Abstract
Although persistent transmission of hepatitis C virus (HCV) from infected mothers to their infants is reported in 4–8%, transient HCV perinatal infection also occurs. This prospective cohort study determined perinatal HCV infection- and early and late clearance-rates in 1,863 mother-infant pairs in rural Egyptian villages. This study found 15.7% and 10.9% of pregnant women had HCV antibodies (anti-HCV) and HCV-RNA, respectively. Among 329 infants born of these mothers, 33 (10.0%) tested positive for both anti-HCV and HCV-RNA 2 months following birth—29 (12.5%) having HCV-RNA positive mothers and 4 (with transient infections) having mothers with only anti-HCV. Fifteen remained HCV-RNA positive at one and/or 2 years (persistent infections), while 18 cleared both virus and antibody by 1 year (transient infections). Among the 15 persistent cases, 7 cleared their infections by 2 or 3 years. At 2- to 6- and at 10- to 12-month maternally acquired anti-HCV was observed in 80% and 5% of infants, respectively. Four perinatally infected and one transiently infected infant were confirmed to be infected by their mothers by the sequence similarity of their viruses. Viremia was 155-fold greater in mothers of infants with persistent than mothers of infants with transient infections. Maternal-infant transmission of HCV is more frequent than generally reported. However, both early and late clearance of infection frequently occurs and only 15 (4.6%) and 8 (2.4%) infants born of HCV-RNA positive mothers had detectable HCV-RNA at one and 2–3 years of age. Investigating how infants clear infection may provide important information about protective immunity to HCV.
Keywords: perinatal transmission, hepatitis C virus, risk factors, viral clearance, transient infection
INTRODUCTION
Global prevalence of hepatitis C virus (HCV) infection is estimated to be 2.2%, with 200 million people having chronic infections, of whom almost 4 million Americans are infected with the virus [Kim, 2002; Group, 2004]. The prevalence of HCV in Egypt is seven- or eightfold greater than in the USA: with 8–10 million having antibodies to HCV (anti-HCV) and 5–7 million having active infections with HCV-RNA [Strickland, 2006].
Extensive studies of risk factors for prevalent and incident infections in Egypt have been conducted [Abdel-Aziz et al., 2000; Habib et al., 2001; Magder et al., 2005; Mohamed et al., 2005; Stoszek et al., 2006; Strickland, 2006; Saleh et al., 2008], and concluded children living with an HCV positive parent are at high risk of infection, particularly if their mother is infected [Mohamed et al., 2005]. The results of a community-based study of perinatal HCV transmission in three rural Egyptian villages are reported where the overall HCV prevalence is greater than 20% [Abdel-Aziz et al., 2000; Habib et al., 2001; Stoszek et al., 2006; Saleh et al., 2008].
MATERIALS AND METHODS
Study Population
Pregnant women during their third trimester residing in three villages in the Nile delta and receiving antenatal care at their village’s Community Health Unit (CHU) were admitted to the study after providing informed consent. Recruitment was initiated in the first village with a population of 9,000 inhabitants in 1997, and was expanded to two other nearby villages with populations of 30,000 each in 1998 and 2001.
Questionnaires that inquired about demographic and health information and potential exposures to HCV were administered. Expectant mothers received antenatal consultation from the project’s obstetrician and a blood sample was collected. Subsequent follow-up of the mother and her child was performed 2 months after birth, and then annually for 5 years. In some cases the initial 2-month visit was delayed up to 4 months. Additional visits when the child was 6 months old were conducted at one village. During follow-up visits, information about mode of delivery, breast-feeding practices, the infant’s and mother’s health, and interim risk factors for HCV infection was obtained. A pediatrician examined the infants and a blood sample was obtained from both mother and child.
Laboratory Tests
Serum samples were tested for anti-HCV with a third-generation enzyme immunoassay (EIA, Abbott Laboratories, Wiesbaden, Delknheim, Germany). Testing for HCV-RNA was performed using a direct nested reverse transcriptase-polymerase chain reaction (RT-PCR) as previously described [Abdel-Hamid et al., 1997]. If the sera were HCV-RNA negative but the EIA was positive for anti-HCV, the RT-RCR was repeated following extraction of RNA. During the first 3½ years of the project, sera were tested for both anti-HCV and HCV-RNA. To reduce cost during the final 1½ years we only performed RT-PCR on EIA positive sera. We did not test for other hepatitis viruses or for HIV which is exceedingly rare in rural Egyptian communities. A UNAIDS/WHO report [2008] estimated that 9,000 Egyptians, predominately men with high risk behaviors, have HIV/AIDS, which is less than 0.1% of the total population. No HIV positive pregnant women were reported during sentinel site surveillance outside of “major urban areas” from 1992 to 1996 and in 2004.
Sequencing and Quantitative PCR
Sera were shipped to the University of Maryland-Baltimore for quantitative RNA determination and sequencing of HCV. RT-PCR was repeated at this time. Samples from 24 HCV-RNA negative subjects, included for quality assurance purposes, were only tested by RT-PCR to confirm they were negative. Among the 31 HCV-RNA positive samples, 7 belonged to children and 24 to mothers. Six of these were from mother-infant pairs and 5 were tested to confirm perinatal mother-to-infant transmission. The remaining mother-infant pair was from a mother and her 5-year-old child to verify whether the mother was source of her child’s infection.
HCV Viral Load Determination
RNA was extracted from 140 µl of serum or plasma using the Qiagen Viral RNA mini kit (Qiagen, Valencia, CA) and eluted in 60 µl. HCV real-time RT-PCR was performed using 5′UTR with a ABI Prism 7700 thermo-cycler as described [Takeuchi et al., 1999]. Quantitative controls consisting of known copy numbers generated from Armored HCV RNA (Ambion, Austin, TX) were run with each reaction. This procedure in our laboratory detects 500 copies of HCV.
Sequencing
This was performed blinded and unmasking was done by another investigator during data analysis. Reverse transcriptase reactions generating sequences were performed with the specific reverse primer HCVE1ROP4a 5′ ACAGGTTTTGGTGAACCCGGTACT using Superscript III (Invitrogen, Gaithersburg, MD). PCR products were generated with primers HCVE1FOP 5′ ACGGCGTGAACTATGCAACAGGGA and primer HCVE1ROP4a in a reaction using Pfu Ultra (Stratagene, La Jolla, CA), generating a 1,216 bp fragment containing HCV E1 and portions of E2 including HVR1. Consensus clones from the PCR were sequenced using DYEnamic ET (Amersham, Piscataway, NJ) and run on an ABI 3730XL (Applied Biosystems, Foster City, CA). Sequences were assembled with Sequencher (Ver4.5, Gene Codes Corp., Ann Arbor, MI) and translated to amino-acids.
Case Definitions
An infant was considered non-infected if he/she was never positive for HCV-RNA and cleared anti-HCV by 18 months of age. Infants were considered to have perinatal mother-infant transmission if they were HCV-RNA positive at any time following birth or had anti-HCV after 18 months of age. An infant was considered to have had transient perinatal HCV infection if he/she was positive for HCV-RNA at the 2- to 4-month visit, but negative for both anti-HCV and HCV-RNA at the 12-month visit. Those children continuing to have HCV-RNA at the 12-month visit were considered to have persistent perinatal HCV infections. Anti-HCV detected in blood of children born to anti-HCV positive mothers 2–6 months after delivery were considered to be maternally acquired antibodies.
Although the children’s visits were scheduled for 2 months after delivery and on their annual birthday thereafter, the actual time varied. Therefore, we calculated clearance of maternally acquired anti-HCV using the actual age. Infants that were HCV-RNA positive at 12 months but cleared their viruses later were considered to have viral clearance, and if they also became anti-HCV negative after 12 months were considered sero-reverted. If anti-HCV disappeared and then re-appeared subsequently, it was considered as decay of maternal antibodies and new production of infantile anti-HCV.
Statistical Analysis and Ethical Review
Chi square or Fisher exact statistics when asymptotic assumptions are not met were used for categorical variables in bivariate analysis. Statistical significance of differences between transmission groups with respect to maternal viral load was assessed using a Kruskal–Walis test. HCV sequences were aligned in MacClade (Ver 4 Sinauer Associates, Sunderland, MA) and genetic distances were calculated using PAUP 4.0 beta10 [Swofford, 2002]. Analysis of amino-acid differences was conducted with each sequence compared to all other sequences. All the analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Possible risk factors of mother-infant transmission of HCV were explored, including mother’s age, appraisal of her health status, Schistosoma infection, parity, stillbirths or abortions, mode of delivery, and who assisted in the deliveries. Predictors related to the child included: gender, birth weight, breast feeding, congenital birth defects, injections, surgical procedures, exposure to jaundiced individuals, and hospital admissions. Initial blood samples were obtained from 52 twin pairs; 35 of these met the criteria for inclusion in the study, we treated each twin as a separate mother–infant pair in our analysis. One pair had HCV-RNA detected: one child having a transient and the other a persistent infection.
This protocol conformed to ethical guidelines of the 1975 Declaration of Helsinki and was approved by the University of Maryland Baltimore’s, Egyptian Ministry of Health and Population’s, and National Liver Institute’s institutional review boards.
RESULTS
A total of 3,457 mother-infant pairs, representing 3,410 different women, consented to participate in the study. Blood samples were available and tested from 2,865 infants seen 2–4 months following birth and 2,017 at the age of 1 and/or 2 years. Of the 1,863 infants having complete baseline and follow-up data, 225 were born to mothers who were both HCV-RNA and anti-HCV positive, 7 were born of mothers who were only HCV-RNA positive, and 97 were born to only anti-HCV positive mothers (Table I).
TABLE I.
Infant Outcomes According to Maternal HCV Status
| Mother’s status | ||||
|---|---|---|---|---|
| Child’s status | HCV RNA positive no. (%) |
Anti-HCV pos. and HCV-RNA neg. no. (%) |
Anti-HCV neg. and HCV-RNA neg. or unknown no. (%)a |
Total no. (%) |
| Total | 232 | 97 | 1,534 | 1,863 |
| Non-infected | 203 (87.5) | 93 (95.9) | 1,534 (100) | 1,830 (98.2) |
| Transient | 14 (6.0) | 4 (4.1) | 0 (0) | 18 (1.0) |
| HCV | ||||
| Persistent | 15 (6.5) | 0 (0) | 0 (0) | 15 (0.8) |
| HCV | ||||
| Prolonged | 8 (3.4) | — | — | 8 (0.4) |
| Cleared | 7 (3.0) | — | — | 7 (0.4) |
pos., positive; neg., negative.
This includes 1,015 who were also HCV-RNA negative and 519 who were not tested for HCV-RNA.
HCV Transmission
HCV-RNA was detected in a total of 33 of the 1,863 infants (Table I). They were classified as:
Transient HCV infection: Among these 18, 14 (6.0%) were born to 232 HCV-RNA positive mothers and 4 (4.1%) to 97 mothers who had HCV antibodies in the absence of detectable HCV-RNA. One of these 4 mothers who delivered a RNA positive infant had detectable viremia at subsequent visits.
Persistent HCV infection: Fifteen (6.5%) infants born to HCV-RNA positive mothers had persistent HCV viremia with 13 having detectable viremia at their 1-year examination. Two children missed their 1-year exam but were both anti-HCV and HCV-RNA positive at 2 years. No infant born of mothers having only anti-HCV had infections that persisted to their first birthday.
Clearance of perinatal HCV infection: Among the 15 children who were HCV-RNA positive at 1–2 years, 7 (46.7%) cleared their HCV-RNA. Six of these 7 children also sero-reverted to anti-HCV negative.
Non-infected children: HCV-RNA was not detected in 203 (87.5%) of children born to HCV-RNA positive mothers and in 93 (95.9%) of infants born to mothers with HCV antibodies without detectable RNA. None of the 1,534 infants born to mothers without anti-HCV had HCV infections; 1,015 of these mothers tested by RT-PCR were also HCV-RNA negative (Table I).
Sequences of Viruses From Mothers and Their Infants
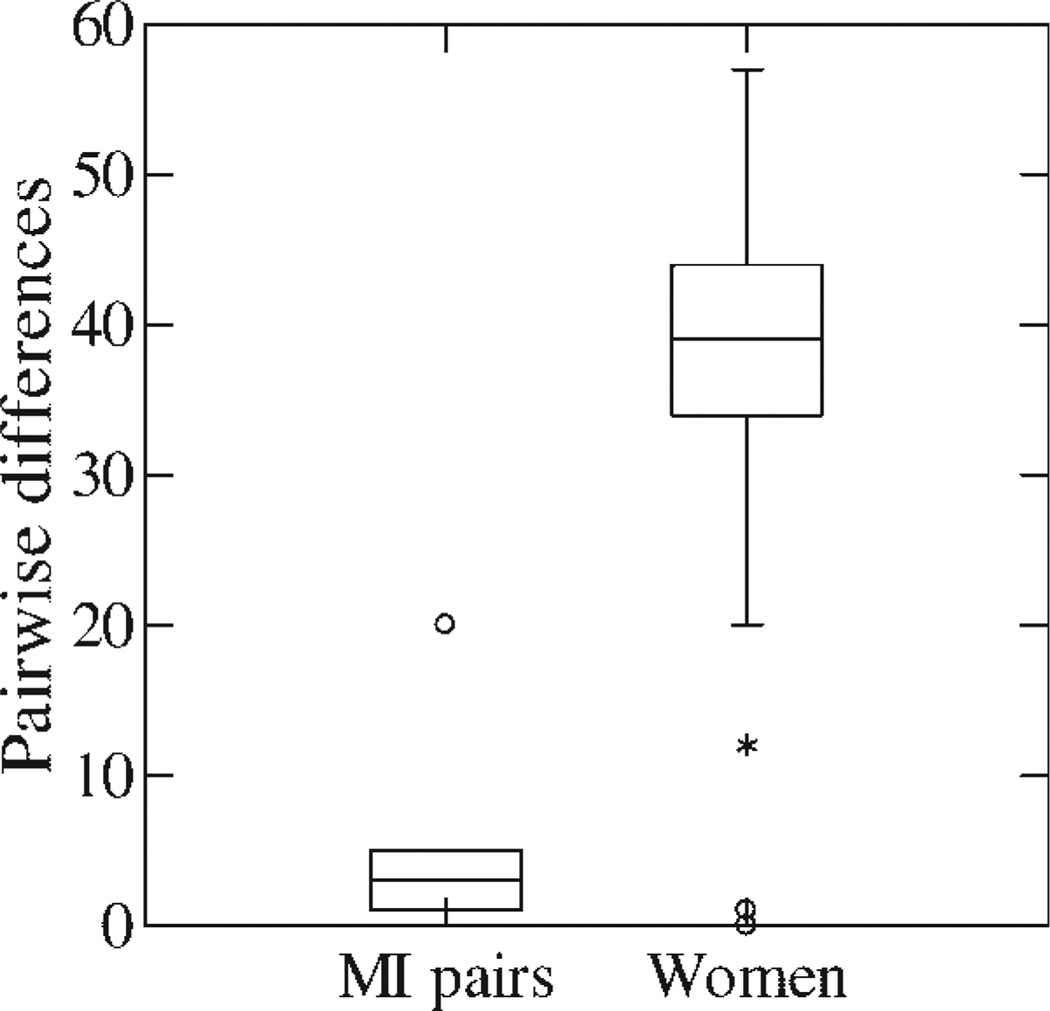
Twenty-seven of the 28 viruses genotyped belonged to genotype-4. Sequences of hypervariable regions of the viruses from the mothers and their infected infants were very similar; much more so than the sequences of the viruses from the pregnant women (P < 0.0001; Fig. 1). Viruses from four infants with infections that persisted to 1 or 2 years differed by 0, 1, 2, and 5 amino-acids from their mothers, while the transiently infected child’s virus differed by 4 amino-acids from his mother. The virus from the child whose infection was detected at his 5-year examination differed by 20 amino-acids from the HCV isolated from his mother during her last trimester of pregnancy. The average distance was 5.3 with a standard deviation of 7.4. In contrast, range of the pair-wise differences between the women averaged 38.9 and had a standard deviation of 7.7. One woman among a pair having only 12 amino-acid differences was the sister and aunt of a mother-infant transmission pair. Only three of 343 pair-wise differences between women were less than 20 amino-acids (Fig. 1).
Fig. 1.
Box and whiskers plot of pair-wise amino acid differences among six mother and infants pairs (MI) and all 343 possible pregnant women (women) pairs. The MI pair (○) with 20 amino acid differences was the 5-year-old child who had probable postnatal transmission from his mother. The two women (*) having 12 amino acid differences were siblings. The amino-acid sequences of the virus isolates will be submitted to GenBank prior to publication.
Anti-HCV Acquisition and Clearance
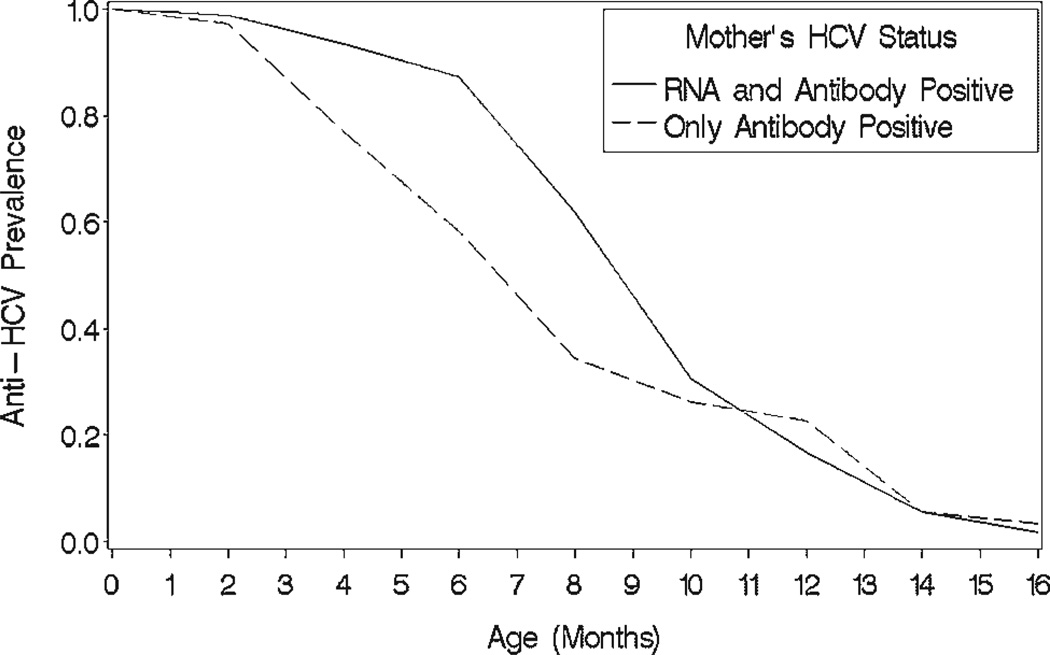
Anti-HCV acquired from their mothers was observed in 79.9% of the 329 infants born to anti-HCV positive mothers at their 2-month visit (Fig. 2). The proportion of anti-HCV positive infants was greater (P < 0.001) when the mother was RT-PCR positive (90.1%) than in infants born of mothers who had only anti-HCV (55.7%).
Fig. 2.
Age related decline in prevalence of hepatitis C antibody in infants who had anti-HCV detected in their initial blood sample according to whether their mothers were positive for both HCV-RNA and anti-HCV or only had anti-HCV.
Among infants never having detectable HCV-RNA the prevalence of anti-HCV declined to 50% in blood samples drawn between 8 and 10 months of age, to 30% at 10–12 months, and to 5% by 12–14 months. It was not present in any blood samples drawn 14–16 months after birth. Among the 18 infants with transient infections, all lost their anti-HCV by 12–14 months. Two infants with persistent perinatal infections lost their antibodies acquired from their mothers by 6 months but produced anti-HCV themselves by their 1-year visit. Another infant lost his anti-HCV by 1 year and subsequently developed his own antibodies by his 2-year visit.
Predictors of HCV Transmission
Infantile and maternal risk factors of transmission were explored using bivariate analysis. Mother’s age or parity, infant’s gender, receiving a blood transfusion, or having an operation or hospitalization, were not statistically significant predictors for either transient or persistent infections. Normal vaginal delivery was borderline protective and physician assisted deliveries in health care facilities trended towards risk for infection (Table II).
TABLE II.
Selected Risk Factors of HCV Transmission in Infants Related to Their Infection Status
| Risk factors/infection status | Non-infected, no. (%) | Transient, no. (%) | Persistent, no. (%) | P-value |
|---|---|---|---|---|
| Maternal viremia (mean ± SD) | 0.23 × 106 ± 0.30 × 106 | 0.01 × 106 ± 0.02 × 106 | 1.55 × 106 ± 2.37 × 106 | 0.006b |
| Male | ||||
| Yes | 159 (89.3) | 10 (5.6) | 9 (5.1) | 0.89a |
| No | 136 (90.7) | 8 (5.3) | 6 (4.0) | |
| Hospital delivery | ||||
| Yes | 155 (86.6) | 13 (7.3) | 11 (6.2) | 0.08a |
| No | 141 (94.0) | 5 (3.3) | 4 (2.7) | |
| Physician-assisted delivery | ||||
| Yes | 156 (86.7) | 13 (7.2) | 11(6.1) | 0.09a |
| No | 139 (93.9) | 5 (3.4) | 4 (2.7) | |
| Normal vaginal delivery | ||||
| Yes | 261(90.3) | 13 (4.5) | 15 (5.2) | 0.05c |
| No | 35 (87.5) | 5 (12.5) | 0 (0.0) | |
| Exclusive breast-feeding | ||||
| Yes | 216 (92.3) | 8 (3.4) | 10 (4.3) | 0.03a |
| No | 79 (84.0) | 10 (10.6) | 5 (5.3) |
Pearson Chi-Square.
Kruskal–Wallis test.
Fisher’s exact test.
The probability of not being infected was higher (OR = 2.28, 95% CI = 1.10, 4.73; P = 0.03) in exclusively breast-fed infants (92.3%) than in infants who received other sources of nourishment (84.0%). Maternal viral loads were higher (P = 0.013) in infants who had persistent infections (1.55 × 106 ± 2.37 × 106) than in infants having transient or no infections (0.19 × 106 ± 0.29 × 106); and the maternal viral load (0.01 × 106 ± 0.02 × 106) in infants with transient infections was 155-times lower than in mothers of infants with persistent infections (P = 0.01; Table II). Three risk factors were marginally associated with delivery, that is, physician assisted (P = 0.09) and surgically assisted (P = 0.05), and hospital-based (P = 0.08) deliveries, suggesting some infants may have been infected during delivery. Five infants that may have been infected during surgical deliveries all had transient infections.
DISCUSSION
Our finding that 15 (6.5%) of children born of mothers who were HCV-RNA positive were infected at 1 or 2 years of age is compatible with the usual reported perinatal infection rate of between 4% and 8% [Zanetti et al., 1995; Resti et al., 1998; Zanetti et al., 1998; Gibb et al., 2000; Ceci et al., 2001; Tajiri et al., 2001; Ferrero et al., 2003; Mast et al., 2005; NEPHCV, 2005a]. In the 329 infants born to anti-HCV- and/or HCV-RNA-positive mothers without known HIV or other factors that increase perinatal transmission, 10% (including four infants with transient infections whose mothers had HCV antibodies without detectable RNA) were positive for both anti-HCV and HCV-RNA 2–4 months following birth. Over half of the infants with virus and antibody during the first months following birth cleared their infections by 1 year. Thus, using our criteria, 18 (5.5%) had transient infections and 15 (4.6%) had persistent perinatal HCV infections. In addition, almost half of these 15 cases cleared HCV-RNA in the next year or two and 6 of 7 children who cleared their viruses after the first year also sero-reverted to anti-HCV negative.
Criteria for perinatal transmission of HCV in the European Pediatric HCV Network (EPHN) multicenter trial of 1,787 mother-child pairs was: two or more positive HCV-RNA PCR test results and/or anti-HCV antibody positive after 18 months of age [NEPHCV, 2005a,b]. They did not include a transient infection category. Their study focused upon risk factors for infection and did not show data about HCV-RNA positive children that did not meet their criteria for infection. However, they had 279 (16%) children classified as “indeterminate infection status” who did not meet their criteria for either infected or non-infected, and classified infants with less than two positive HCV-RNA results as non-infected [NEPHCV, 2005a]. Members of the EPHN group subsequently reported on accuracy of HCV-RNA PCR testing for the diagnosis of vertically acquired HCV infections [Polywka et al., 2006]. Since they used the presence of anti-HCV at 18 months as their “gold standard” for mother-to-infant transmission, it is not surprising they concluded the test was not reliable during the first few months of life. Sensitivity and positive predictive value of the PCR testing was poor since children who had positive tests for HCV-RNA without persistence of HCV antibodies and RNA were considered to be false positives.
Another article published in the same journal issue reported on risk factors and natural history of perinatal HCV infection in 244 infants born of HCV positive mothers in Houston and Honolulu [Mast et al., 2005]. Nine (3.7%) infants met their criteria for infection: “serum was found to be positive for HCV-RNA on at least two follow-up visits or was found to be anti-HCV positive at age ≥24 months.” They reported 57 (38%) of 151 uninfected infants, compared with 5 (62.5%) of 8 infected infants, had HCV-RNA in their cord blood; and 6 of 81 uninfected infants, compared with 4 of 6 infected infants, had HCV-RNA in their initial peripheral blood samples. None of their uninfected infants had HCV-RNA detected in subsequent blood samples and all their infected infants were HCV-RNA positive at 2 months. Three of their nine infected infants appeared to clear their infections between 10 and 20 months, which is similar to our finding viral clearance after 12 months in 7 of 15 perinatal infected infants. They noted, as we did in some infants, passively transferred anti-HCV was lost in four infected children and the anti-HCV returned in response to infection [Mast et al., 2005].
Ketzinel-Gilad et al. [2000] reported “transient” transmission of HCV in 5 of 23 infants born of 22 HCV infected mothers. Anti-HCV was detected in all the newborns shortly after birth but it disappeared or diminished to very low levels by 7 months of age. HCV-RNA detected in five infants on the second day of life was undetectable at 6 months. One of these infants had HCV-RNA in his cord blood and peripheral blood samples taken at 2 days and 2 months before becoming negative on three subsequent occasions. Sequence analysis performed upon HCV isolated from this child and his mother confirmed the similarity of the viruses and subsequent sequencing of the infant’s virus isolated at 2 months of age suggested it had a low replication rate [Ketzinel-Gilad et al., 2000].
So, what is the rate of perinatal transmission of HCV from HCV-infected mothers to their infants? Herein, we propose that a higher proportion of infants born of HCV-infected mothers have infections and then clear their infections than is generally reported. In our study this was 12.5% in infants whose mothers were HCV-RNA positive, but it may have been greater if we had sampled the infants earlier and more frequently. When Ketzinel-Gilad et al. [2000] did this, they detected transient HCV-RNA in 22%. Ceci et al. [2001] reported during a 2-year follow-up of 60 HCV-infected infants born of HCV-RNA positive mothers that 75% (decreasing from 13.3% to 3.3%) cleared their infections. Ruiz-Extremera et al. [2000] reported 7 of 8 Spanish infants born of anti-HCV positive mothers followed for an average of 29 months had detectable HCV-RNA and anti-HCV, both of which cleared. Others reported that 2 of 3 infants with perinatal HCV cleared their viremia by 2 months of age [Sasaki et al., 1997].
The results reported herein could be caused by cross-contamination or laboratory error due to false-positive RT-PCR testing for HCV-RNA. This is unlikely to explain all the results because: (1) data and samples from the mothers and their tests were performed during their last trimester of pregnancy. The data and samples from the children were taken and their tests were performed after the children were born. (2) In most cases there was more than one positive blood sample; (3) none of 1,534 children born of mothers who did not have HCV antibodies had HCV infections; (4) only 4 (4.1%) of children born of the 97 mothers who had HCV antibody in the absence of HCV-RNA had detectable viremia, and all of these were transient infections; (5) there was a much higher HCV-RNA viral titer in mothers who delivered infants having persistent infections than those who delivered infants having no or transient infections; and (6) analysis of five mothers’ and their infected infants’ (including one with a transient infection) viruses confirmed the viral sequences was nearly identical, indicating the mothers were the source of their infants’ viruses.
Although most studies have only reported transmission of HCV to infants from mothers who have detectable HCV-RNA[Resti et al., 1998; Ceci et al., 2001; Tajiri et al., 2001; Ferrero et al., 2003; Mast et al., 2005], a large multicenter study reported five infections in infants born of mothers who only had HCV antibodies [Resti et al., 2002]. This could be due to a fluctuating viremia that at the time of testing was below the level of detection. This could explain our finding of transient infections in children whose mothers had HCV antibodies in the absence of RNA and is supported by the very low viral loads in mothers of these infants with transient infections.
Previous investigators have used persistence of anti-HCV for greater than 18–24 months in the absence of detectable HCV-RNA as criteria for mother-to-infant transmission of HCV [Resti, 1999; Gibb et al., 2000; Resti et al., 2003; Mast et al., 2005]. The reasoning behind this, that is, viral levels were fluctuating and below the level of detection when the blood samples were drawn, is plausible. It is unlikely even in breast-feeding infants that passively transferred antibodies from the mother could persist for over a year in the infant. Gibb et al. [2000] reported that among infants born of 441 HCV-infected mothers, half of uninfected infants cleared their anti-HCV by 8 months and 95% were anti-HCV negative at 13 months. Others reported that anti-HCV transmitted passively disappears in infants by 6 or 8 months [Ni et al., 1994; Ketzinel-Gilad et al., 2000; Ferrero et al., 2003], and three of our- and four of Mast’s-cases who were infected perinatally lost anti-HCV transferred passively from their mothers by 6–12 months before making their own anti-HCV [2005]. All these results suggest some infants having anti-HCV persisting longer than 12 months could be undetected infected cases with low and transient levels of viremia.
Infants infected transiently usually cleared their HCV antibodies concomitantly with their HCV-RNA. Others reported that infants cleared their viruses without having persistent antibodies [Ni et al., 1994; Sasaki et al., 1997; Ketzinel-Gilad et al., 2000], which could have several explanations. One of these could be the passage of neutralizing antibody along with low titers of viral particles from mothers to their newborns clears the infection and either aborts or diminishes the child’s antibody production [Zibert et al., 1995]. Children being more likely to clear HCV infections than adults is supported by this paper’s finding that only 8 of 33 children sampled in years 2 and/or 3 continued to be HCV-RNA positive [Ruiz-Extremera et al., 2000; NEPHCV, 2005b]. The 76% (25 of 33) HCV clearance rate was much greater than the 23% clearance among the children in the EPHN multicenter project [NEPHCV, 2005b]. However, these authors had different criteria for classifying initial infection and only considered clearance occurring in children who remained infected until their first birthday. Using their criteria about half the children in this report with HCV-RNA cleared their infections by their third birthday.
Genotype-4 was present in 96% of the HCV isolated from the mothers and their infants which is higher than the 80–90% prevalence in the entire country [Abdel-Hamid et al., 2007]. This very high prevalence of genotype-4 can be explained by extensive transmission of this genotype in these and other rural Egyptian villages during campaigns to control schistosomiasis using intravenous chemotherapy [Frank et al., 2000; Tanaka et al., 2004]. All five mother-infant pairs with virus isolated during the perinatal period had more than 98% amino-acid similarities. The similarity was 93% for the pair in which the child was 5 years old when his HCV-RNA was detected. This could represent maternal to-child postnatal household transmission [Magder et al., 2005; Mohamed et al., 2005]. There was also 94% nucleotide similarity between two mothers who were sisters, which could also represent interfamilial transmission. The pair-wise differences between mothers’ and their infants’ amino-acids in the hypervariable regions were much less than the pair-wise differences between different women (P < 0.0001; Fig. 1). Therefore, the conclusion was that the mothers were the source of their children’s viruses, including the child with the transient infection.
Although this is probably the largest single site study of HCV perinatal mother-to-infant transmission, the sample size is inadequate for statistically identifying some of potential risk factors for infant infection. It was unable to show that most maternal or infant characteristics, including the infant’s gender, were a risk for infection. Some studies reported that elective delivery by caesarian section may protect the infant from infection during delivery [Gibb et al., 2000], while others have not found this relationship [Mast et al., 2005]. Too few infants born of HCV-infected mothers had caesarian sections for evaluation but a procedure used locally for complicated deliveries, ventouse suction extraction, was associated with an increased risk of infection. Infants having complicated deliveries by physicians in a health care facility tending to be infected suggests they were iatrogenic induced infections. Another observation, the lower odds of viremia in exclusively breast-fed children, suggest HCV-specific immunoglobulins in colostrum and breast milk might protect against HCV transmission during infancy [Hanson and Korotkova, 2002; Van de Perre, 2003]. However, children who received other sources of nourishment other than breast milk were also more likely to have the above risk factors. The effect of breast-feeding upon the infants’ infection status has been uncertain and variable [Sasaki et al., 1997; Gibb et al., 2000; Ketzinel-Gilad et al., 2000; Mast et al., 2005; NEPHCV, 2005a].
Maternal viral load, similar to other reports [Ruiz-Extremera et al., 2000; Ceci et al., 2001; Ferrero et al., 2003; Steininger et al., 2003], was higher (about 150-times greater) in infants that had persistent infections than in both children who had no- or transient-infections. This suggests infants with transient infections were exposed to low levels of HCV and agrees with reports that chimpanzees challenged with low doses of the virus often clear their infections [Shata et al., 2003]. Investigating how infants clear HCV transmitted from their mothers could provide useful information about protective immunity to HCV.
ACKNOWLEDGMENTS
We thank the physicians and staff of the Community Health Units in the three villages and the deans and staff of the National Liver Institute who assisted in collecting data and samples. Dr. Robert Purcell and Dr. Suzanne Emerson kindly critiqued the manuscript. Our good friend, the late Dr. Shaker Narooz, was instrumental in all aspects of data collection and interactions with the mothers and infants in the CHU.
Grant sponsor: National Institutes of Health; Grant numbers: NIAID/NICHD:1U01 HD39164, NIAID: U01 AI-58372; Grant sponsor: Wellcome Trust-Burroughs Wellcome Fund Infectious Disease Initiative; Grant number: RO1 DA13324; Grant sponsor: United States Aid for International Development; Grant number: 263-G-00-96-00043-00.
REFERENCES
- Abdel-Aziz F, Habib M, Mohamed MK, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Thomas D, Fix AD, Strickland GT, Anwar W, Sallam I. Hepatitis C virus (HCV) infection in a community in the Nile Delta: Population description and HCV prevalence. Hepatology. 2000;32:111–115. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- Abdel-Hamid M, Edelman DC, Highsmith WE, Constantine NT. Optimization, assessment, and proposed use of a direct nested reverse transcription-polymerase chain reaction protocol for the detection of hepatitis C virus. J Hum Virol. 1997;1:58–65. [PubMed] [Google Scholar]
- Abdel-Hamid M, El-Daly M, Molnegren V, El-Kafrawy S, Abdel-Latif S, Esmat G, Strickland GT, Loffredo C, Albert J, Widell A. Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J Gen Virol. 2007;88:1526–1531. doi: 10.1099/vir.0.82626-0. [DOI] [PubMed] [Google Scholar]
- Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, Impedovo L, Ierardi E, Mastroianni M, Bettocchi S, Selvaggi L. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: A 24-month prospective study. J Pediatr Gastroenterol Nutr. 2001;33:570–575. doi: 10.1097/00005176-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Lungaro P, Bruzzone BM, Gotta C, Bentivoglio G, Ragni N. Prospective study of mother-to-infant transmission of hepatitis C virus: A 10-year survey (1990–2000) Acta Obstet Gynecol Scand. 2003;82:229–234. doi: 10.1034/j.1600-0412.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W, Sallam I. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, Cafferkey M, Butler K. Mother-to-child transmission of hepatitis C virus: Evidence for preventable peripartum transmission. Lancet. 2000;356:904–907. doi: 10.1016/s0140-6736(00)02681-7. [DOI] [PubMed] [Google Scholar]
- Group The Global Burden of Hepatitis C Working. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT, Fix AD, Sallam I. Hepatitis C virus infection in a community in the Nile Delta: Risk factors for seropositivity. Hepatology. 2001;33:248–253. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7:275–281. doi: 10.1016/s1084-2756(02)90124-7. [DOI] [PubMed] [Google Scholar]
- Ketzinel-Gilad M, Colodner SL, Hadary R, Granot E, Shouval D, Galun E. Transient transmission of hepatitis C virus from mothers to newborns. Eur J Clin Microbiol Infect Dis. 2000;19:267–274. doi: 10.1007/s100960050474. [DOI] [PubMed] [Google Scholar]
- Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:S30–S34. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- Magder LS, Fix AD, Mikhail NN, Mohamed MK, Abdel-Hamid M, Abdel-Aziz F, Medhat A, Strickland GT. Estimation of the risk of transmission of hepatitis C between spouses in Egypt based on seroprevalence data. Int J Epidemiol. 2005;34:160–165. doi: 10.1093/ije/dyh370. [DOI] [PubMed] [Google Scholar]
- Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, Alter MJ. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–1889. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- Mohamed MK, Abdel-Hamid M, Mikhail NN, Abdel-Aziz F, Medhat A, Magder LS, Fix AD, Strickland GT. Intrafamilial transmission of hepatitis C in Egypt. Hepatology. 2005;42:683–687. doi: 10.1002/hep.20811. [DOI] [PubMed] [Google Scholar]
- Network European Paediatric Hepatitis C Virus. A significant sex–but not elective cesarean section–effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis. 2005a;192:1872–1879. doi: 10.1086/497695. [DOI] [PubMed] [Google Scholar]
- Network European Paediatric Hepatitis C Virus. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005b;41:45–51. doi: 10.1086/430601. [DOI] [PubMed] [Google Scholar]
- Ni YH, Lin HH, Chen PJ, Hsu HY, Chen DS, Chang MH. Temporal profile of hepatitis C virus antibody and genome in infants born to mothers infected with hepatitis C virus but without human immunodeficiency virus coinfection. J Hepatol. 1994;20:641–645. doi: 10.1016/s0168-8278(05)80353-8. [DOI] [PubMed] [Google Scholar]
- Polywka S, Pembrey L, Tovo PA, Newell ML. Accuracy of HCV-RNA PCR tests for diagnosis or exclusion of vertically acquired HCV infection. J Med Virol. 2006;78:305–310. doi: 10.1002/jmv.20540. [DOI] [PubMed] [Google Scholar]
- Resti M. Mother-to-infant transmission of hepatitis C virus. Ital J Gastroenterol Hepatol. 1999;31:489–493. [PubMed] [Google Scholar]
- Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, Vierucci A. Mother to child transmission of hepatitis C virus: Prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. Br Med J. 1998;317:437–441. doi: 10.1136/bmj.317.7156.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resti M, Azzari C, Galli L, Zuin G, Giacchino R, Bortolotti F, Marcellini M, Moriondo M, de Martino M, Vierucci A. Maternal drug use is a preeminent risk factor for mother-to-child hepatitis C virus transmission: Results from a multicenter study of 1372 mother-infant pairs. J Infect Dis. 2002;185:567–572. doi: 10.1086/339013. [DOI] [PubMed] [Google Scholar]
- Resti M, Bortolotti F, Vajro P, Maggiore G. Guidelines for the screening and follow-up of infants born to anti-HCV positive mothers. Dig Liver Dis. 2003;35:453–457. doi: 10.1016/s1590-8658(03)00217-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Extremera A, Salmeron J, Torres C, De Rueda PM, Gimenez F, Robles C, Miranda MT. Follow-up of transmission of hepatitis C to babies of human immunodeficiency virus-negative women: The role of breast-feeding in transmission. Pediatr Infect Dis J. 2000;19:511–516. doi: 10.1097/00006454-200006000-00004. [DOI] [PubMed] [Google Scholar]
- Saleh DA, Shebl F, Abdel-Hamid M, Narooz S, Mikhail N, El-Batanony M, El-Kafrawy S, El-Daly M, Sharaf S, Hashem M, El-Kamary S, Magder LS, Stoszek SK, Strickland GT. Incidence and risk factors for hepatitis C infection in a cohort of women in rural Egypt. Trans RSoc Trop Med Hyg. 2008;102:921–928. doi: 10.1016/j.trstmh.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Matsui A, Momoi M, Tsuda F, Okamoto H. Loss of circulating hepatitis C virus in children who developed a persistent carrier state after mother-to-baby transmission. Pediatr Res. 1997;42:263–267. doi: 10.1203/00006450-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Shata MT, Tricoche N, Perkus M, Tom D, Brotman B, McCormack P, Pfahler W, Lee DH, Tobler LH, Busch M, Prince AM. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–616. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger C, Kundi M, Jatzko G, Kiss H, Lischka A, Holzmann H. Increased risk of mother-to-infant transmission of hepatitis C virus by intrapartum infantile exposure to maternal blood. J Infect Dis. 2003;187:345–351. doi: 10.1086/367704. [DOI] [PubMed] [Google Scholar]
- Stoszek SK, Abdel-Hamid M, Narooz S, El Daly M, Saleh DA, Mikhail N, Kassem E, Hawash Y, El Kafrawy S, Said A, El Batanony M, Shebl FM, Sayed M, Sharaf S, Fix AD, Strickland GT. Prevalence of and risk factors for hepatitis C in rural pregnant Egyptian women. Trans R Soc Trop Med Hyg. 2006;100:102–107. doi: 10.1016/j.trstmh.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Strickland GT. Liver disease in Egypt: Hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43:915–922. doi: 10.1002/hep.21173. [DOI] [PubMed] [Google Scholar]
- Swofford DL. Phylogenetic analysis using parsimony (*and other methods) Sunderland, Massachusetts: Sinauer Associates; 2002. PAUP*. [Google Scholar]
- Tajiri H, Miyoshi Y, Funada S, Etani Y, Abe J, Onodera T, Goto M, Funato M, Ida S, Noda C, Nakayama M, Okada S. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10–14. doi: 10.1097/00006454-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Katsume A, Tanaka T, Abe A, Inoue K, Tsukiyama-Kohara K, Kawaguchi R, Tanaka S, Kohara M. Real-time detection system for quantification of hepatitis C virus genome. Gastroenterology. 1999;116:636–642. doi: 10.1016/s0016-5085(99)70185-x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Agha S, Saudy N, Kurbanov F, Orito E, Kato T, Abo-Zeid M, Khalaf M, Miyakawa Y, Mizokami M. Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol. 2004;58:191–195. doi: 10.1007/s00239-003-2541-3. [DOI] [PubMed] [Google Scholar]
- Egypt: Epidemiological Fact Sheet on HIV and AIDS; Core data on epidemiology and response; 2008. UNAIDA/WHO, Working Group on Global HIV/AIDA and STI Surveillance; p. 7. http://www.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_EG.pdf. [Google Scholar]
- Van de Perre P. Transfer of antibody via mother’s milk. Vaccine. 2003;21:3374–3376. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- Zanetti AR, Tanzi E, Paccagnini S, Principi N, Pizzocolo G, Caccamo ML, D’Amico E, Cambie G, Vecchi L. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet. 1995;345:289–291. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]
- Zanetti AR, Tanzi E, Romano L, Zuin G, Minola E, Vecchi L, Principi N. A prospective study on mother-to-infant transmission of hepatitis C virus. Intervirology. 1998;41:208–212. doi: 10.1159/000024938. [DOI] [PubMed] [Google Scholar]
- Zibert A, Schreier E, Roggendorf M. Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology. 1995;208:653–661. doi: 10.1006/viro.1995.1196. [DOI] [PubMed] [Google Scholar]