression of a cDNA encoding copper/zinc superoxide dismutase from cultured cells of cassava (Mannihot esculenta Crantz) Mol Gen Genet. 1999;262:807–814. doi: 10.1007/pl00013819. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH. Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol. 2007;164:1626–1638. doi: 10.1016/j.jplph.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee KW, Kim KY, Choi GJ, Yoon SH, Ji HC, Seo S, Lim YC, Ahsan N. Identification of salt-stress induced differentially expressed genes in barley leaves using the annealing control-primer-based GeneFishing technique. Afr J Biotechnol. 2009;8:1326–1331. [Google Scholar]
- Leshem Y. Plant membranes: a biophysical approach to structure, development and senescence. Dordrecht: Kluwer Academic Publishers; 1992. pp. 1–266. [Google Scholar]
- Lindquist S. The heat shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Neta-Sharir I, Isaacson T, Lurie S, Weiss D. Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell. 2005;17:1829–1938. doi: 10.1105/tpc.105.031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prändl R, Hinderhofer K, Eggers-Schumacher G, Schöffl F. HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol Gen Genet. 1998;258:269–278. doi: 10.1007/s004380050731. [DOI] [PubMed] [Google Scholar]
- Sabehat A, Weiss D, Lurie S. The correlation between heat shock protein accumulation and persistence and chilling tolerance in tomato fruit. Plant Physiol. 1996;110:531–537. doi: 10.1104/pp.110.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A, Allona I, Collada C, Guevara MA, Cerezo R, Casado ER, Aragoncillo C, Gomez L. Heterologous expression of a plant small heat shock protein enhances Escherichia coli viability under heat and cold stress. Plant Physiol. 1999;120:521–528. doi: 10.1104/pp.120.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto S, Nakayama J, Fukuda D, Sonezaki S, Watanabe M, Towskhong A, Sonomoto K. Effect of heterologous expression of molecular chaperone DnaK from Tetragenococcus halophilus on salinity adaptation of Escherichia coli. J Biosci Bioeng. 2003;96:129–133. doi: 10.1016/s1389-1723(03)90114-9. [DOI] [PubMed] [Google Scholar]
- Temsamani KR, Bouchta D, Fahmi T, Benicha M, Bennouna M, Azmani A. Evaluation of the photosynthesis inhibitory effect of new sulfonylureas derivatives on Oxalis pes-caprae. Bioelectrochem Bioenerg. 1995;38:63–66. doi: 10.1016/0302-4598(95)01826-Z. [DOI] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev. 1991;42:579–620. [Google Scholar]
- Washington JA, Sutter VL. Dilution test procedures. In: Lennette EH, Balows A, Hausler WJ, Truant JP, editors. Manual of clinical microbiology. Washington, DC: American Society for Microbiology; 1981. pp. 549–555. [Google Scholar]
- Wollgiehn R, Newmann D. Stress response of tomato cell cultures to toxic metals and heat shock: differences and similarities. J Plant Physiol. 1995;146:736–742. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


