Abstract
Background
The healthcare system is a key channel for delivering treatment to tobacco users. Brief clinic-based interventions are effective but not reliably offered. Population management strategies might improve tobacco treatment delivery in a healthcare system.
Purpose
To test the effectiveness of supplementing clinic-based care with a population-based direct-to-smoker (DTS) outreach offering easily accessible free tobacco treatment.
Design
RCT, conducted in 2009–2010, comparing usual clinical care to usual care plus DTS outreach.
Setting/participants
A total of 590 smokers registered for primary care at a community health center in Revere, MA.
Interventions
Three monthly letters offering a free telephone consultation with a tobacco coordinator who provided free treatment including up to 8 weeks of nicotine patches (NRT) and proactive referral to the state quitline for multisession counseling.
Main Outcome Measures
Use of any tobacco treatment (primary outcome) and tobacco abstinence at 3 month follow-up; cost-per-quit.
Results
Of 416 eligible smokers, 43 (10.3%) in the DTS group accepted the treatment offer; 42 (98%) requested NRT and 30 (70%) requested counseling. In intention-to-treat analyses adjusted by logistic regression for age, gender, race, insurance, diabetes, and coronary heart disease, a higher proportion of the DTS group, compared to controls, had used NRT (11.6% vs 3.9%, OR 3.47; 95% CI 1.52, 7.92) or any tobacco treatment (14.5% vs 7.3%, OR 1.95, 95% CI 1.04, 3.65) and reported being tobacco abstinent for the past 7 days (5.3% vs 1.1%, OR 5.35, 95% CI 1.23, 22.32) and past 30 days (4.1% vs 0.6%, OR 8.25, 95% CI 1.08, 63.01). The intervention did not increase smokers’ use of counseling (1.7% vs 1.1%) or non-NRT medication (3.6% vs 3.9%). Estimated incremental cost-per-quit was $464.
Conclusions
A population-based outreach offering free tobacco treatment to smokers in a health center was a feasible, cost-effective way to increase the reach of treatment (primarily NRT) and to increase short-term quit rates.
Introduction
The healthcare system is a key channel for delivering treatment to tobacco users.1 Clinical interventions at office visits increase smoking-cessation rates but are not consistently offered.2,3 Even if universally provided, they can reach only those smokers making a visit. A healthcare system might increase its delivery of tobacco treatment by supplementing visit-based interventions with proactive population-based outreach,4,5 using methods proven effective in public health settings.6–13 Whether this strategy is feasible and effective for promoting smoking cessation in a healthcare setting is unclear. Two randomized trials of population-based outreach in primary care improved smokers’ use of tobacco treatment and quit attempts but not smoking-cessation rates.14,15
This RCT tested the effectiveness of a population-based Direct-to-Smoker (DTS) outreach program in a healthcare system. Smokers, identified by the electronic health record (EHR), were proactively offered free tobacco treatment that did not require an office visit. The hypothesis was that adding the DTS program to usual primary care would increase the proportion of smokers who used treatment and thereby stopped smoking.
Methods
Study Design, Setting, and Participants
The study was conducted among smokers with a primary care provider (PCP) at a community health center in Revere, Massachusetts, belonging to Partners HealthCare System. Partners’ EHR identified adults (aged ≥18 years) who had made an office visit to a PCP in the year before May 2009, had a telephone, and had in the past 5 years the entry of “current smoker” in the health monitoring grid or “smoking” on the problem list. Two cohorts of subjects, DTS1 and DTS2, were drawn sequentially from the population during 1 year (July 2009–June 2010), randomly assigned to receive the DTS program or usual care, and followed for 3 months to assess outcomes.
Recruitment and Randomization
All 19 PCPs participated. Each received a list of their patients identified as smokers by the EHR and excluded those who were nonsmokers or not appropriate to contact for telephone counseling. Those not excluded received a letter from the medical director confirming that they were current smokers and offering the option to decline further study contact. Individuals who were not excluded were randomly assigned to intervention or usual care control groups (1:1 for DTS1; 3:1 for DTS2). Randomization was stratified by PCP.
Intervention
Intervention group participants were mailed one letter monthly for 3 months beginning in August 2009 (DTS1) or January 2010 (DTS2). Letters, signed by the PCP, encouraged the smoker to quit and offered a free telephone consultation by Partners’ Tobacco Treatment Coordinator (TTC), free nicotine patches, and referral to additional treatment resources. Additionally, in DTS2, the TTC made 2 proactive calls to a randomly selected one third of participants (Intervention Plus group) to try to increase uptake of the offer.
Participants who contacted the TTC received a 15-minute consultation following clinical guidelines.2 She offered a free 4-week supply of 21-mg nicotine patches mailed to their home (refillable once), helped smokers obtain prescriptions for other smoking-cessation medication, used a fax-referral system to connect smokers to free multisession counseling from the Massachusetts Smokers Quitline, and referred to in-person counseling programs. The TTC documented the encounter in the EHR and emailed the smoker’s PCP.
Control
Smokers assigned to usual care received a letter from their PCP advising them to stop smoking, encouraging them to make a visit to discuss quitting, and telling them to expect a telephone call in 3 months.
Assessment
Baseline characteristics were obtained from the EHR. The primary outcome was the proportion of participants who reported using any tobacco treatment during the 3-month study period, defined as (1) any smoking-cessation counseling contact (with the TTC, Quitline, or in-person counseling) or (2) FDA-approved smoking-cessation pharmacotherapy (nicotine patch, gum, lozenge, inhaler, or nasal spray; bupropion; or varenicline). The secondary outcome was tobacco abstinence, defined as self-reported 7-day and 30-day point-prevalence abstinence at 3-month follow-up. Additional outcomes included use of individual components of tobacco treatment and having made a quit attempt (intentional tobacco abstinence lasting ≥24 hours) in the past 3 months. DTS program response was measured as the percentage of eligible subjects who responded to the offer by contacting the TTC.
Outcomes were assessed by telephone survey (5 call attempts, no incentive for completion) at 3-month follow-up. Due to resource constraints, follow-up in DTS1 was attempted for a randomly selected half of control group participants (n=98). Response rates were calculated using the American Association for Public Opinion Research response-rate calculation #4.16
Statistical Analysis
Results were examined in 2010 separately by cohort, then pooled for analysis using an intention-to-treat approach that included all eligible randomized subjects, with two exceptions. It excluded control-group subjects for whom follow-up was not attempted (n=97) and individuals who after randomization were found to be ineligible because they were nonsmokers or did not speak English. These individuals were included in a subsequent sensitivity analysis. Subjects with missing follow-up data were classified as smokers and considered not to have used tobacco treatment during the study. Baseline factors between study arms and between survey respondents and nonrespondents were compared, and variables related either to group assignment or to survey response were included as covariates in logistic regression models assessing outcomes.
Cost-effectiveness analysis
Personnel and material costs of the intervention, excluding research costs, were tracked prospectively. The analysis took the perspective of a provider organization. Cost effectiveness was calculated as the incremental cost divided by the incremental number of quits in the intervention group. The incremental number of quits is the risk difference for 7-day point-prevalence abstinence at follow-up multiplied by the number of intervention patients.
Results
Participants
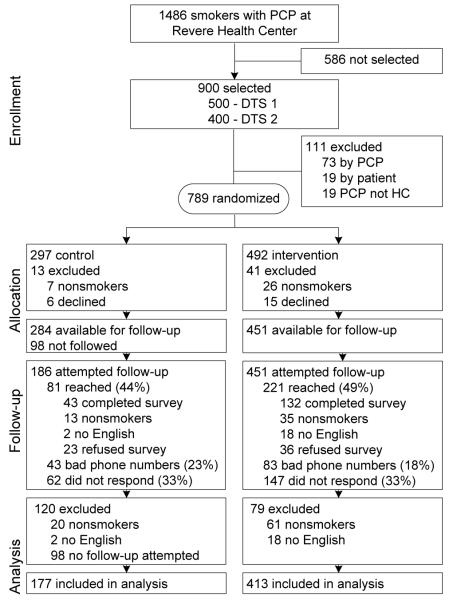
Figure 1 displays the flow of participants through the trial. The 3-month adjusted survey response rate, accounting for estimated ineligibility among nonrespondents and refusals, was 35% (28% in the control group, 39% in the intervention group).16 Table 1 displays baseline characteristics of the 590 study participants. Control and intervention groups differed only in gender.
Figure 1.
Flow diagram
Note: Intervention Plus consists of the standard intervention (three mailed letters offering treatment) plus two proactive telephone calls offering the same treatment options.
DTS, direct to smoker; HC, health center; PCP, primary care provider
Table 1.
Baseline characteristics of Direct-to-Smoker (DTS) Study participants, % unless otherwise indicated
| Full Study (both cohorts) |
DTS 1 (1st cohort) |
DTS 2 (2nd cohort) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Control n=177 |
All Intervention n=413 |
p- value |
Control n=98 |
Intervention n=174 |
p- valu e |
Control n=79 |
Intervention n=165 |
Intervention Plusa n=74 |
p- value |
| Age (years) | ||||||||||
| M (SD) | 47.8 (14.6) | 47.2 (15.3) | .667 | 48.6 (14.7) | 47.7 (15.2) | .611 | 46.7 (14.6) | 47.3 (16.0) | 46.7 (15.2) | .954 |
| <50 | 57.1 | 58.6 | .729 | 52.0 | 55.8 | .556 | 63.3 | 62.4 | 56.8 | .649 |
| Gender - male | 46.3 | 33.4 | .003 | 52.0 | 32.8 | .002 | 39.2 | 34.6 | 32.4 | .656 |
| Race | ||||||||||
| White | 82.5 | 83.8 | .879 | 83.7 | 84.5 | .561 | 81.0 | 81.2 | 87.8 | .506 |
| Black | 5.1 | 3.2 | 7.1 | 3.5 | 2.5 | 2.4 | 4.1 | |||
| Hispanic | 6.2 | 5.3 | 3.1 | 5.8 | 10.1 | 5.5 | 4.1 | |||
| Asian | 4.5 | 4.6 | 5.1 | 3.5 | 3.8 | 6.7 | 2.7 | |||
| Other | 0.6 | 1 | 1.0 | 1.6 | 0 | 1.8 | 0 | |||
| Unknown or refused | 1.1 | 1.8 | 0 | 1.1 | 2.5 | 2.4 | 1.4 | |||
| Health insurance | ||||||||||
| Private | 49.7 | 44.8 | .698 | 53.1 | 44.3 | .605 | 45.6 | 44.9 | 46.0 | .775 |
| Medicare | 17.0 | 21.6 | 18.4 | 24.7 | 15.2 | 18.2 | 21.6 | |||
| Commonwealth Connectorb | 5.1 | 6.1 | 5.1 | 7.5 | 5.1 | 5.5 | 4.1 | |||
| Medicaid | 23.7 | 23.5 | 19.4 | 19.0 | 29.1 | 29.1 | 21.6 | |||
| Uninsured or self-pay | 4.5 | 4.1 | 4.1 | 4.6 | 5.1 | 2.4 | 6.8 | |||
| Health insurance (combined) (Medicaid, uninsured, self-pay) vs other |
28.3 | 27.6 | .873 | 23.5 | 23.6 | .986 | 34.2 | 31.5 | 28.4 | .742 |
| Medical history | ||||||||||
| Diabetes | 11.9 | 12.8 | .745 | 14.3 | 12.1 | .600 | 8.9 | 14.6 | 10.8 | .408 |
| Coronary heart disease | 4.0 | 3.9 | .963 | 2.0 | 3.5 | .510 | 6.3 | 3.6 | 5.4 | .618 |
| Office visits in past year – M (SD) | ||||||||||
| To primary care provider at RHCc | 2.3 (2.5) | 2.5 (2.9) | .458 | 2.3 (2.4) | 2.5 (2.8) | .587 | 2.4 (2.7) | 2.4 (2.8) | 2.7 (3.3) | .666 |
| To any provider at RHCc | 8.2 (11.0) | 9.0 (11.0) | .445 | 8.0 (11.7) | 8.6 (10.3) | .645 | 8.5 (10.2) | 8.4 (10.3) | 11.1 (13.5) | .195 |
Intervention Plus group received two proactive phone calls from the Tobacco Treatment Coordinator in addition to three monthly letters offering treatment.
State-subsidized private insurance available to low-income residents of Massachusetts whose incomes exceeds limits to qualify them for Medicaid
Revere Health Center
Response to Treatment Offer
Forty-three (10.3%) of 416 intervention subjects offered treatment contacted the TTC. Forty-two (98%) requested nicotine patches (41 passed a medical screen and were sent patches) and 30 (70%) requested counseling. Response did not differ by season (11.5% in August–October vs 9.6% in January–March). Adding two proactive telephone calls to three letters offering treatment in DTS2 did not improve response (9.5% vs 9.7%, respectively).
Outcomes
Table 2 displays study outcomes at 3-month follow-up. The treatment offer increased the proportion of smokers who used any tobacco treatment and who used NRT but not non-nicotine medications or counseling. At 3-month follow-up, the treatment offer increased self-reported abstinence for the past 7 days and past 30 days. An alternative analysis retaining all randomized subjects, even those later found to be ineligible for the study, produced the same pattern of significant results (data not shown). Limiting the analysis to respondents only (n=209) produced similar ORs, but reduced statistical power to detect some differences (data not shown).
Table 2.
Outcomes at 3-month follow-up, n (%) unless otherwise indicated
| Outcome measure | Control n=177 |
Intervention n=413 |
p-value | AORa | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Used any tobacco treatment in past 3 months | 13 (7.3) | 60 (14.5) | 0.026 | 1.95 | 1.04, 3.65 | 0.036 |
| Any counseling | 2 (1.1) | 7 (1.7) | 0.731b | 1.19 | 0.29, 4.77 | 0.811 |
| Any medication | 12 (6.8) | 55 (13.3) | 0.022 | 2.2 | 1.14, 4.27 | 0.019 |
| Nicotine replacement therapy | 7 (3.9) | 48 (11.6) | 0.003 | 3.47 | 1.52, 7.92 | 0.003 |
| Bupropion or varenicline | 7 (3.9) | 15 (3.6) | 0.859 | 0.91 | 0.36, 2.28 | 0.841 |
| Made a quit attempt of ≥24 hours | 20 (11.3) | 72 (17.4) | 0.066 | 1.64 | 0.96, 2.80 | 0.07 |
| Tobacco abstinence (self-report) | ||||||
| Past 7 days | 2 (1.1) | 22 (5.3) | 0.021 b | 5.35 | 1.23, 22.32 | 0.026 |
| Past 30 days | 1 (0.6) | 17 (4.1) | 0.019 b | 8.25 | 1.08, 63.01 | 0.042 |
ORs were adjusted for age (<50/>50), gender, race (white/other), insurance (Medicaid or uninsured vs other), CHD, and diabetes in multiple logistic regression analysis. Covariates were chosen to adjust for group differences in baseline characteristics (gender) and in the response rate to the outcome survey (age, race, health insurance, and history of CHD or diabetes).
F exact test.
Cost-Effectiveness Analysis
The total cost of intervention implementation was $9,981. The incremental number of intervention-attributable quits was 21.5. The incremental cost per quit was $464.
Conclusion
A population-based outreach program offering free tobacco treatment to smokers in a community health center was a feasible, cost-effective way to increase the reach of treatment (primarily NRT) and to increase short-term quit rates. The 10% response to a mailed offer of free treatment was similar to the 9% rate in a nonrandomized trial in New Zealand.17 The study extends two previous randomized studies that used the components of our population-based intervention model.14,15
The proactive offer of free NRT and counseling nearly doubled smokers’ likelihood of using pharmacotherapy and more than tripled their odds of using NRT specifically, but did not increase the use of counseling or other smoking-cessation medications. The intervention increased rates of self-reported tobacco abstinence for past 7 days and 30 days at 3-month follow-up. The intervention’s effect might be strengthened by identifying a way to increase smokers’ acceptance of counseling along with pharmacotherapy, because combined treatment is more effective than single treatment.2
The study has several limitations. Follow-up lasted only 3 months; therefore, the results reflect short-term rather than long-term tobacco abstinence. Further study is needed to determine the effect on long-term abstinence. Abstinence was not verified biochemically, although validation is not required in low-intensity population-based interventions such as this study.18 Response to the outcome assessment was only 35%. To avoid bias, an intent-to-treat analysis was used, conservative assumptions were made about nonrespondents (that they all continued smoking and did not access treatment), and analyses were adjusted for systematic differences between respondents and nonrespondents.19 Finally, the study was conducted in one site, limiting generalizability.
The DTS program is a promising strategy that extends the reach of tobacco treatment beyond the office and leverages a health system’s resources to improve the health of the population it serves. It deserves further study to determine its long-term impact. If effective, it could be incorporated into emerging models of healthcare delivery like patient-centered medical homes and accountable care organizations and potentially serve as a model for managing chronic diseases other than tobacco dependence.
Acknowledgements
We are grateful to the clinicians at Revere Health Center, especially Roger Pasinski, MD, and Amy Wheeler, MD, who assisted with this study; to Eric Campbell, PhD, for calculating survey response rates; to Yuchiao Chang, PhD, for the randomization scheme and advising on study design; to Christine Hahn for assistance with mailings and manuscript preparation; and to Jennifer Pandiscio, Michelle Blitzman, and Nina Mayer, MD for helping to conduct the follow-up assessments.
Partners Health Care, Inc., funded the DTS program, study data collection and data management. Data analysis was conducted by an independently funded investigator (Dr. Hoeppner). Study investigators’ efforts on the project were supported by grants #T32HP10251 from the Health Resources and Services Administration for the Harvard General Medicine Fellowship (Dr. Bitton); #1K01DA027097-01A1 (Dr. Hoeppner), and #K24-HL08880 (Dr. Rigotti). The funders of these grants had no role in study design, conduct, data collection, analysis, interpretation, or manuscript preparation, review, or approval.
NAR receives partial salary support from Partners HealthCare Inc. for role as Chair, Partners Tobacco Task Force. NAR has research grants from Nabi Biopharmaceuticals for testing an investigational smoking-cessation aid; unpaid consultant for Pfizer, Inc., which makes a smoking-cessation product, and Free & Clear, Inc., which provides telephone smoking-cessation services.
EM receives partial salary support from Partners HealthCare, Inc., for role as Director for High Performance Medicine Team 3. JAK receives salary support from Partners HealthCare, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other authors reported financial disclosures.
References
- 1.Curry SJ, Keller PA, Orleans CT, Fiore MC. The role of health care systems in increased tobacco cessation. Annu Rev Public Health. 2008;29:411–428. doi: 10.1146/annurev.publhealth.29.020907.090934. [DOI] [PubMed] [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, et al. [Accessed March 20, 2011];Treating tobacco use and dependence: 2008 update. clinical practice guideline. http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf.
- 3.Thorndike AN, Regan S, Rigotti NA. The treatment of smoking by U.S. physicians during ambulatory visits: 1994 2003. Am J Public Health. 2007;97(10):1878–1883. doi: 10.2105/AJPH.2006.092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman SE. A framework for tobacco control: Lessons learnt from Veterans Health Administration. BMJ. 2008;336(7651):1016–1019. doi: 10.1136/bmj.39510.805266.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigotti NA. The future of tobacco treatment in the health care system. Ann Intern Med. 2009;150(7):496–497. doi: 10.7326/0003-4819-150-7-200904070-00011. [DOI] [PubMed] [Google Scholar]
- 6.Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY. Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addict Behav. 2001;26(4):583–602. doi: 10.1016/s0306-4603(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 7.Velicer WF, Friedman RH, Fava JL, et al. Evaluating nicotine replacement therapy and stage-based therapies in a population-based effectiveness trial. J Consult Clin Psychol. 2006;74(6):1162–1172. doi: 10.1037/0022-006X.74.6.1162. [DOI] [PubMed] [Google Scholar]
- 8.Tzelepis F, Paul CL, Wiggers J, et al. A randomised controlled trial of proactive telephone counselling on cold-called smokers’ cessation rates. Tob Control. 2010 doi: 10.1136/tc.2010.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Land T, Warner D, Paskowsky M, et al. Medicaid coverage for tobacco dependence treatments in Massachusetts and associated decreases in smoking prevalence. PLoS One. 2010;5(3):e9770. doi: 10.1371/journal.pone.0009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller N, Frieden TR, Liu SY, et al. Effectiveness of a large-scale distribution programme of free nicotine patches: A prospective evaluation. Lancet. 2005;365(9474):1849–1854. doi: 10.1016/S0140-6736(05)66615-9. [DOI] [PubMed] [Google Scholar]
- 11.An LC, Schillo BA, Kavanaugh AM, et al. Increased reach and effectiveness of a statewide tobacco quitline after the addition of access to free nicotine replacement therapy. Tob Control. 2006;15(4):286–293. doi: 10.1136/tc.2005.014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinkelman D, Wilson SM, Willett J, Sweeney CT. Offering free NRT through a tobacco quitline: Impact on utilisation and quit rates. Tob Control. 2007;16(Suppl 1):i42–i46. doi: 10.1136/tc.2007.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16(Suppl 1):i53–i59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray RL, Coleman T, Antoniak M, et al. The effect of proactively identifying smokers and offering smoking cessation support in primary care populations: A cluster-randomized trial. Addiction. 2008;103(6):998–1006. doi: 10.1111/j.1360-0443.2008.02206.x. discussion 1007–8. [DOI] [PubMed] [Google Scholar]
- 15.Ellerbeck EF, Mahnken JD, Cupertino AP, et al. Effect of varying levels of disease management on smoking cessation: A randomized trial. Ann Intern Med. 2009;150(7):437–446. doi: 10.7326/0003-4819-150-7-200904070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Association for Public Opinion Research [Accessed November 30, 2010];Standard definitions: Final dispositions of case codes and outcome rates for surveys. http://www.aapor.org/Content/NavigationMenu/ResourcesforResearchers/StandardDefinitions/StandardDefinitions2009new.pdf.
- 17.Watson D, Bullen C, Glover M, McRobbie H, Parag V, Walker N. Impact on quit attempts of mailed general practitioner ‘brief advice’ letters plus nicotine replacement therapy vouchers. J Primary Health Care. 2010;2:4–10. [PubMed] [Google Scholar]
- 18.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 19.Judd CM, Kenny DA. Data analysis in social psychology: Recent and recurring issues. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of social psychology. 5th ed Vol 1. John Wiley & Sons Inc.; Hoboken NJ: 2010. pp. 115–139. [Google Scholar]