Abstract
Immunoglobulin class switch recombination (CSR) occurs in activated mature B cells, and causes an exchange of the IgM isotype for IgG, IgE or IgA isotypes, which increases the effectiveness of the humoral immune response. DNA double-stranded breaks (DSBs) in recombining switch (S) regions, where CSR occurs, are required for recombination. Activation-induced cytidine deaminase (AID) initiates DSB formation by deamination of cytosines in S regions. This reaction requires reactive oxygen species (ROS) intermediates such as hydroxyl radicals. In this study we show that the ROS scavenger N-acetyl-cysteine (NAC) inhibits CSR. We also demonstrate that interferon-γ (IFNγ) treatment, which is used to induce IgG2a switching, increases intracellular ROS levels, and activates p53 in switching B cells, and show that p53 inhibits IgG2a class switching through its antioxidant-regulating function. Finally, we show that p53 inhibits DNA breaks and mutations in S regions in B cells undergoing CSR, suggesting that p53 inhibits the activity of AID.
Keywords: activation-induced cytidine deaminase, S region DNA double-strand breaks, interferon-γ, reactive oxygen species, immune response to Polyoma virus, S region mutations
INTRODUCTION
In mature B cells the immunoglobulin heavy chain locus undergoes two major genetic alterations. Somatic hypermutation (SHM) and class switch recombination (CSR) shape the antibody repertoire by increasing the affinity for antigen, and changing the isotype of the expressed immunoglobulin (Ig), respectively. CSR diversifies the effector function of the humoral immune response by changing the expressed Ig isotype from IgM to IgG, IgE, or IgA. Both SHM and CSR are dependent on the activation-induced cytidine deaminase (AID) enzyme (1, 2). During CSR, AID initiates the formation of DNA double-stranded breaks (DSBs) in the switch (S) regions that are located upstream of each of the constant regions (CH), which encode the different Ig-isotypes (with the exception of Cδ, encoding IgD) (3–6). AID deaminates cytidines in the S regions, thereby generating dU bases, which are removed by the base excision and mismatch repair systems, resulting in either mutations or DSBs (5, 7–11). AID-instigated DSBs in Sμ and in the downstream S regions are recombined by non-homologous or microhomology-mediated end joining, generating the S-S junction and resulting in the excision of the intervening DNA sequences (12).
We have previously shown that AID-dependent S region blunt DSBs occur predominantly in the G1-phase of the cell-cycle (13). How this cell-cycle restriction is enforced is, however, unknown. Clearly, DNA damage response pathways are activated by CSR-associated DSBs, since efficient class switching requires DNA damage sensing proteins such as the ataxia telangiectasia mutated kinase (ATM), the variant histone H2AX, p53-binding protein-1 (53BP1) and the Mre11-Rad50-Nbs1 (MRN) complex (12, 14–22). The p53 protein plays a central role downstream in the DNA damage response pathways, essentially acting as a gatekeeper. Unresolved DNA breaks activate p53, which, depending on the context, regulates the cell-cycle, DNA repair and cell death (23).
Additionally, recent reports have shown that p53 has a role in the regulation of the levels of intracellular reactive oxygen species (ROS) (24, 25). Mammalian cells are continuously subjected to ROS, which can result in proliferation, cell death or growth arrest, depending on the cellular context and the level of ROS. In cells that have sustained extensive damage, p53 can stimulate the expression of genes that increase intracellular ROS as part of a proapoptotic response (26). At low stress levels, p53 can also activate antioxidant genes that lower ROS levels as part of a protective response (24). The role of intracellular ROS levels in B cells undergoing class switching is unknown. However, both enzymatic and spontaneous cytidine deamination are achieved through oxidation, which involves hydroxyl radicals, showing that ROS are required intermediates in this process (27, 28). Here, we present a novel role for p53 in limiting class switching. We find that although p53 does not affect AID levels, cell-cycle progression, proliferation, or apoptosis in B cells induced to switch, it has a redox-dependent inhibitory function in CSR, resulting in inhibition of S region breaks, mutations, and isotype switching.
MATERIALS AND METHODS
Mice
All mouse strains were backcrossed to C75BL/6. p53−/− mice were obtained from Dr Stephen Jones, and were previously described (29). AID-deficient mice were obtained from T. Honjo (Kyoto University, Kyoto, Japan) (1). p53 S18A knock-in mice have been described (30). Prdx6-transgenic mice have been described (31). Mice were housed in the Institutional Animal Care and Use Committee-approved specific pathogen-free facility at the University of Massachusetts Medical School. The mice were bred and used according to the guidelines from University of Massachusetts Animal Care and Use committee. For each experiment, splenic B cells from WT mice and their mutant littermates were analyzed.
Splenic B-cell isolation and culture
Single-cell suspensions were prepared from spleens of 6 to 12 week old mice by mechanical dispersion, and RBCs were lysed in Gey’s solution. B cells were enriched by guinea pig complement lysis of T cells using a cocktail of anti-T-cell antibodies, as described previously (32). For CSR analysis, cells stained with carboxyfluorescein diacetate succinimidyl diester (CFSE) (Molecular Probes, Invitrogen, Carlsbad, CA), were cultured at 1×105/mL in 24-well plates and activated for switching to the different Ig-isotypes. Cultures contained LPS (50 µg/mL; Sigma-Aldrich, St. Louis, MO) and human BLyS (100 ng/mL; Human Genome Sciences, Rockville, MD). For IgG1 switching, recombinant mouse IL-4 (20 ng/mL, eBioscience, San Diego, CA) was added; for IgG2a switching, IFNγ 20 ng/mL; PeproTech Inc., Rocky Hill, NJ) was added; for IgG2b switching, TGF-β (2 ng/mL; R&D Systems, Minneapolis, MN) was added; for IgG3 switching, anti–δ-dextran (0.3 ng/mL; FinaBio, Rockville, MD) was added; and for IgA switching, TGF-β 2 ng/mL; R&D Systems, Minneapolis, MN), IL-4 (800 U/mL), IL-5 (1.5 ng/mL; BD Biosciences, San Jose, CA), and anti–δ-dextran (0.3ng/mL) (FinaBio) were added. For LM-PCR analysis, cells were cultured at 2×105/mL in 6-well plates and activated for two days as described previously (5). In the indicated experiments, 2.5 µM Nutlin-3 (Sigma Aldrich) was added at the start of the culture, and as control the solvent dimethyl sulfoxide (DMSO) was added. In the indicated experiments N-acetyl-cysteine (NAC) (Sigma Aldrich) was dissolved in PBS + 25 mM HEPES (pH = 7.0), and added at the start of the culture and again at 24 h.
Infection of mice with polyoma virus
Mice were infected intraperitoneally with 2×106 pfu/mouse polyoma virus strain A2. Mice were sacrificed 12–20 days after infection. Isotype-switched splenic germinal center B cells were analyzed by flow cytometry, using allophycocyanin (APC) conjugated mouse-anti-mouse B220 (RA3-6B2; Caltag Laboratories, Invitrogen, Carlsbad, CA) and fluorescein isothiocyanate (FITC) conjugated mouse-anti-mouse GL7 (BD Pharmingen, San Jose, CA), in combination with either phycoerythrin (PE) conjugated goat F(ab’)2 anti-mouse IgG1, IgG2a, IgG2b or IgG3 (SouthernBiotech, Birmingham, AL).
Flow cytometry
For FACS analysis, cells were washed twice with PBS, 1% FCS, 0.2% NaN3, and incubated for 30 minutes on ice with PE-goat F(ab')2 anti–mouse IgG1, IgG2b, IgG2a, or IgG3, or PE-goat anti–mouse IgA (SouthernBiotech, Birmingham, AL). For CFSE labeling, cells were washed in Hank’s Balanced Salt Solution (Invitrogen) and resuspended at 40 × 106/mL. An equal volume of 2.0 µM CFSE was added and cells were incubated at 37°C for 15 minutes, quenched in 100% FCS, and then washed twice with medium containing 10% FCS. For splenic B-cell subset analysis, cells were stained with anti-B220 APC (RA3-6B2; Caltag Laboratories), anti-CD23 PE (2G8; SouthernBiotech), anti-CD21 FITC (7G6; BD Pharmingen). CFSE fluorescence and antibody staining were acquired on a LSR flow cytometer (BD Biosciences) and analyzed using the FlowJo software package (Tree Star Inc., Ashland, OR).
ROS detection by flow cytometry
Splenic B cells activated for 48 h were stained with 1 µM 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2-DCFDA) (Molecular Probes, Invitrogen) for 15 minutes at 37°C, followed by a 10 minutes recovery period at 37°C. Extracellular CM-H2-DCFDA was quenched with 0.2% trypan-blue. Fluorescence resulting from intracellular oxidation of CM-H2-DCFDA was assessed by flow cytometry. Dead cells were excluded by 7-amino-actinomycin D (7-AAD) (BD Pharmingen) staining.
Western blotting
Cytoplasmic and nuclear extracts were prepared by resuspending cells in hypotonic buffer (10 mM HEPES pH8, 1 mM EDTA, 10 mM KCl, 0.1 mM EGTA, 1 mM DTT, 2 µM pepstatin) and Complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). After 15 minutes incubation on ice, cells were lysed by addition of Nonidet P-40 to a final concentration of 0.625%. Supernatants were taken as cytoplasmic extracts. Nuclei were washed once with hypotonic buffer and resuspended in hypertonic buffer (20 mM HEPES pH8, 1 mM EDTA, 400 mM NaCl, 1 mM EGTA, 1 mM DTT, 2 µM pepstatin and complete protease inhibitor cocktail). For whole-cell extracts, cells were lysed in RIPA buffer (150 mM NaCl, 0.5% deoxycholate, 0.1% SDS, 1.0% NP-40, 50 mM Tris-HCl pH7.4, complete protease and phosphatase inhibitor cocktail) and subjected to 3 freeze-thaw cycles. Protein content of cytoplasmic, nuclear and whole-cell extracts were determined using Bradford assay (Bio-Rad, Hercules, CA). Equal amounts of proteins were electrophoresed on 8% SDS-polyacrylamide gels or 4–20% gradient SDS-polyacrylamide gels (Pierce, Rockford, IL), and blotted onto Immobilon-P polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). Antibodies used were: rabbit-anti-APEX1 (33), rabbit-anti-APE2 (33) rabbit anti-Ung (33), rabbit anti-phospho-18 p53 (Anaspec Inc, Fremont CA), rabbit-anti-LC3B (Abcam, Cambridge, MA) rabbit-anti-GAPDH (Santa Cruz Biotechnology), and mouse-anti-TBP1 (TATA- box binding protein; Abcam), followed by goat-anti-rabbit horseradish peroxidase, or donkey-anti-mouse horseradish peroxidase (Santa Cruz Biotechnology) and enhanced chemiluminescent substrate (Pierce).
Reverse-transcriptase (RT)-PCR
RNA was isolated using TRI reagent (Applied Biosystems/Ambion, Austin, TX) as recommended by the manufacturer. One to 5 µg RNA was reverse transcribed in a 30 µL reaction with 0.8 µM p(dT)10 primer (Roche Applied Science) and 200 U Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) (Invitrogen) for 1 h at 42°C. Germline Sγ 1 and Sγ 2a transcript and aicda mRNA were assessed by radioactive ([α32P]-dCTP) semi-quantitative RT-PCR on threefold cDNA dilutions, normalized for input by hprt RT-PCR. p21 transcripts were quantified by LightCycler PCR using SYBR Green (Roche Applied Science). PCR primers used in this study: AID-sense (5’-ATATGGACAGCCTTCTGATGAAGC-3’), AID-antisense (5’-TGCTTTCAAAATCCCAACATACG-3’), 5’-HPRT (5’-GTTGGATACAGGCCAGACTTTGTTG-3’), 3’-HPRT (5’-TACTAGGCAGATGGCCACAGGACTA-3’), GLγ1-FW (5’-CAGCCTGGTGTCAACTAG-3’), GLγ1-REV (5’-CTGTACATATGCAAGGCT-3’), GLγ2a-FW (5’-GTCCACCTTGGTGCTGCTT-3’), GLγ2a-REV (5’-GCTGATGTACCTACCTGAGAGAG-3’). P21-FW (5’-GAACTTTGACTTCGTCACGG-3’), P21-REV (5’-AGTGATAGAAATCTGTCAGGCT-3’), Sestrin-1 FW (5’-CCAGGACGAGGAACTTGG-3’), Sestrin-1 REV (5’-CCAGGTAGGAACACTGATGC-3’). These primers do not amplify genomic DNA (not shown).
Genomic DNA preparation and linker ligation-mediated PCR (LM-PCR)
After culture for two days, viable cells were isolated by flotation on Ficoll/Hypaque gradients (ρ=1.09), or Lympholyte (Cedar Lane, Ontario, Canada), cells were imbedded in low melt agarose plugs, and DNA isolated as described (5). For linker ligation, 50 µL 1X ligase buffer was added to the plugs which were then heated to 62°C to melt the agarose. 20 µL DNA (about 200,000 cell equivalents) was added to 2 µL T4 DNA Ligase (2 Weiss units, MBI Fermentas, Hanover, MD), 10 µL ds annealed linker in 1X ligase buffer, 3 µL 10x Ligase buffer and 30 µL dH20 and incubated overnight at 18°C. Linker was prepared by annealing 5 nmoles each of LMPCR.1 (5’-GCGGTGACCCGGGAGATCTGAATTC-3’) and LMPCR.2 (5’-GAATTCAGATC-3’) in 300 µL 1X ligase buffer, which results in a ds oligo with a 14 nt ss overhang that can only ligate unidirectionally. Ligated DNA samples were heated at 70°C for 10 min, diluted 5x in dH20 and then assayed for gapdh DNA by PCR to adjust DNA input prior to LM-PCR. The primer 5’Sμ (5’-GCAGAAAATTTAGATAAAATGGATACCTCAGTGG-3’) or 5’Sγ2a (5’-ATGGTTCCTGCAGTACCAATACA-3’) (Integrated DNA Technologies, Coralville, IA) were used in conjunction with linker primer (LMPCR.1) to amplify DNA breaks in Sμ and Sγ2a, respectively. Three-fold dilutions of input DNA (0.5, 1.5 and 4.5 µL for Sμ LM-PCR, 1.5, 4.5 and 13.5 µL for Sγ2a LM-PCR) were amplified by HotStar Taq (Qiagen) using a touchdown PCR program (28 cycles after touchdown for Sμ LM-PCR and 35 cycles after touchdown for Sγ2a LM-PCR). PCR products were electrophoresed on 1.25% agarose gels and blotted onto nylon membranes (GeneScreen Plus, Perkin Elmer, Waltham, MA). Blots were hybridized with an Sμ-specific oligonucleotide probe (mu probe5’: 5’-AGGGACCCAGGCTAAGAAGGCAAT-3’) for 5’ Sμ LM-PCR, or an Sγ2a-specific probe (Sγ2a probe: 5’-CAGCTCTCAGTAGGGAACGG-3’) for Sγ2a LM-PCR, end-labeled with [γ32P]-ATP at 42°C overnight and washed at 55°C with 2X SSC/0.1% SDS. Semi-quantitative assessment of DSB frequency was achieved by densitometry scanning of autoradiographs, combining all three dose titration lanes for each mouse.
PCR amplification and cloning of Sμ-Sγ3 junctions
Genomic DNA was isolated from purified splenic B cells after culturing for 4 days (8). Sμ-Sγ3 junctions were amplified from genomic DNA by PCR using the Expand Long Template Taq and Pfu polymerase mix (Roche, Piscataway NJ) and the primers μ3-H3 and g3-2 (8). PCR products were cloned into the vector pCR4-TOPO (Invitrogen, Carlsbad, CA). For measurement of the mutation frequency and spectrum within recombined Sμ-Sγ3 segments, only clones whose Sμ or Sγ3 segments were completely sequenced were included.
RESULTS
p53 inhibits IgG2a class switching
To examine if p53 has a role in CSR, splenic B cells from p53−/− and WT littermates were stimulated to switch to several different isotypes in vitro by culturing with lipopolysaccharide (LPS), anti-IgD dextran, and various cytokines (see Materials and Methods). Surface expression of switched Ig isotypes and proliferation were assayed by flow cytometric analysis of carboxy-fluorescein succinimidyl-ester (CFSE) stained splenic B cells cultured for 2.5 days. Switching to most isotypes was not affected by the absence of p53, but IgG2a switching was increased by 2.2-fold in p53−/− B cells relative to WT cells (Fig 1). A slight but consistent increase in IgG2b and IgA switching was also observed. No appreciable differences in the number of cell divisions, as assessed by dilution of the CFSE signal, were observed in p53−/− versus WT B cells for any of the culture conditions (Suppl Fig S1A). Also, the cell-cycle profiles of WT and p53−/− splenic B cells induced to undergo CSR were indistinguishable (Suppl Fig S1B), indicating that the inhibitory effect of p53 on IgG2a switching is not mediated through perturbation of proliferation or the cell-cycle. The absolute numbers and proportions of total B cells and newly formed, follicular, and marginal zone B-cell subsets in the spleens of p53−/− mice were not different from WT littermates (Suppl Fig S2).
Figure 1. p53-deficient splenic B cells show increased IgG2a switching in culture.
CFSE-loaded splenic B cells from p53−/− and wild-type littermate mice were cultured for 2.5 days with LPS, cytokines and/or anti-δ-dextran to induce Ig class switching to the indicated isotypes. (A) Cells were analyzed for CSFE-dilution and surface Ig by flow cytometry; representative FACS plots are shown; percentages of switched cells are indicated within the gates. (B) Data from 8 experiments (8 sets of mice) were normalized to the percent of wild-type littermate (WT) switching, indicated as 100%. Mean percentages of switching in p53−/− relative to WT (+SEM) for the different isotypes are shown. Significance was determined by the one-sample t test.
Increased in vivo IgG2a switching in polyoma virus infected p53−/− mice
To determine if p53 has an effect on isotype expression during an immune response in vivo, we infected p53−/− and WT littermate mice with polyoma virus, which elicits a robust protective antibody response that is characterized by the induction of predominantly IgG2a and IgG2b antibodies (34). Splenic germinal center B cells were analyzed by flow-cytometry 12–20 days after infection. A 2-fold increase in IgG2a-expressing splenic germinal center B cells was observed in polyoma virus infected p53−/− mice compared with infected wild-type littermates (p = 0.018), whereas the proportions of germinal center B cells expressing IgG1, IgG2b, or IgG3 were not significantly different (Fig 2). Thus, p53-deficiency appears to specifically increase IgG2a CSR in vitro and in vivo, but to have little effect on other isotypes.
Figure 2. Polyoma virus infected p53−/− mice show increased in vivo IgG2a switching.
p53−/−, aid−/−, and wild-type littermate mice were inoculated i.p. with 2×106 pfu of polyoma virus strain A2. Splenic germinal center B cells were analyzed for surface isotype expression by flow cytometry 12–20 days after inoculation. (A) FACS plots show surface Ig expression on B220+ GL7+ splenic B cells from an experiment in which cells were harvested 14 days after infection. Percentages of switched cells are depicted next to the gates. (B) Data points and bars represent individual mice and means (n=7 for wild-type, n=5 for p53−/−) of the percentages of isotype switched cells within the B220+ GL7+ splenic germinal center B-cell subset. Three independent experiments were performed using: 3, 2, and 2 wild-type mice, 1, 2, and 2 p53−/− mice, and in the second experiment (shown) 2 aid−/− mice were included. Significance was determined by the Mann-Whitney U test.
Increased p53 levels inhibits CSR to multiple isotypes
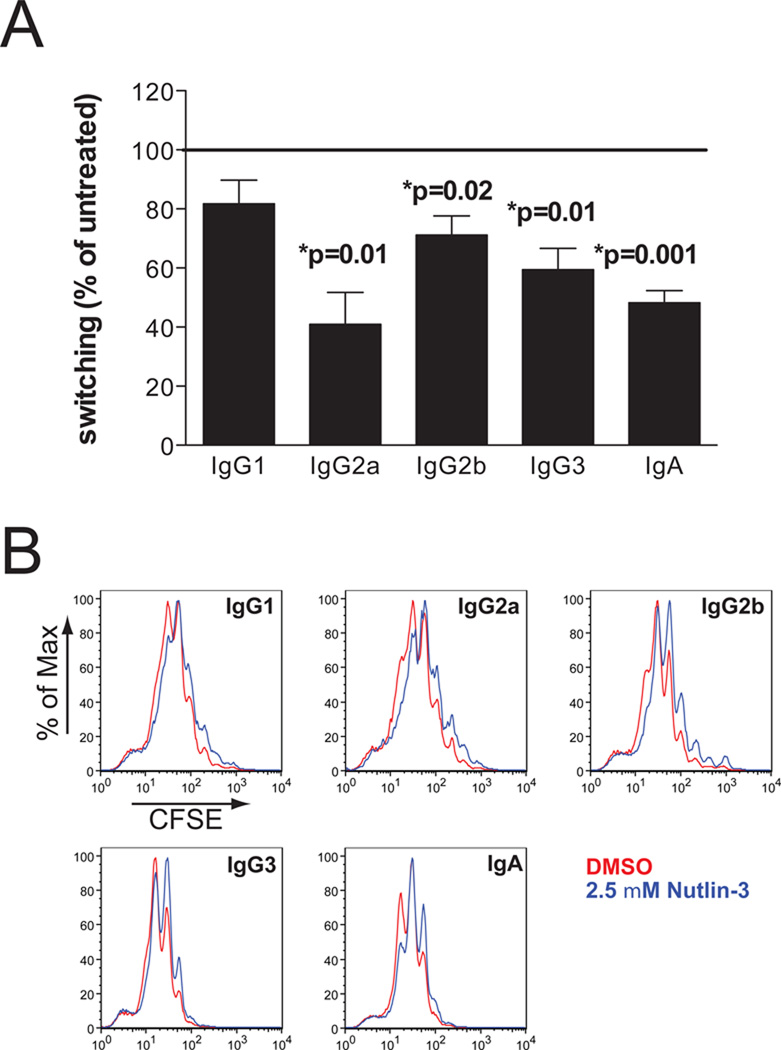
To determine the consequences of increased p53 levels on CSR, we assessed in vitro class switching in Nutlin-3 treated splenic B cells. Nutlin-3 is a potent MDM2 antagonist, stabilizing p53 by inhibiting MDM2-mediated degradation of p53, thereby strongly activating the p53 pathway (35). In B cells activated for IgG2a switching, Nutlin-3 treatment resulted in a 5-fold increase in p21 mRNA expression, which is a direct transcriptional target of p53 (see Fig 5B). Nutlin-3 treatment significantly inhibited CSR to IgG2a, IgG2b, IgG3 and IgA, indicating that supraphysiological p53 levels decrease switching to multiple Ig isotypes (Fig 3A). Cell proliferation was largely unaffected by this inhibitor at the concentration used in this study and did not account for the decreased switching, as determined by assessing switching in each cell division using CSFE staining (Fig 3B and data not shown).
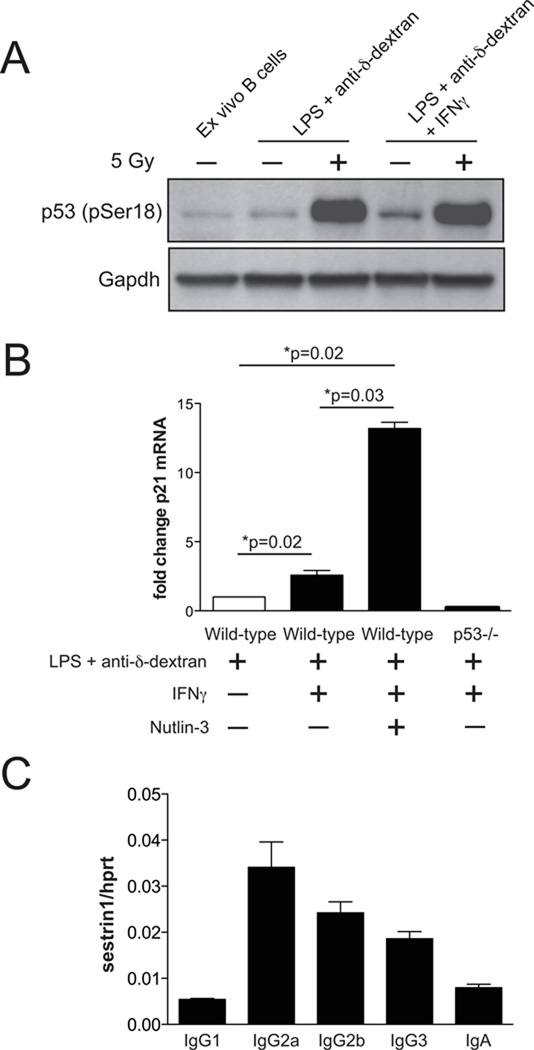
Figure 5. IFNγ+LPS activates p53 in switching B cells.
(A) Western blot analysis of Ser18 phosphorylated p53 (pSer18) in ex vivo splenic B cells, B cells stimulated for 2 days with LPS+anti-δ-dextran, or LPS+anti-δ-dextran+IFNγ. Activated splenic B cells irradiated at 5 Gy were used a positive control. Gapdh was used for protein loading control. This experiment was performed twice, using cells from one mouse each time. (B) Quantitative PCR analysis for p21 mRNA expression in WT splenic B cells stimulated for 48 h with LPS+anti-δ-dextran or LPS+anti-δ-dextran+IFNγ. Cells treated with 2.5 µM Nutlin-3 were used as a positive control, p53−/− cells as a negative control. Bar graph depicts relative p21 mRNA abundance normalized to wild-type splenic B cells stimulated with LPS+anti-δ-dextran. Significance was determined by the one-sample t test. Four independent experiments were performed, using 4 sets of mice. (C) Quantitative PCR analysis for sestrin 1 mRNA expression relative to hprt mRNA in WT splenic B cells stimulated to switch to the indicated isotypes as described in Methods. Data are from one experiment, PCR was performed in triplicate.
Figure 3. Nutlin-3 treatment inhibits CSR to multiple isotypes without affecting cell proliferation.
CFSE-loaded wild-type splenic B cells were activated for CSR to the indicated Ig isotypes. 2.5 µM Nutlin-3 or solvent control (DMSO) was added at the start of the cultures. Class switching was assessed by flow cytometry for surface Ig after 2.5 days of culture. (A) Data from 4 experiments were normalized to the percent of switching in untreated (DMSO) splenic B cells, indicated as 100%. Mean percentages (+SEM) of switching in Nutlin-3 treated splenic B cells relative to untreated for the different isotypes are shown. Significance was determined by the one-sample t test. (B) Overlay CSFE histograms of splenic B cells activated for switching to the indicated isotypes, treated with 2.5 µM Nutlin-3 (blue profiles) or DMSO vehicle control (red profiles) are shown.
Cell-death and survival of switching splenic B cells are not affected by p53-deficiency
In addition to regulation of the cell-cycle, p53 also coordinates cell-death pathways such as apoptosis and autophagy (36, 37). However, in splenic B cells activated for CSR, we detected no effect of p53-deficiency on cell-death, as measured by 7-AAD staining (Suppl Fig S3A), or on autophagy, as determined by LC3-I/II immunoblotting (Suppl Fig S3B). These results show that p53 does not act in CSR through its well-established functions in the regulation of the cell-cycle and cell-death.
AID expression and germline S region transcription in activated p53−/− splenic B cells is unaltered
The amount of CSR observed is related to the expression level of AID, which in turn correlates with aicda mRNA levels (38, 39). However, by immunoblotting we found no effect of p53 deficiency on levels of AID protein in splenic B cells activated to undergo CSR under several different conditions (Suppl Fig S4A). Concomitantly, no differences were found in the aicda transcript levels, as measured by semi-quantitative RT-PCR (Suppl Fig S4B). S region transcription is also required for CSR (41). To address whether this is inhibited by p53, we measured γ1 and γ2a germline transcripts after stimulation with LPS+IL4 or LPS+IFNγ, respectively (Suppl Fig S4C). Neither γ1 nor γ2a germline transcripts were increased in p53−/− splenic B cells.
S region DSBs are increased in switching p53−/− splenic B cells
As S region DSBs are required for CSR, we asked whether they are increased in p53−/− B cells induced to switch. As shown in Fig 4, the frequency of AID-dependent DSBs in Sμ and Sγ2a is increased by 2- and 2.3-fold, respectively, in p53−/− cells relative to WT cells stimulated to switch to IgG2a (LPS+IFNγ) for 2 days. There was little or no increase in Sμ DSBs in cells induced with LPS+IL-4 to switch to IgG1. These results are consistent with the increased CSR to IgG2a, but not to IgG1, in p53−/− cells. We have previously shown that AID-dependent Sμ DSBs are restricted to the G1 phase of the cell cycle in splenic B cells induced to switch in culture (13). As p53 is essential for the G1-S checkpoint, we asked whether the increased S region DSBs were due to delayed repair, but found that the Sμ DSBs in p53−/− cells are still almost entirely restricted to G1 phase (data not shown).
Figure 4. S region DSBs are increased p53−/− splenic B cells induced for IgG2a switching.
Sμ and Sγ2a LM-PCR were performed on threefold dilutions of GAPDH-normalized DNA isolated from wild-type (WT), p53−/−, and aid−/− splenic B cells that had been stimulated for 2 days with LPS+IL4 or LPS+IFNγ. Blunt DSBs in Sμ and Sγ2a were assessed by use of 5’ Sμ or 5’ Sγ2a primer, respectively, in combination with a linker specific primer. PCR products were blotted and hybridized with an internal Sμ or Sγ2a probe, respectively. (A) Sμ DSBs in cells activated for IgG1 switching. (B) Sμ DSBs in cells activated for IgG2a switching. (C) Sγ2a DSBs in cells activated for IgG2a switching. (D) Bar graph depicts means (+SEM) of densitometry measurements of autoradiographic films, normalized to WT, which was set at 1.0 (n=4 for all experiments, except for Sγ2a DSBs under IgG2a conditions, n=3.) All 3 titration lanes were scanned together. Each replicate experiment was performed on material from a separate set of mice. Two independent experiments using two sets of mice were performed. (E) Mutations in Sμ-Sγ3 junctions from WT and p53−/− splenic B cells activated to undergo IgG3 switching. Mutation frequency per 50-nucleotide segment is plotted for WT (black bars) and p53−/− (white bars). Overall mutation frequency is 3.1-fold increased in p53−/− versus WT (p = 0.00001). Total mutations/nucleotides analyzed for WT: 31/10,231; for p53: 30/3,182.
Increased mutation frequencies at S-S junctions
Mutations are frequently found in the nucleotides surrounding the junctions of recombined switch regions (S-S junctions). We examined whether the increased S region breaks in p53−/− B cells were accompanied by an increase in S region mutations by sequencing Sμ-Sγ3 junctions from p53−/− and WT B cells induced to switch to IgG3. Indeed, we observed a 3.1-fold increase in mutation frequencies in cloned Sμ-Sγ3 junction fragments from p53−/− cells induced to switch to IgG3 (mutation frequency: WT = 30.4 × 10−4 versus p53−/− = 95.2 × 10−4, p = 0.00001). There is no apparent difference in the distribution of the mutations between WT and p53−/− cells relative to the Sμ-Sγ3 junctions (Fig 4E). Taken together with the finding of increased S region DSBs in p53−/− cells, these data suggest that p53 affects switching by inhibiting AID activity and/or stimulating DNA repair.
Base excision repair (BER) is unperturbed in p53−/− splenic B cells
Our data indicate that S region DSBs and mutations are increased in p53−/− cells. This could be due to an increase in activities that increase mutations and breaks, or alternatively, to a decrease in DNA repair activity. An essential component of BER, DNA polymerase β (Pol β), was shown to be stimulated by p53 in cell-free repair experiments (40). Moreover, Polβ levels and activity correlate with p53 levels and function in several human cell lines and mouse embryonic fibroblasts (41). Polβ-deficient B cells show a modest increase (1.6-fold) in IgG2a switching, but no changes in other isotypes. This finding, along with the finding that Polβ associates with S regions in B cells undergoing CSR, suggests that Polβ attempts to repair AID-instigated lesions but is simply overwhelmed by the large number of lesions (42). We considered the possibility that the role of p53 in CSR could be mediated through its effects on Polβ levels and activity. To address this issue, nuclear protein extracts from WT and p53−/− B cells activated for IgG2a switching were tested for BER competence. A 43 nt double-stranded (ds) oligonucleotide containing a single tetrahydrofuran moiety (mimics an abasic site) within one of the strands was incubated with nuclear extract in the presence of [32P]α -dCTP. Abasic site cleavage and Polβ activity, followed by ligation, results in the incorporation of radioactive dCTP into the 43 nt oligo. As shown in Suppl Fig S5A, p53 deficiency does not affect the efficiency of BER in these extracts. Consistent with this result, immunoblotting experiments showed that p53 does not affect Polβ levels in splenic B cells, nor the levels of AP endonucleases (Apex1 and Apex2) (Suppl Fig S5B, C), which we have shown to be important for CSR and for Sμ DSBs (33). Moreover, using a [32P] γ-ATP end-labeled ss oligonucleotide containing a tetrahydrofuran residue, we were unable to detect any differences in the abasic site cleavage activity in nuclear protein extracts from WT or p53−/− B cells (data not shown). Thus, we find no evidence for reduction in BER activity in p53−/− B cells.
IFNγ activates p53 in switching B cells
It has been shown in several different cell-types that IFNγ activates p53, which can result in cell-cycle arrest and even senescence (43–46). Since IFNγ is used to stimulate IgG2a switching, we hypothesized that the isotype-specific effect of p53 is due to enhanced activation of p53 in the presence of IFNγ. Note that in Nutlin-3 treated cells, all isotypes were reduced, suggesting that if p53 were induced under other CSR conditions, these other isotypes would also be enhanced in its absence. DNA damage induces phosphorylation of p53 on Ser18, the mouse-equivalent of Ser15 in human p53, which is a substrate for ataxia telangiectasia mutated (ATM) and ATM-and-Rad3 related (ATR) kinases (47–49). We assessed p53 Ser-18 phosphorylation in naïve ex vivo splenic B cells and in splenic B cells activated in vitro with either LPS+anti-δ-dextran (stimulating IgG3 switching) or LPS+anti-δ-dextran+IFNγ (stimulating IgG2a switching). As shown in Fig 5A, p53 phosphorylation is slightly elevated in LPS+anti-δ-dextran stimulated cells compared to unstimulated ex vivo B cells, but is approximately 3-fold increased in B cells activated in the presence of IFNγ. In comparison, gamma-irradiation of activated B cells results in at least 10-fold more p53 Ser18 phosphorylation. Stimulation of splenic B cells to switch to other Ig isotypes, using different cytokines, results in smaller increases in p53 Ser-18 phosphorylation than does IFNγ treatment (data not shown). Although IFNγ stimulates p53 phosphorylation, we did not observe an increase in p53 protein or mRNA levels in these cultures (data not shown).
p53 Ser18 phosphorylation regulates IgG2a switching
To test whether phosphorylation of p53 on Ser18 impacts CSR, we examined CSR in cultured splenic B cells obtained from mice harboring knock-in alleles in which p53 Ser18 was mutated to Ala (p53 S18A) (30, 50). IgG2a was increased by 1.8-fold in p53 S18A knock-in splenic B cells, slightly less than the effect of p53 knockout, indicating that p53 Ser18 phosphorylation is important for the inhibition of IgG2a class switching in IFNγ treated B cells, although other amino acids might also be involved (Fig 6). Also, a slight but significant increase in IgA switching was observed in p53 S18A B cells, similar to what was found in p53−/− B cells.
Figure 6. p53 Ser18 phosphorylation regulates IgG2a switching.
CFSE-loaded splenic B cells from p53S18A and wild-type control mice were cultured for 2.5 days with LPS, cytokines and/or anti-δ-dextran to induce Ig class switching to the indicated isotypes. Class switching was determined by flow cytometry for surface Ig staining. (A) Representative FACS plots are shown. Upper panel shows equal CFSE loading in wild-type mice from the same colony and p53S18A splenic B cells. Percentages of switched cells are indicated within the gates. (B) Bar graph depicts data from 4 sets of mice, which were all analyzed in one experiment. Percent CSR in p53S18A relative to WT (+SEM) for the different isotypes are shown. Significance was determined by the one-sample t test.
Increased levels of reactive oxygen species (ROS) in p53−/− cells and reduction of IgG2a switching in p53−/− splenic B cells treated with the antioxidant N-acetyl-cysteine
In a number of different cell types, IFNγ treatment results in increased ROS production, which in turn can activate p53 (43, 44, 51, 52). In phagocytic cells, IFNγ induces the expression of several NADPH oxidases, which are responsible for the production of ROS as part of a microbicidal host response (55). IFNγ might also increase mitochondrial ROS production in B cells. ROS-induced DNA damage can activate the p53 pathway in an ATM-dependent manner (53). p53 induces the expression of antioxidant genes, thereby decreasing the levels of intracellular ROS (24, 54). Following from this, one would expect increased levels of intracellular ROS in IFNγ-treated p53−/− splenic B cells relative to WT cells. Using the fluorescent probe CM-H2-DCFDA to assess intracellular ROS levels, we established that this is indeed the case (Fig 7A). Other CSR induction conditions also cause increased ROS levels (not shown), most likely because B-cell activation stimulates mitochondria, which generate ROS (55–57). However, we find that ROS levels are higher in cells in which the induction conditions include IFNγ.
Figure 7. Increased ROS in switching p53−/− splenic B cells; NAC inhibits CSR and abolishes increased IgG2a switching in p53−/− splenic B cells.
(A) Shown are overlay histograms of wild-type (red profile) and p53−/− (blue profile) splenic B cells loaded with the CM-H2-DCFDA fluorescent ROS probe after activation with LPS + anti-δ-dextran + IFNγ for 48 h. Treatment with 10 mM (blue profile) and 20 mM NAC (green profile) decreases intracellular ROS in activated wild-type and p53−/− splenic B cells. (B) CFSE-loaded cells were activated for IgG2a switching and treated with 10 mM NAC. IgG2a switching was assessed by flow cytometry for surface Ig staining after 2.5 days. Representative FACS plots of 3 independent experiments are shown. Percentages of IgG2a switched cells are given within the gates. (C) CFSE-loaded wild-type splenic B cells were activated with LPS + cytokines and/or anti-δ-dextran to induce CSR to the indicated Ig isotypes. CSR was assessed by flow cytometry for surface Ig staining. Percentages of switched cells are indicated within the gates. Top row shows untreated control cultures, middle row shows cultures treated with 10 mM NAC. Bottom row shows overlay histograms of CSFE fluorescence in control cultures (red profiles) and 10 mM NAC treated cultures (blue profiles). Representative FACS plots of three experiments are shown. (D) Bar graph shows data from the 3 experiments normalized to untreated cells, shown as 100%. Means (+SEM) of percentages of switched cells in NAC-treated cutures relative to untreated cultures are shown. Significance was determined by the one-sample t test. (E) Sμ LM-PCR was performed on threefold dilutions of Gapdh-normalized DNA from wild-type and aid−/− splenic B cells activated with LPS + anti-δ-dextran with or without 10 mM NAC for 2 days. LM-PCR products were blotted and hybridized with an internal Sμ probe. Densitometry scanning was performed on all three lanes representing the threefold dilutions. Untreated sample was set at 1.0 to determine relative density. Representative result of 2 experiments is shown.
We also examined levels of the mRNA for Sestrin 1, an antioxidant protein that is induced by ROS and is also transcriptionally upregulated by p53 (58). We found that Sestrin 1 mRNA is expressed most highly in cells treated for two days to induce IgG2a switching, but to a lesser extent in cells treated to switch to other isotypes (Fig 5C). Although we find a small but significant increase in IgA CSR, Sestrin 1 mRNA is not induced under IgA switching conditions. We speculate that ROS might also be elevated in these cultures, but since p53 is known to induce other antioxidant genes besides Sestrin 1 (22, 54), this might account for the effect of p53 on IgA CSR. Taken together, our results suggest that ROS levels are increased during CSR, and that this effect is strongest in cells in which the activation conditions include IFNγ.
Addition of the thiol-containing antioxidant N-acetylcysteine (NAC) to the B cell cultures decreased the levels of intracellular ROS, as expected (Fig 7A). NAC is a low molecular weight thiol-containing compound that detoxifies ROS and enhances glutathione synthesis, which also reduces ROS (59). In preliminary experiments, we optimized the amount of NAC to add (not shown). Importantly, the increase in IgG2a switching in p53−/− relative to WT splenic B cells was nearly abolished when 10 mM NAC was added to the cultures (Fig 7B and data not shown). Specifically, NAC treatment inhibited IgG2a CSR in WT cells by 36%, but inhibited CSR in p53−/− cells to a greater extent, by 53% (2 independent experiments), suggesting that the antioxidant function of p53 might be responsible for the inhibitory effect on IgG2a switching.
N-acetyl-cysteine (NAC) inhibits S region breaks and class switching in wild-type splenic B cells
To further establish the importance of the redox state of B cells for CSR, we tested the effect of adding 10 mM NAC to cultures of WT cells induced to switch to several isotypes. CFSE-stained mouse splenic B cells were activated to induce class switching to IgG1, IgG2a, IgG2b, IgG3, and IgA. We found that 10 mM NAC inhibits class switching by 30–80%, depending on the isotype, with the exception of IgG2b (Fig 7C, D). As shown by CFSE-dilution, NAC has no effect on cell-division rate, except under IgG2b induction conditions (Fig 7C).
Since reactive oxygen species (ROS) also function as second messengers in several signaling cascade pathways, we analyzed whether NAC affected Ig germline transcription. Germline transcripts for γ1 and γ2a were not affected by NAC treatment (Suppl Fig S6A). Also, aicda mRNA levels were similar in NAC-treated versus untreated control B-cell cultures stimulated for IgG1 and IgG2a switching (Suppl Fig S6A).
We have previously shown that formation of S region DSBs requires the BER pathway enzymes uracil-N-glycosylase (Ung) and Apex1/Apex2 (5, 33). Both the levels and the subcellular localization of these enzymes are unchanged upon NAC-treatment of B cells stimulated for CSR (Suppl Fig S6B). However, by use of LM-PCR we found that AID-dependent blunt DSBs in Sμ are approximately 2-fold decreased in NAC-treated B cells compared to untreated cultures (Fig 7E), consistent with the decrease in CSR upon NAC-treatment. We conclude that NAC inhibits the formation of S region DSBs, but does not affect S region germline transcription or the expression and subcellular localization of proteins involved in DSB-formation during CSR.
S-S junctions are normal in p53−/− splenic B cells
We considered the possibility that end-joining might be altered due to the increased ROS levels, as it has been shown that increased ROS can reduce the levels and activity of DNA-dependent DNA protein kinase (DNA-PKcs) (60). To assess this, we examined the sequences of Sμ-Sγ3 junctions from p53−/− and WT B cells induced to switch to IgG3. S-S junctions from B cells deficient in DNA-PKcs show increased lengths of microhomologies, suggesting defective non-homologous end joining (61). However, we found no differences in the frequency of blunt S-S junctions or lengths of junction microhomology between p53−/− and WT B cells (Suppl Table S1). These data suggest that end-processing and recombination of S regions are normal in p53−/− B cells.
Increased ROS levels modestly enhance IgG2a class switching
We asked whether increased ROS levels in switching B cells could enhance class switching. To that end, we tried several different reagents, such as adding H2O2 and L-buthionine sulfoximine (BSO) to the cultures. These experiments can be problematic as these chemicals are highly toxic to activated B cells (data not shown). Nonetheless, lowering the concentration of β-mercaptoethanol in the culture medium from 50 µM to 20 µM resulted in a modest (1.3-fold, p=0.017) increase in IgG2a switching, while switching to other isotypes was not affected. Further lowering of β-mercaptoethanol concentrations inhibited class switching, as it decreased cell proliferation (data not shown). This result is consistent with our hypothesis that increased ROS levels cause the increased CSR to IgG2a that we observe in p53−/− cells.
DISCUSSION
Although it has been shown that p53 prevents the formation of chromosomal translocations that derive from CSR activity (62), whether p53 affects the CSR mechanism itself has not been previously addressed. This is important since it is not known if the response to genotoxic stress in the form of programmed DSBs limits gene diversifications that are an integral part of the adaptive immune system. In human germinal center B cells, which presumably represent the population that undergoes CSR and SHM in vivo, it has been shown that Bcl6 represses transcription of p53 and p21, suggesting that the induction of apoptosis and cell-cycle arrest might be blunted in cells sustaining DNA DSBs occurring during CSR and SHM (63, 64).
Here we show that p53 inhibits IgG2a class switching in vitro and in vivo, and that this is not mediated through its canonical effects on the cell-cycle or cell death, but most likely through regulating the redox state of switching B cells. These results raise the question of how intracellular ROS might affect S region DSBs and class switching. Interestingly, the chemical pathway resulting in deamination of cytidine to uracil involves an oxidation reaction. ROS such as hydroxyl radicals (•OH) are required intermediates in this reaction (27, 28). We reasoned that the intracellular redox state, and perturbations thereof, could potentially influence the cytidine deaminase activity of AID in B cells, which could be the basis of the observed effect of p53 deficiency on IgG2a class switching. Our finding that both S region mutations and DSBs are increased in p53−/− cells supports the hypothesis that the increased levels of ROS in these cells stimulate AID activity. Most importantly, these data suggest that the role of p53 in suppressing chromosomal translocations and B cell oncogenesis might be due to its ability to inhibit AID-induced DNA mutations and breaks, in addition to its ability to prevent selection and outgrowth of cells that show dysregulated growth.
Alternatively, it might be that increased ROS levels in IFNγ-stimulated p53-deficient B cells cause DNA lesions that lead to strand breaks in S regions, which in concert with AID-induced breaks, stimulate CSR. Of note, we did not observe any class switching activity in aid−/− p53−/− double knock-out B cells (data not shown), showing that AID is absolutely required, and that potential ROS-initiated DNA strand breaks are not sufficient to support class switching.
In the absence of DNA-PKcs, AID-dependent chromosomal IgH breaks accumulate in switching B cells. Interestingly, it was shown that p53 only limits chromosomal IgH breaks in LPS-stimulated DNA-PKcs-deficient B cells and not in CD40-stimulated B cells (65). The authors suggested that this might be because CD40 ligation might cause stronger Bcl6 upregulation and concomitant p53 suppression than LPS stimulation. However, we find that IgG2a switching is similarly increased in LPS+IFNγ and anti-CD40+IFNγ treated p53−/− B cells, compared to WT (data not shown).
We speculate that the isotype-specific effect for IgG2a is probably due to a combination of increased p53 activation upon IFNγ-treatment, which is used to induce IgG2a CSR, and the increased production of ROS in IFNγ-treated cells. We found that induction of p53 Ser18-phosphorylation is stronger upon IFNγ treatment than under conditions used to stimulate switching to other isotypes, and that p53 Ser18-phosphorylation is important for the inhibitory effect on IgG2a switching, as demonstrated by the increased in vitro IgG2a switching of p53 S18A knock-in B cells. A concomitant increase in the expression of the p53 target gene sestrin 1 was found in IFNγ treated B cells, relative to other induction conditions (Fig 5C). However, the finding that mutations are increased in segments surrounding S-S junctions in cells induced to switch to IgG3 suggests that p53 also represses AID activity in the absence of IFNγ, although apparently to a lesser degree.
The induction of p21 observed in WT B cells treated with LPS+IFNγ did not result in a measurable effect on proliferation or the cell-cycle, although if only a small proportion of the cells were affected we would not have detected it. Also, p21 induction does not appear to activate the cell-cycle checkpoint kinase Chk2, as we find that class switching is unaltered in chk2−/− splenic B cells (data not shown). The finding that Chk2 does not regulate CSR was also recently reported by Jankovic et al. (66).
A low level of p53 activation has been shown to be important for some of its functions. For instance, p53 regulates stem cell renewal and pluripotency potential in the absence of any obvious stressors (67). Also, p53 affects reproductive success by regulating the expression of leukemia inhibitory factor (LIF), which is essential for fetal implantation (68). These functions are not typically associated with DNA damage, indicating that p53 also acts in normal homeostatic processes. Based on the results presented in this study, CSR can be added to the ever-growing list of processes modulated by p53.
In a broader perspective, how would the organism benefit from the p53-restricted IgG2a switching in B cells? The IgG2a isotype is involved in the pathology of most of the antibody-mediated autoimmune disease models in the mouse, so one can envision that limiting IgG2a switching might be beneficial. Aging p21-deficient animals develop autoimmune disease (69), and antigen-induced arthritis is more severe in p53−/− mice, without altering the antigen-specific Ig responses (70). Upon polyoma virus challenge, p53-deficient animals have significantly more IgG2a-expressing germinal center B cells in the spleen than polyoma-infected wild-type littermates, but this did not result in a measurable increase in virus-specific IgG2a titers in the serum (data not shown). This could be due to compensatory mechanisms. We did not detect any differences in the serum titers of spontaneous antinuclear anti-bodies (ANA) in 8 wild-type versus p53−/− mice (data not shown). Spontaneous ANA titers usually rise with age, but due to the high incidence of malignant tumors in p53−/− mice, we could not assess ANA titers in older mice. Taken together with our data, these results indicate that p53 regulates the switching event per se, but it is unclear whether it affects subsequent production of antibody.
IgG2a CSR appears to be particular sensitive to increased ROS levels. Increasing intracellular ROS by culturing B cells in a lower concentration of the reducing agent β-mercaptoethanol resulted in a slight, but consistent and significant increase in IgG2a switching, showing that this isotype is particularly sensitive to changes in the intracellular redox state. In addition to our finding that ROS levels appear highest in IFNγ-treated cells, it is also possible that this sensitivity is due to the fact that the Sγ2a switch region has the lowest number of AID hotspots of all S regions, which might make AID-instigated lesions particularly limiting for IgG2a switching (42). Increased efficiency of AID-mediated cytidine deamination might therefore especially impact IgG2a switching. Consistent with this, we previously found that DNA Polβ deficiency, the major DNA polymerase involved in correcting AID lesions as part of the base excision repair pathway, results in a small but consistent increase in IgG2a switching, specifically (42).
Supplementary Material
ACKNOWLEDGMENTS
We thank Anna Ucher and Cara West for excellent technical support. We thank Dr Aron B. Fisher, Univ of Pennsylvania, Philadelphia PA for advice on the manuscript.
Footnotes
This work was supported by grants from NIH: RO1 AI023283 and AI063026 (J.S.), AI065639 (C.E.S.), GM084361 (M.H.B.), and CA06664 (E.S-T). J.E.J.G. was supported by the Irvington Institute Fellowship Program of the Cancer Research Institute.
The authors have no conflicting financial interests.
REFERENCES
- 1.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 2.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Petersen S, Casellas R, Reina-San-Martin B, Chen HT, Difilippantonio MJ, Wilson PC, Hanitsch L, Celeste A, Muramatsu M, Pilch DR, Redon C, Ried T, Bonner WM, Honjo T, Nussenzweig MC, Nussenzweig A. AID is required to initiate Nbs1/gamma-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush JS, Fugmann SD, Schatz DG. Staggered AID-dependent DNA double strand breaks are the predominant DNA lesions targeted to S mu in Ig class switch recombination. Int.Immunol. 2004;16:549–557. doi: 10.1093/intimm/dxh057. [DOI] [PubMed] [Google Scholar]
- 5.Schrader CE, Linehan EK, Mochegova SN, Woodland RT, Stavnezer J. Inducible DNA breaks in Ig S regions are dependent on AID and UNG. J.Exp.Med. 2005;202:561–568. doi: 10.1084/jem.20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalan N, Selz F, Imai K, Revy P, Fischer A, Durandy A. The block in immunoglobulin class switch recombination caused by activation-induced cytidine deaminase deficiency occurs prior to the generation of DNA double strand breaks in switch mu region. J.Immunol. 2003;171:2504–2509. doi: 10.4049/jimmunol.171.5.2504. [DOI] [PubMed] [Google Scholar]
- 7.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 8.Schrader CE, Vardo J, Stavnezer J. Role for mismatch repair proteins Msh2, Mlh1, and Pms2 in immunoglobulin class switching shown by sequence analysis of recombination junctions. J.Exp.Med. 2002;195:367–373. doi: 10.1084/jem.20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol.Cell. 2004;16:163–171. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The biochemistry of somatic hypermutation. Annu.Rev.Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 11.Stavnezer J, Schrader CE. Mismatch repair converts AID-instigated nicks to double-strand breaks for antibody class-switch recombination. Trends Genet. 2006;22:23–28. doi: 10.1016/j.tig.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu.Rev.Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrader CE, Guikema JE, Linehan EK, Selsing E, Stavnezer J. Activation-induced cytidine deaminase-dependent DNA breaks in class switch recombination occur during G1 phase of the cell cycle and depend upon mismatch repair. J.Immunol. 2007;179:6064–6071. doi: 10.4049/jimmunol.179.9.6064. [DOI] [PubMed] [Google Scholar]
- 14.Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J.Exp.Med. 2004;200:1103–1110. doi: 10.1084/jem.20041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reina-San-Martin B, Difilippantonio S, Hanitsch L, Masilamani RF, Nussenzweig A, Nussenzweig MC. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J.Exp.Med. 2003;197:1767–1778. doi: 10.1084/jem.20030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumsden JM, McCarty T, Petiniot LK, Shen R, Barlow C, Wynn TA, Morse HC, III, Gearhart PJ, Wynshaw-Boris A, Max EE, Hodes RJ. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J.Exp.Med. 2004;200:1111–1121. doi: 10.1084/jem.20041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manis JP, Morales JC, Xia Z, Kutok JL, Alt FW, Carpenter PB. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat.Immunol. 2004;5:481–487. doi: 10.1038/ni1067. [DOI] [PubMed] [Google Scholar]
- 18.Kracker S, Bergmann Y, Demuth I, Frappart PO, Hildebrand G, Christine R, Wang ZQ, Sperling K, Digweed M, Radbruch A. Nibrin functions in Ig class-switch recombination. Proc.Natl.Acad.Sci.U.S.A. 2005;102:1584–1589. doi: 10.1073/pnas.0409191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinkelmann M, Spehalski E, Stoneham T, Buis J, Wu Y, Sekiguchi JM, Ferguson DO. Multiple functions of MRN in end-joining pathways during isotype class switching. Nat Struct Mol Biol. 2009 doi: 10.1038/nsmb.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reina-San-Martin B, Nussenzweig MC, Nussenzweig A, Difilippantonio S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc.Natl.Acad.Sci.U.S.A. 2005;102:1590–1595. doi: 10.1073/pnas.0406289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan-Hammarstrom Q, Lahdesmaki A, Zhao Y, Du L, Zhao Z, Wen S, Ruiz-Perez VL, Dunn-Walters DK, Goodship JA, Hammarstrom L. Disparate roles of ATR and ATM in immunoglobulin class switch recombination and somatic hypermutation. J.Exp.Med. 2006;203:99–110. doi: 10.1084/jem.20050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahdesmaki A, Taylor AM, Chrzanowska KH, Pan-Hammarstrom Q. Delineation of the role of the Mre11 complex in class switch recombination. J Biol Chem. 2004;279:16479–16487. doi: 10.1074/jbc.M312796200. [DOI] [PubMed] [Google Scholar]
- 23.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 24.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snider MJ, Reinhardt L, Wolfenden R, Cleland WW. 15N kinetic isotope effects on uncatalyzed and enzymatic deamination of cytidine. Biochemistry. 2002;41:415–421. doi: 10.1021/bi011410i. [DOI] [PubMed] [Google Scholar]
- 28.Labet V, Grand A, Cadet J, Eriksson LA. Deamination of the radical cation of the base moiety of 2'-deoxycytidine: a theoretical study. Chemphyschem. 2008;9:1195–1203. doi: 10.1002/cphc.200800154. [DOI] [PubMed] [Google Scholar]
- 29.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 30.Armata HL, Garlick DS, Sluss HK. The ataxia telangiectasia-mutated target site Ser18 is required for p53-mediated tumor suppression. Cancer Res. 2007;67:11696–11703. doi: 10.1158/0008-5472.CAN-07-1610. [DOI] [PubMed] [Google Scholar]
- 31.Phelan SA, Wang X, Wallbrandt P, Forsman-Semb K, Paigen B. Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med. 2003;35:1110–1120. doi: 10.1016/s0891-5849(03)00462-3. [DOI] [PubMed] [Google Scholar]
- 32.Schrader CE, Edelmann W, Kucherlapati R, Stavnezer J. Reduced isotype switching in splenic B cells from mice deficient in mismatch repair enzymes. J.Exp.Med. 1999;190:323–330. doi: 10.1084/jem.190.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guikema JE, Linehan EK, Tsuchimoto D, Nakabeppu Y, Strauss PR, Stavnezer J, Schrader CE. APE1- and APE2-dependent DNA breaks in immunoglobulin class switch recombination. J.Exp.Med. 2007;204:3017–3026. doi: 10.1084/jem.20071289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szomolanyi-Tsuda E, Le QP, Garcea RL, Welsh RM. T-Cell-independent immunoglobulin G responses in vivo are elicited by live-virus infection but not by immunization with viral proteins or virus-like particles. J Virol. 1998;72:6665–6670. doi: 10.1128/jvi.72.8.6665-6670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 36.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 37.Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa M, Tolarova H, Li Z, Dubois W, Lim S, Callen E, Franco S, Mosaico M, Feigenbaum L, Alt FW, Nussenzweig A, Potter M, Casellas R. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. J Exp Med. 2008;205:1949–1957. doi: 10.1084/jem.20081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sernandez IV, de Yebenes VG, Dorsett Y, Ramiro AR. Haploinsufficiency of activation-induced deaminase for antibody diversification and chromosome translocations both in vitro and in vivo. PLoS One. 2008;3:e3927. doi: 10.1371/journal.pone.0003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Ahn J, Wilson SH, Prives C. A role for p53 in base excision repair. EMBO J. 2001;20:914–923. doi: 10.1093/emboj/20.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo YR, Fishel ML, Amundson S, Kelley MR, Smith ML. Implication of p53 in base excision DNA repair: in vivo evidence. Oncogene. 2002;21:731–737. doi: 10.1038/sj.onc.1205129. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Stavnezer J. DNA polymerase beta is able to repair breaks in switch regions and plays an inhibitory role during immunoglobulin class switch recombination. J.Exp.Med. 2007;204:1677–1689. doi: 10.1084/jem.20070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KS, Kang KW, Seu YB, Baek SH, Kim JR. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev. 2009;130:179–188. doi: 10.1016/j.mad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of ATM pathway: a culture study. Free Radic Res. 2008;42:625–632. doi: 10.1080/10715760802244768. [DOI] [PubMed] [Google Scholar]
- 45.Mann-Chandler MN, Kashyap M, Wright HV, Norozian F, Barnstein BO, Gingras S, Parganas E, Ryan JJ. IFN-gamma induces apoptosis in developing mast cells. J Immunol. 2005;175:3000–3005. doi: 10.4049/jimmunol.175.5.3000. [DOI] [PubMed] [Google Scholar]
- 46.Zhang HM, Yuan J, Cheung P, Chau D, Wong BW, McManus BM, Yang D. Gamma interferon-inducible protein 10 induces HeLa cell apoptosis through a p53-dependent pathway initiated by suppression of human papillomavirus type 18 E6 and E7 expression. Mol Cell Biol. 2005;25:6247–6258. doi: 10.1128/MCB.25.14.6247-6258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 48.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 49.Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller SP, Lavin MF. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 50.Sluss HK, Armata H, Gallant J, Jones SN. Phosphorylation of serine 18 regulates distinct p53 functions in mice. Mol Cell Biol. 2004;24:976–984. doi: 10.1128/MCB.24.3.976-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonoda J, Laganiere J, Mehl IR, Barish GD, Chong LW, Li X, Scheffler IE, Mock DC, Bataille AR, Robert F, Lee CH, Giguere V, Evans RM. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moiseeva O, Mallette FA, Mukhopadhyay UK, Moores A, Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol Biol Cell. 2006;17:1583–1592. doi: 10.1091/mbc.E05-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 56.Josse C, Legrand-Poels S, Piret B, Sluse F, Piette J. Impairment of the mitochondrial electron chain transport prevents NF-kappa B activation by hydrogen peroxide. Free Radic Biol Med. 1998;25:104–112. doi: 10.1016/s0891-5849(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 57.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 58.Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, Skaliter R, Gudkov AV, Chumakov PM, Feinstein E. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 59.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–127. [PubMed] [Google Scholar]
- 60.Lu HR, Zhu H, Huang M, Chen Y, Cai YJ, Miao ZH, Zhang JS, Ding J. Reactive oxygen species elicit apoptosis by concurrently disrupting topoisomerase II and DNA-dependent protein kinase. Mol Pharmacol. 2005;68:983–994. doi: 10.1124/mol.105.011544. [DOI] [PubMed] [Google Scholar]
- 61.Cook AJ, Oganesian L, Harumal P, Basten A, Brink R, Jolly CJ. Reduced switching in SCID B cells is associated with altered somatic mutation of recombined S regions. J Immunol. 2003;171:6556–6564. doi: 10.4049/jimmunol.171.12.6556. [DOI] [PubMed] [Google Scholar]
- 62.Ramiro AR, Jankovic M, Callen E, Difilippantonio S, Chen HT, McBride KM, Eisenreich TR, Chen J, Dickins RA, Lowe SW, Nussenzweig A, Nussenzweig MC. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 64.Phan RT, Saito M, Basso K, Niu H, Dalla-Favera R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol. 2005;6:1054–1060. doi: 10.1038/ni1245. [DOI] [PubMed] [Google Scholar]
- 65.Franco S, Murphy MM, Li G, Borjeson T, Boboila C, Alt FW. DNA-PKcs and Artemis function in the end-joining phase of immunoglobulin heavy chain class switch recombination. J.Exp.Med. 2008;205:557–564. doi: 10.1084/jem.20080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jankovic M, Robbiani DF, Dorsett Y, Eisenreich T, Xu Y, Tarakhovsky A, Nussenzweig A, Nussenzweig MC. Role of the translocation partner in protection against AID-dependent chromosomal translocations. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0908946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Elf SE, Miyata Y, Sashida G, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, Reva B, Koff A, Nimer SD. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 69.Salvador JM, Hollander MC, Nguyen AT, Kopp JB, Barisoni L, Moore JK, Ashwell JD, Fornace AJ., Jr Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16:499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]
- 70.Leech M, Xue JR, Dacumos A, Hall P, Santos L, Yang Y, Li M, Kitching AR, Morand EF. The tumour suppressor gene p53 modulates the severity of antigen-induced arthritis and the systemic immune response. Clin Exp Immunol. 2008;152:345–353. doi: 10.1111/j.1365-2249.2008.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.