Abstract
South Asians from India and Pakistan represent one of the fastest growing immigrant populations in the US, yet there are limited data assessing breast cancers for this distinct ethnic sub-group. The aim of this study was to analyze clinical-pathologic, treatment and outcome characteristics of U.S.-residing Indian-Pakistani (IP) versus non-Hispanic white (NHW) female breast cancer patients to assess if any differences/disparities exist. The study cohort consisted of 2,393 IP and 555,832 NHW women (diagnosed 1988–2006) in the SEER database. Differences between the two populations were analyzed using chisquared and multivariate regression analysis. Age-adjusted incidence, mortality, and relative survival rates were calculated for the two groups. Significant differences in the characteristics of the IP cohort’s invasive disease included: younger median age at presentation; larger tumor size; higher stage, higher grade, more involved lymph-nodes, and more hormone receptor negative disease (all P < 0.01). The age-adjusted incidence and breast cancer mortality were lower in IP women. The relative survival at 5 years was statistically significant at 84% for IP versus 89% for NHW women, but was not significantly different on multivariate analysis (P > 0.05). Within each stage (Tis, I, II), there were no disparities in the rate of breast conservation surgery (BCS) or in the percentage of patients receiving adjuvant radiation after BCS for the 2 cohorts. Post-mastectomy radiation was delivered significantly more often in stage I/II IP patients undergoing mastectomy. In conclusion, this analysis suggests that while there appear to be significant differences in the features of breast cancers of US-residing IP women, no disparities were noted in the rates of breast conserving surgery or adjuvant radiation, as seen in some other ethnicities. The more aggressive clinical-pathologic features stage-for-stage in IP women may partially explain the more frequent use of post-mastectomy RT in this patient population. These findings warrant further investigation.
Keywords: Breast cancer, Ethnicity Indian, Disparities, SEER Radiation, Pakistan, Breast conservation, Asian
Introduction
The U.S. Census Bureau estimates that the number of residents in the US identified as “Asian” is approximately 15 million, representing 5% of the nation’s population, and making it the third largest minority group in the US [1]. Historically, Asians were grouped by the US census classification as “Asian/Pacific Islander”, which corresponded to more than 50 ethnic/cultural subgroups. While this classification later divided the “Pacific Islanders” from “Asians”, the term remained limited in that it still represented a large aggregate of people from a wide array of countries. Recognizing this constraint, several large national databases including the U.S. Census Bureau and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program, have evolved to sub-classify ethnic groups by the country of origin, sub-dividing “Asians” into Chinese, Japanese, Filipino, Korean, Vietnamese and Indian/Pakistani, among others. Several studies have shown that when the Asians are divided into these constituents, significant differences in incidence and mortality of various cancers are unveiled [2, 3].
Descendants from India and Pakistan are often cohesively referred to as “South Asian”, defined as immigrants from the Indian sub-continent who are geographically and culturally distinct from the rest of the Asian population [4-6]. Between the years 1990 and 2000, the numbers of Indians and Pakistanis in the US increased by over 100%, making this population one of the fastest growing immigrant sub-groups in the nation [7]. Interestingly, the Indian/Pakistani (IP) ethnic sub-group is reported to be amongst the most highly educated, highest earning and most insured populations in the US [7, 8]. Despite these facts, adherence to breast cancer screening recommendations has been reported to be lower in U.S.-residing IP women, particularly in recent immigrants [9-11].
For the most part, data regarding breast cancers in U.S.-residing IP immigrants are limited and fragmented, with the majority of the literature focusing on IP breast cancer patients originating from abroad [12-20]; such studies cannot be extrapolated to IP immigrants residing in the US because it has been shown that once immigrants leave their native country of origin, subsequent generations espouse breast cancer risk profiles similar to their adopted country [21-23]. Furthermore, these statistics from foreign countries are difficult to interpret given the differences in environmental, dietary, reproductive and lifestyle factors that often occur after migration that can potentially alter patterns of development and cancer traits.
To date, no studies have specifically examined all available clinical-pathologic, treatment and outcome breast cancer parameters in the SEER database for the IP cohort. Notably, a recent publication [24] looked at clinical-pathologic features of a small subset of IP patients in the SEER database, but did not analyze any treatment variables to determine if any disparities exist (such as rates of breast conserving surgery [BCS] or the receipt of adjuvant radiation after BCS or mastectomy).
This study was conducted to establish if there are any significant differences in presentation, clinical-pathologic features, mortality/survival for US-residing IP versus NHW breast cancer patients. Furthermore, available treatment-related factors were analyzed to determine if any disparities exist in the delivery of treatment for US-residing IP women. To the best of our knowledge, this is the most comprehensive study of breast cancer presentation, treatment and outcomes of US-residing IP residents to date.
Materials and methods
Our population-based sample was obtained from the Surveillance, Epidemiology and End Results (SEER) database [25], which comprises data from 18 population-based regional and state cancer registries nationwide. We identified all women diagnosed with a first primary breast cancer between the years of 1988 (the year in which SEER began to collect detailed data on race) and 2006. All SEER registries (excluding the Alaska Native, Arizona Indian and Rural Georgia registries) were included in this analysis, although not all registries contributed cases throughout the entire study period. Patients identified as being of Hispanic ethnicity (in combination with IP or white race) were excluded from analysis, as were patients with Paget’s disease of the breast (due to staging/coding issues). “Asian Indian” and “Pakistani” racial groups were combined as one sub-category “Asian Indian or Pakistani” (IP) as per SEER coding rules [26]. Breast cancer patients identified as NHW were used as the reference cohort. The clinical-pathologic variables evaluated included: age at diagnosis, American Joint Committee on Cancer (AJCC) staging, size, grade, nodal involvement, number of involved lymph nodes and estrogen/progesterone receptor status. Rates of breast conserving surgery (BCS) versus mastectomy, and percentage of patients receiving adjuvant radiation after BCS or mastectomy, were also analyzed for the 2 cohorts. When comparing differences between different racial groups, the χ2 test and multivariate logistic regression analysis were utilized. All analysis was performed using SPSS [27] and SYSTAT [28] analytic software packages. Age-adjusted breast cancer incidence and mortality rates for women with breast cancer in the period 1998-2002 were calculated using SEER*Stat software [29], utilizing 2000-centered population data made available by the SEER program.1 The registries/states included in the SEER dataset are described in detail elsewhere [2]. Five year relative survival for the 2 cohorts was calculated for those breast cancers that were diagnosed from 1988 to 2002 and followed up through December 2006, adjusting for stage, year of diagnosis, age, and ER/PR status. Relative survival estimates were computed by SEER*Stat [26, 29] using well-established actuarial methodology [31].
Results
558,225 cancers were eligible for detailed analysis in this study (2,393 IP and 555,832 NHW women). The mean ages of the cohorts were 53.7 years IP versus 62.3 years NHW (P = 0.019). IP women presented at a younger age for both in situ (age < 40: 7.2% IP vs. 3.4% NHW, P < 0.001) and invasive cancers (age < 40: 14.8% IP vs. 4.5% NHW, P < 0.001).
Age-adjusted breast cancer incidence and mortality rates for IP and NHW are shown in Table 1. Age-adjusted breast cancer incidence rates for the IP cohort were lower than those of the NHW for both invasive and in situ cancers for the “all ages” group and within each individual age group (even when the “maximum” rate for the IP women was considered; see Footnote 1). Similarly, the breast cancer mortality rates for IP women were lower than those for NHW women, although the mortality rates for women aged ≤40 years were not significantly different between the two cohorts.
Table 1.
Breast cancer incidence and mortality rates (age adjusted) in IP and NHW women
| IPhigh Rate per 100,000 (95% CI) |
IPlow | NHW | |
|---|---|---|---|
| Incidence–in situ | |||
| All ages | 14.9 (12.8–17.3) | 16.9 (14.4–19.6) | 34.6 (34.1–35.1) |
| <40 years | 1.1 (0.6–1.9) | 1.2 (0.6–2.1) | 2.3 (2.2–2.5) |
| 40–64 years | 36.9 (31.3–43.3) | 41.6 (35.2–48.7) | 71.3 (70.1–72.5) |
| 65+ years | 24.4 (15.4–37.6) | 28.1 (17.7–43.2) | 91.7 (89.7–93.8) |
| Incidence–invasive | |||
| All ages | 72.3 (67.0–77.9) | 82.1 (76.1–88.5) | 149.5 (148.5–150.4) |
| <40 years | 10.0 (8.3–11.9) | 11.2 (9.2–13.4) | 13.6 (13.2–14.1) |
| 40–64 years | 141.5 (130.2–153.6) | 159.3 (146.5–172.9) | 256.0 (253.7–258.3) |
| 65 + years | 186.8 (157.1–220.8) | 216.4 (181.8–256.1) | 505.5 (500.8–510.1) |
| Mortality# | |||
| All ages | 9.9 (8.3–11.6) | 11.2 (9.4–13.2) | 27.8 (27.6–28.0) |
| <40 years | 1.1 (0.7–1.7) | 1.2 (0.7–1.9) | 1.6 (1.5–1.6) |
| 40–64 years | 18.9 (15.7–22.6) | 21.3 (17.7–25.4) | 36.4 (35.9–36.9) |
| 65+ years | 27.7 (18.8–39.5) | 32.2 (21.8–45.9) | 125.4 (124.1–126.8) |
Diagnoses/deaths in the period 1998–2002
IP Asian Indian/Pakistani (the “high” and “low” subscripts denote which population database was used to calculate the rate, see Footnote 1 in text), NHW non-Hispanic white
Mortality data are presented for Asian Indians only for the following states based on the availability of death and population data by detailed race: CA, HI, IL, NJ, NY, TX and WA. See reference 2 for further details
The clinical-pathologic characteristics for the two cohorts are shown in Table 2. While the distribution of invasive to non-invasive cancers did not differ significantly between the two ethnicities (P > 0.05), the IP cohort presented with significantly higher stages of invasive disease, larger invasive tumor size, higher invasive grade, and more lymph node involvement (all P > 0.001). The absolute number of lymph nodes involved (1–3, 4–9, ≥10) as a function of racial group is shown in Table 2. The IP cohort had significantly more hormone receptor negative tumors (P < 0.001) as shown in Fig. 1.
Table 2.
Clinical-pathologic features of breast cancer by race
| IP n (%) |
NHW n (%) |
P value | |
|---|---|---|---|
| Behavior | 0.229 | ||
| Invasive | 1,965 (82.1) | 461,588 (83.0) | |
| In situ | 428 (17.9) | 94,244 (17.0) | |
| Stage (invasive) | <0.001 | ||
| I | 697 (35.5) | 219,188 (47.5) | |
| II | 852 (43.4) | 159,916 (34.6) | |
| III | 213 (10.8) | 31,578 (6.8) | |
| IV | 98 (5.0) | 19,942 (4.3) | |
| Other/unknown | 105 (5.3) | 30,964 (6.7) | |
| Grade | |||
| In situ | 0.011 | ||
| I | 47 (11.0) | 8,071 (8.6) | |
| II | 119 (27.8) | 21,143 (22.4) | |
| III | 68 (15.9) | 15,198 (16.1) | |
| IV | 36 (8.4) | 9,418 (10.0) | |
| Unknown | 158 (36.9) | 40,414 (42.9) | |
| Invasive | <0.001 | ||
| I | 226 (11.5) | 77,505 (16.8) | |
| II | 647 (32.9) | 160,516 (34.8) | |
| III | 798 (40.6) | 127,942 (27.7) | |
| IV | 45 (2.3) | 9,223 (2.0) | |
| Unknown | 249 (12.7) | 86,402 (18.7) | |
| Tumor size (invasive) | <0.001 | ||
| No mass | 0 (0) | 902 (0.2) | |
| Microscopic focus/foci | 34 (1.7) | 7,353 (1.6) | |
| <1.0 cm | 246 (12.5) | 79,515 (17.2) | |
| 1.0 to <2.0 cm | 534 (27.2) | 160,930 (34.9) | |
| ≥2.0 cm | 978 (49.8) | 170,723 (37.0) | |
| Unknown/other | 173 (8.8) | 42,165 (9.1) | |
| Nodal status (invasive) | <0.001 | ||
| Positive | 708 (36.0) | 126,497 (27.4) | |
| 1–3 nodes positive | 409 (20.8) | 80,725 (17.5) | |
| 4–9 nodes positive | 180 (9.2) | 28,355 (6.1) | |
| 10 nodes positive | 119 (6.1) | 17,417 (3.8) | |
| Negative | 1,000 (50.9) | 250,646 (54.3) | |
| No nodes examined | 230 (11.7) | 77,848 (16.9) | |
| Not known | 27 (1.4) | 6,597 (1.4) |
Cancers diagnosed 1988–2006
IP Asian Indian/Pakistani, NHW non-Hispanic white
Fig. 1.
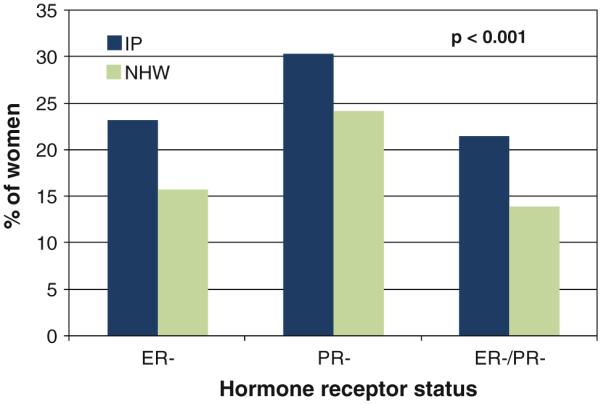
Differences in estrogen and progesterone receptor negative disease for invasive cancers of IP and NHW cohorts. Abbreviations: IP Indian-Pakistani, NHW non-Hispanic white, ER– estrogen receptor negative, PR– progesterone receptor negative, ER–/PR– both estrogen and progesterone receptor negative
An age-adjusted analysis of the parameters in Table 2 was conducted for each ethnicity (<40 years, 41–64 years, 65+ years). In summary, these analyses suggest that the differences of higher stage of disease, larger tumor size, and more nodal involvement for IP versus NHW remained significant across the three age groups. Higher tumor grade and more hormone receptor negative disease was no longer significantly different in the ≤40 age groups, but remained significantly different in the older 2 age groups.
In comparing treatment delivered, there appeared to be no disparity in the percentages of patients whose ER/PR test results were classified as “not done” within the database for IP and NHW (P > 0.05). Analysis of breast conservation rates for early-stage breast cancer using multivariate logistic regression analysis, taking into account age at diagnosis, stage of disease, and hormone receptor status, showed that there was no disparity in the percentage of patients who underwent a breast conservation approach (vs. mastectomy) as shown in Table 3 (P > 0.05). Additionally, there were no disparities in the proportion of patients who received adjuvant radiation therapy after breast conserving surgery for either ductal carcinoma in situ or invasive Stage I–II tumors (P > 0.05). However, post-mastectomy radiation was delivered more often in Stage I–II IP patients and remained highly significant when adjusted for stage of disease, age at diagnosis, and hormone receptor status (P < 0.001, Table 3).
Table 3.
Treatment: Breast conserving surgery (BCS) versus mastectomy and receipt of adjuvant radiation after BCS/mastectomy by race and stage at diagnosis
| Surgery type by stage | IP | NHW |
||
|---|---|---|---|---|
| n (%) | n (%) | P value* | ||
| Stage I | BCS | 441 (63.3) | 139,207 (63.5) | 0.445 |
| Mastectomy | 251 (36.0) | 77,953 (35.6) | ||
| Stage II | BCS | 352 (41.3) | 66,942 (41.9) | 0.158 |
| Mastectomy | 489 (57.4) | 90,543 (56.6) | ||
| Stage III | BCS | 34 (16.0) | 5,262 (16.7) | 0.510 |
| Mastectomy | 169 (79.3) | 23,857 (75.5) | ||
| Surgery type by stage |
IP | NHW |
||
|---|---|---|---|---|
| Radiation received | n (%) | n (%) | P value* | |
| BCS | Yes | 146 (50.9) | 29,209 (47.5) | 0.690 |
| In situ | No/UK | 141 (49.1) | 32,283 (52.5) | |
| BCS | Yes | 577 (72.8) | 147,695 (71.6) | 0.211 |
| Stage I & II | No/UK | 216 (27.2) | 58,454 (28.4) | |
| Mastectomy | Yes | 154 (20.8) | 18,588 (11.0) | <0.001 |
| Stage I & II | No/UK | 586 (79.2) | 149,908 (89.0) | |
| Mastectomy | Yes | 98 (58.0) | 11,507 (48.2) | 0.266 |
| Stage III | No/UK | 71 (42.0) | 12,350 (51.8) | |
Cancers diagnosed 1988–2006
Adjusted for race, age, stage at diagnosis and ER and PR status using multivariate logistic regression analysis
IP Asian Indian/Pakistani, NHW non-Hispanic white, BCS breast conserving surgery, No/UK did not receive or unknown whether received radiation
A significantly lower relative survival at 5 years for the entire IP cohort compared with NHW is shown in Fig. 2. But when analyzing the relative survival using ethnicity, age, stage, year of diagnosis and ER/PR status in the Cox proportion hazard model, the differences in survival outcomes for IP versus NHW were no longer significant (Fig. 2, analysis).
Fig. 2.
5 year relative survival. Graph shows relative survival for overall population of IP versus NHW. Table shows 5 year relative survival for the 2 cohorts by ethnicity, when adjusted by stage, age, year of diagnosis and ER/PR status (P > 0.05)
Discussion
Several publications originating from India and Pakistan indicate that a high proportion of their breast cancer patients present with aggressive features such as locally advanced disease, higher tumor grade, and more hormone receptor-negative tumors [17-19]; for Indians and Pakistanis living in the United States, breast cancer ethnicity-based studies assessing clinical and pathologic characteristics and (potential) disparities in treatment delivery have not been extensively conducted to date. While not specifically focusing on the US-residing IP immigrants, there are scattered data reported some characteristics of IP patients from population-based studies that have addressed different cancer related-questions. Examples include: variations of breast cancer incidence across the different Asian sub-groups [2, 32], incidences of all cancer-types for the IP cohort in comparison to other ethnicities [3, 33], or differences in breast cancer stage and survival across multiple races/ethnicities [26, 34].
In this study, we compared a large cohort of US-residing IP breast cancer patients to NHW using all available parameters from the SEER database to comprehensively assess breast cancer characteristics and possible disparities. Our findings include a lower age-adjusted breast cancer incidence for IP versus NHW women, which was also reported in another study that assessed the incidence of specific cancer types in a variety of Asian sub-groups using SEER data [2]. We further stratified the incidence by age and invasive/non-invasive disease, and observed a lower incidence of breast cancers in IP women for every age group for both in situ and invasive tumors, though the proportion of in situ to invasive tumors for both cohorts was similar.
Our data demonstrate some significant differences in the clinical-pathologic features of the IP and NHW breast cancers; these included younger age at presentation, larger primary tumor size, more advanced stage of disease at presentation, more lymph node positivity, higher absolute number of involved nodes and higher grade. Because the general IP immigrant population in the United States is of a younger median age compared with that of the American NHW population, we conducted an age-adjusted analysis of the breast cancer characteristics that differed between the two cohorts to determine these differences were secondary to the overall younger IP population. Nevertheless, the majority of observed differences remained statistically significant when age-adjusted, thus cannot be explained by the younger mean age of the IP immigrant population. These findings are supported, in part, by several publications that have reported certain breast cancer characteristics by ethnicity and included some IP patients [3, 24, 35].
The current study also demonstrated that the IP cohort present with more hormone receptor negative disease, a finding which has also been suggested in other studies that have included IP women [24, 34]. One possible explanation for this observation may be the younger age at presentation, which is known to be associated with more ER negative tumors [36, 37]. Another theory that has been postulated is the reduced exogenous estrogen exposures in IP patients (i.e., birth control pills [BCP] and hormone replacement therapy [HRT]) [38]. As is well documented, exogenous estrogens are associated with increased ER positive breast cancers [38, 39], and thus in a patient population who have less exogenous estrogen exposure, there could theoretically be a shift in the proportion of estrogen positive to negative tumors. Data on ethnic variability of birth control methods suggest that rate of birth control pill usage in Indians is very low compared to their white counterparts [38, 40-42].
Unique to our study was the comparison of evaluable treatments delivered in the IP compared to NHW breast cancer populations. It was reassuring that there did not appear to be any apparent treatment-related disparities for the IP versus NHW cohorts. In fact, there were no significant differences in the frequency of reporting of the hormone receptor status (i.e., “not done” category) or in the percentage of patients who were coded as “unknown nodal status” for IP versus NHW. In fact, the percentage of patients in the “no nodes examined” category for axillary lymph node involvement was lower in IP compared with NHW cohorts (given similar invasive to in situ proportions). Furthermore, the percentages of early-stage breast cancer patients who underwent a breast conserving approach (versus mastectomy) in the current study did not differ between the two populations and the proportions of patients who received adjuvant breast radiation as a component of their breast conservation therapy did not differ. This apparent lack of disparities in treatment delivery for the IP population may be due, in part, to the higher socioeconomic status of the US-residing IP population, with higher education levels and higher levels of health insurance [43, 44]. While no such published data exists specifically assessing treatment-related factors for US-residing IP patients in comparison to NHW to our knowledge, these data are encouraging in light of other such ethnicity-based studies that have unveiled significant disparities. For example, similar studies conducted in African Americans compared to whites have exposed that greater proportions of AA patients are reported with “unknown receptor status”, have higher mastectomy rates/lower breast conservation rates, and less receipt of adjuvant radiation after conservative surgery [35, 45-48].
Interestingly, IP have been reported to perform less breast self-examinations than their other Asian counterparts and are less likely to have ever had a mammogram compared with whites and other Asian subgroups [9-11, 49, 50]. While this may potentially explain the more advanced stage of disease at presentation for IP patients, there still appear to be some biologic differences in terms of higher grade, more HR negative disease, and younger age at presentation that cannot be explained by screening or access to care alone. Certainly, these findings warrant further investigation.
Lastly, the IP cohort appeared to receive more post-mastectomy radiotherapy (PMRT) for early-stage disease compared to NHW when logistic regression analysis was performed accounting for stage, age at diagnosis and hormone receptor status. While this may be, in part, due to cultural differences between the two cohorts, the higher use of PMRT may also be potentially explained by the more aggressive tumor features, specifically higher node positivity and larger tumor size, that have been demonstrated in the IP cohort.
Our analysis revealed an overall lower 5-year relative survival for the IP cohort in comparison to the NHW population, which is consistent with outcomes reported by others [35]. However, it is important to recognize that this difference may be confounded by other factors including age, higher grade, hormone receptor status and more advanced stages of disease. When adjusting the relative survival for these specific factors, ethnicity did not detrimentally influence the overall survival, suggesting that the individual tumor factors may be more significant in determining relative survival than ethnicity.
Limitations of using large, public databases such as SEER to report ethnic differences in tumor characteristics must be acknowledged. The evaluation of the accuracy of tumor registry data on race/ethnicity has been shown to have varying levels of misclassification [51-55]. There is a lack of patient-level information on etiologic risk factors and cancer screening. The SEER database, specifically, does not contain some important pathologic and treatment characteristics such as margin status, systemic therapy details, HER2-neu status, and detailed outcome data (such as local, regional and distant relapse) that are relevant when studying disparities in breast cancer populations. Additionally, the SEER program covers only 26% of the US population [56]. Hence data for IP women included in the SEER database may not be representative of the overall IP breast cancer population in the US.
Despite these limitations, these data allow for comparison of large numbers of IP patients with relatively long follow-up. To our knowledge, this is the largest and most comprehensive study to cohesively compare the incidence, mortality, survival, clinical-pathologic and treatment parameters of breast cancer in US-residing IP compared with NHW women, and to assess whether disparities exist in this distinct immigrant population.
Conclusion
In summary, US-residing IP breast cancer patients present at a younger age, with higher stages of disease, more nodal involvement, high grade tumors and hormone receptor negative tumors compared to NHW women, though these clinical-pathologic differences do not appear to translate into a detrimental difference in overall survival as a function of ethnicity alone. Reassuringly, there do not appear to be any evident disparities in the evaluable treatments delivered. Notably, the rates of breast conservation versus mastectomy do not differ for the two cohorts, nor does the rate of receipt of adjuvant radiation after breast conserving surgery. The more aggressive clinical-pathologic features in IP women may partially explain the more frequent use of post-mastectomy RT for early-stage disease in this patient population. In future studies, additional clinically relevant parameters of IP breast cancers (i.e., local–regional relapse after BCS, utilization/delivery of systemic therapy, etc.), as well as cultural, cancer screening, dietary, hormonal/reproductive and other lifestyle factors should be analyzed with efforts to discern the causality of the above-noted differences. Furthermore, differential expression of biologic markers and genetics should also be examined to potentially elucidate some of these ethnic variations found in the IP immigrant population.
Acknowledgment
The Connecticut Tumor Registry is supported by a contract (No. N01-PC-35133) between the National Cancer Institute and the Connecticut Department of Public Health.
Abbreviations
- IP
Indian-Pakistani
- NHW
Non-Hispanic White
- RS
Relative survival
- CI
Confidence interval
Footnotes
Both the ‘low population’ and ‘high population’ databases were used. In the former, the denominator included persons who identified themselves as a single race only; in the latter, persons who identified themselves as being of a particular race alone or in combination with other races were included. Thus, the calculated incidence rates represent minimum and maximum rates for the IP population [2, 30].
Conflict of interest The authors of this manuscript have no actual or potential conflicts of interest to disclose.
Contributor Information
Meena S. Moran, Department of Therapeutic Radiology, Yale University School of Medicine, 333 Cedar Street, P.O. Box 208040, New Haven, CT 06520-8040, USA; Yale New Haven Hospital, New Haven, CT, USA; William W. Backus Hospital, Norwich, CT, USA
Lou Gonsalves, Connecticut Tumor Registry, Hartford, CT, USA.
Donna M. Goss, William W. Backus Hospital, Norwich, CT, USA
Shuangge Ma, School of Public Health, Yale University, New Haven, CT, USA.
References
- 1.US Census Bureau . 2007 ACS Demographic and Housing Estimates 2007. US Census Bureau; Washington, DC: [Accessed 2 May 2010]. 2007. http://factfinder.census.gov/ [Google Scholar]
- 2.Miller BA, Chu KC, Hankey BF, Ries LAG. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goggins WB, Wong G. Cancer among Asian Indians/ Pakistanis living in the United States: low incidence and generally above average survival. Cancer Causes Control. 2009;20:635–643. doi: 10.1007/s10552-008-9275-x. [DOI] [PubMed] [Google Scholar]
- 4.Meyer MW. South Asia: a short history of the subcontinent. Adams Littlefield; Totowa: 1976. [Google Scholar]
- 5.Sukhwal BL. South Asia: a region of conflicts and contradictions. In: Norwine J, González A, editors. The third world: states of mind and being. Unwin Hyman; London: 1988. [Google Scholar]
- 6.Shankar LD, Srikanth R. A part, yet apart: South Asians in Asian America. Temple University Press; Philadelphia: 1998. [Google Scholar]
- 7.Le CN. Asian-Nation: the landscape of Asian America—population statistics & demographics. [Accessed 9 Oct 2009]. 2009. http://www.asiannation.org/population.shtml. [Google Scholar]
- 8.Reeves TJ, Bennett CE. Census 2000 special reports. US Census Bureau; Washington, DC: 2004. We the people: Asians in the United States. [Google Scholar]
- 9.Glenn BA, Chawla N, Surani Z, Bastani R. Rates and sociodemographic correlates of cancer screening among South Asians. J Community Health. 2009;34:113–121. doi: 10.1007/s10900-008-9129-1. [DOI] [PubMed] [Google Scholar]
- 10.Gomez SL, Tan S, Keegan TH, Clarke CA. Disparities in mammographic screening for Asian women in California: a cross-sectional analysis to identify meaningful groups for targeted intervention. BMC Cancer. 2007;7:201. doi: 10.1186/1471-2407-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boxwala FI, Bridgemohan A, Griffith DM, Soliman AS. Factors associated with breast cancer screening in Asian Indian women in metro-detroit. J Immigr Minor Health. 2009 Jul 23; doi: 10.1007/s10903-009-9277-0. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack RH, Davies EA, Moller H. Breast cancer incidence, stage, treatment and survival in ethnic groups in South East England. Br J Cancer. 2009;100:545–550. doi: 10.1038/sj.bjc.6604852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormack VA, Mangtani P, Bhakta D, McMichael AJ, dos Santos Silva I. Heterogeneity of breast cancer risk within the South Asian female population in England: a populationbased case-control study of first-generation migrants. Br J Cancer. 2004;90:160–166. doi: 10.1038/sj.bjc.6601440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.dos Santos Silva I, Mangtani P, De Stavola BL, Bell J, Quinn M, Mayer D. Survival from breast cancer among South Asian and non-South Asian women resident in South East England. Br J Cancer. 2003;89:508–512. doi: 10.1038/sj.bjc.6601097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velikova G, Booth L, Johnston C, Forman D, Selby P. Breast cancer outcomes in South Asian population of West Yorkshire. Br J Cancer. 2004;90:1926–1932. doi: 10.1038/sj.bjc.6601795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinshaw KA, Sarin R, Budrukkar AN, Shrivastava SK, Deshpande DD, Chinoy RF, Badwe R, Hawaldar R. Safety and feasibility of breast conserving therapy in Indian women: two decades of experience at Tata Memorial Hospital. J Surg Oncol. 2006;94:105–113. doi: 10.1002/jso.20497. [DOI] [PubMed] [Google Scholar]
- 17.Shet T, Agrawal A, Nadkarni M, Palkar M, Havaldar R, Parmar V, Badwe R, Chinoy RF. Hormone receptors over the last 8 years in a cancer referral center in India: what was and what is? Ind J Pathol Microbiol. 2009;52:171–174. doi: 10.4103/0377-4929.48909. [DOI] [PubMed] [Google Scholar]
- 18.Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF. Hormone receptor status of breast cancer in India: a study of 798 tumors. Breast. 2000;9:267–270. doi: 10.1054/brst.2000.0134. [DOI] [PubMed] [Google Scholar]
- 19.Dey S, Boffetta P, Mathews A, Brennan P, Soliman A, Mathew A. Risk factors according to estrogen receptor status of breast cancer patients in Trivandrum. South India Int J Cancer. 2009;125:1663–1670. doi: 10.1002/ijc.24460. [DOI] [PubMed] [Google Scholar]
- 20.Bhurgri Y, Kayani N, Faridi N, Pervez S, Usman A, Bhurgri H, Malik J, Bashir I, Bhurgri A, Hasan SH, Zaidi SH. Pathoepidemiology of breast cancer in Karachi ‘1995-1997’. Asian Pac J Cancer Prev. 2007;8:215–220. [PubMed] [Google Scholar]
- 21.Dunn JE., Jr Breast cancer among American Japanese in the San Francisco Bay area. Natl Cancer Inst Monogr. 1977;47:157–160. [PubMed] [Google Scholar]
- 22.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 23.Hensley Alford S, Schwartz K, Soliman A, Johnson C, Gruber S, Merajver S. Breast cancer characteristics at diagnosis and survival among Arab-American women compared to European- and African-American women. Breast Cancer Res Treat. 2009;114:339–346. doi: 10.1007/s10549-008-9999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakarala M, Rozek L, Cote M, Liyanage S, Brenner DE. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.—a SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use? Hurricane Katrina Impacted Louisiana Cases, Nov 2008 Sub (1973-2006 varying)—Linked To County Attributes—Total U.S., 1969-2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2008 submission
- 26.Johnson CH, Adamo M, editors. SEER Program Coding, Staging Manual 2007. National Cancer Institute; Bethesda: 2008. NIH Publication number 07-5581. [Google Scholar]
- 27.SPSS Statistics 17.0 . SPSS Inc.; Chicago: 2008. Release 17.0.0. [Google Scholar]
- 28.SYSTAT 13 for Windows® . SYSTAT Software, Inc.; Chicago: 2009. [Google Scholar]
- 29.Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 6.5.2. Incidence databases: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—Racial Ethnic Mono, SEER 18 (excl AZ,AK,RG) Limited-Use, Nov 2005 for Detailed API Races Only (1998-2002) < Low 2000 Pops by 5 >, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006, based on the November 2005 submission; Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—Racial Ethnic Mono, SEER 18 (excl AZ,AK,RG) Limited-Use, Nov 2005 for Detailed API Races Only (1998-2002) < High 2000 Pops by 5 >, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006, based on the November 2005 submission; Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 13 Regs Research Data, Nov 2008 Sub (1992-2006) < Katrina/Rita Population Adjustment > Linked To County Attributes—Total U.S., 1969-2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2008 submission
- 30.Detailed Asian/Pacific Islander Databases (2000-Centered) [Accessed 2 May 2010]; http://seer.cancer.gov/seerstat/databases/api.races.2000pops/2005submission.html.
- 31.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 32.Keegan TH, Gomez SL, Clarke CA, Chan JK, Glaser SL. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California. Int J Cancer. 2007;120:1324–1329. doi: 10.1002/ijc.22432. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal N, Deka D, Takkar D. Contraceptive status and sexual behavior in women over age 35 in India. Adv Contracept. 1999;15:235–244. doi: 10.1023/a:1006705718070. [DOI] [PubMed] [Google Scholar]
- 34.International Institute for Population Sciences (IIPS) Macro International . National Family Health Survey (NFHS-3), 2005-06: India. I. IIPS; Mumbai: 2007. [Google Scholar]
- 35.Saxena S, Copas AJ, Mercer C, Johnson AM, Fenton K, Erens B, Nanchahal K, Macdowall W, Wellings K. Ethnic variations in sexual activity and contraceptive use: national cross-sectional survey. Contraception. 2006;74:224–233. doi: 10.1016/j.contraception.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 37.Anderson WF, Jatoi I, Sherman ME. Qualitative age interactions in breast cancer studies: mind the gap. J Clin Oncol. 2009;27:5308–5311. doi: 10.1200/JCO.2009.22.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tewari M, Pradhan S, Singh U, Shukla HS. Estrogen and progesterone receptor status in breast cancer: effect of oral contraceptive pills and hormone replacement therapy. Breast Oct. 2007;16(5):540–545. doi: 10.1016/j.breast.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Lower EE, Blau R, Gazder P, Stahl DL. The effect of estrogen usage on the subsequent hormone receptor status of primary breast cancer. Breast Cancer Res Treat. 1999;58(3):205–210. doi: 10.1023/a:1006315607241. [DOI] [PubMed] [Google Scholar]
- 40.Hossain A, Sehbai A, Abraham R, Abraham J. Cancer health disparities among Indian and Pakistani immigrants in the United States. Cancer. 2008;113:1423–1430. doi: 10.1002/cncr.23686. [DOI] [PubMed] [Google Scholar]
- 41.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 42.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 43.Hughes D. Quality of health care for Asian Americans: findings from the Commonwealth Fund 2001 Health Care Quality Survey. The Commonwealth Fund; New York: 2002. [Google Scholar]
- 44.Improving Health Coverage and Access for Asians and Pacific Islanders [Accessed 2 Apr 2010];Minority Health Initiatives, Families USA. 2006 January; www.familiesusa.org/assets.
- 45.Du Xianglin L, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women, 1992 to 2002. Ethn Dis. 2007;17:122–128. [PMC free article] [PubMed] [Google Scholar]
- 46.Hausauer A, Keegan T, Chang E, Clarke C. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007;9:R90. doi: 10.1186/bcr1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 48.Prehn AW, Topol B, Stewart S, Glaser SL, O’Connor L, West DW. Differences in treatment patterns for localized breast carcinoma among Asian/Pacific islander women. Cancer. 2002;95:2268–2275. doi: 10.1002/cncr.10965. [DOI] [PubMed] [Google Scholar]
- 49.Wu TY, Bancroft J, Guthrie B. An integrative review on breast cancer screening practice and correlates among Chinese, Korean, Filipino, and Asian Indian American Women. Health Care Women Int. 2005;26:225–246. doi: 10.1080/07399330590917780. [DOI] [PubMed] [Google Scholar]
- 50.Wu TY, West B, Chen YW, Hergert C. Health beliefs and practices related to breast cancer screening in Filipino, Chinese and Asian-Indian women. Cancer Detect Prev. 2006;30:58–66. doi: 10.1016/j.cdp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 52.Frost F, Taylor V, Fries E. Racial misclassification of Native Americans in a surveillance, epidemiology, and end results cancer registry. J Natl Cancer Inst. 1992;84:957–962. doi: 10.1093/jnci/84.12.957. [DOI] [PubMed] [Google Scholar]
- 53.Stewart SL, Swallen KC, Glaser SL, Horn-Ross PL, West DW. Adjustment of cancer incidence rates for ethnic mis-classification. Biometrics. 1998;54:774–781. [PubMed] [Google Scholar]
- 54.Swallen KC, Glaser SL, Stewart SL, West DW, Jenkins CN, McPhee SJ. Accuracy of racial classification of Vietnamese patients in a population-based cancer registry. Ethn Dis. 1998;8:218–227. [PubMed] [Google Scholar]
- 55.Stewart SL, Swallen KC, Glaser SL, Horn-Ross PL, West DW. Comparison ofmethodsfor classifying Hispanic ethnicity in a population-based cancer registry. Am J Epidemiol. 1999;149:1063–1071. doi: 10.1093/oxfordjournals.aje.a009752. [DOI] [PubMed] [Google Scholar]
- 56. [Accessed 2 May 2010];Overview of the SEER Program. http://seer.cancer.gov/about/



