Abstract
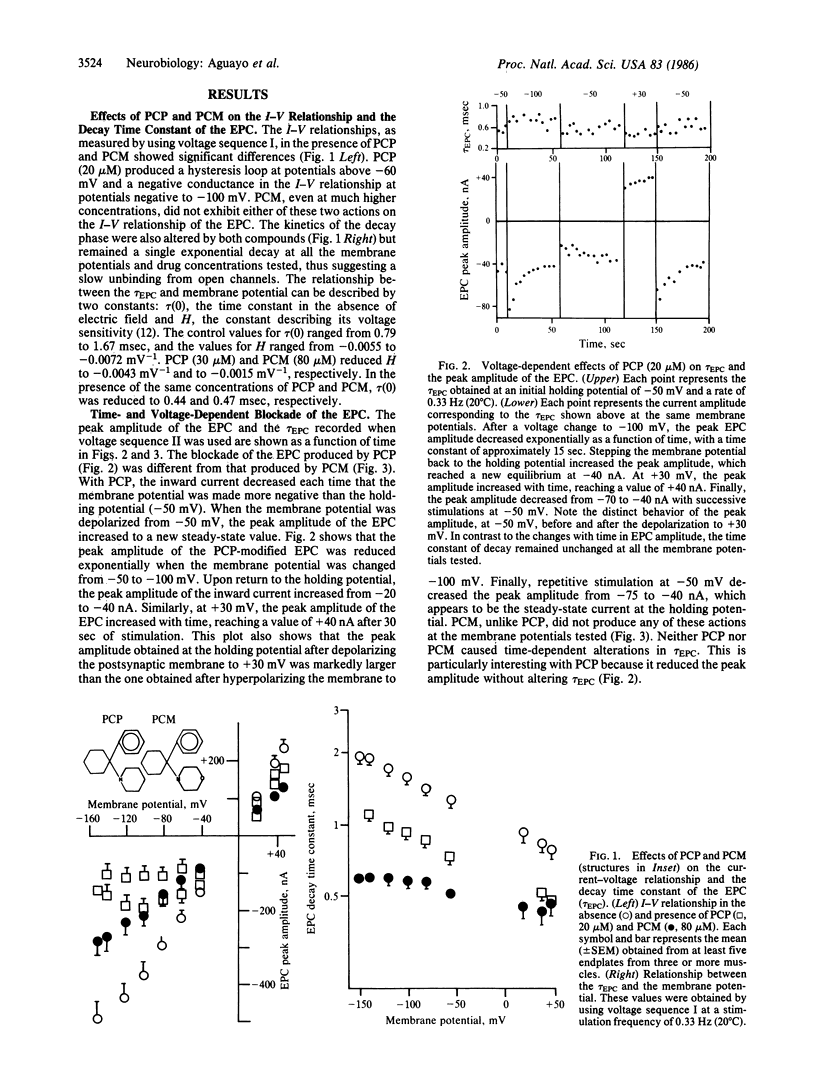
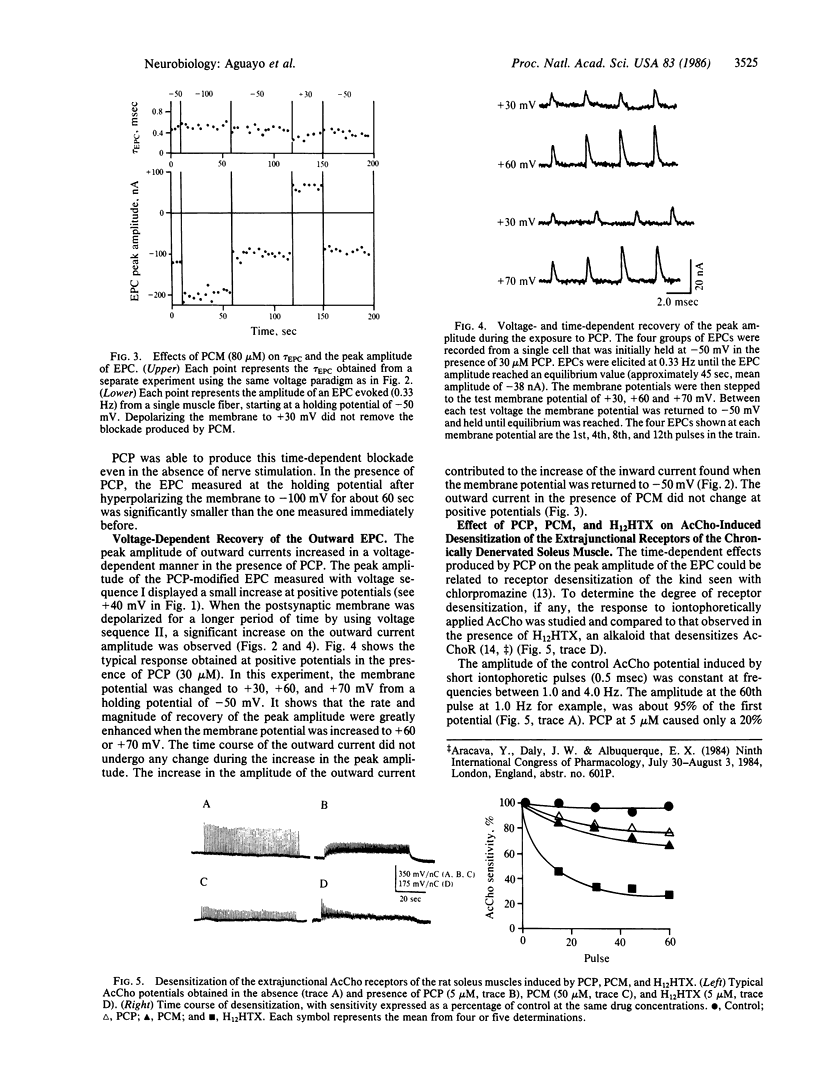
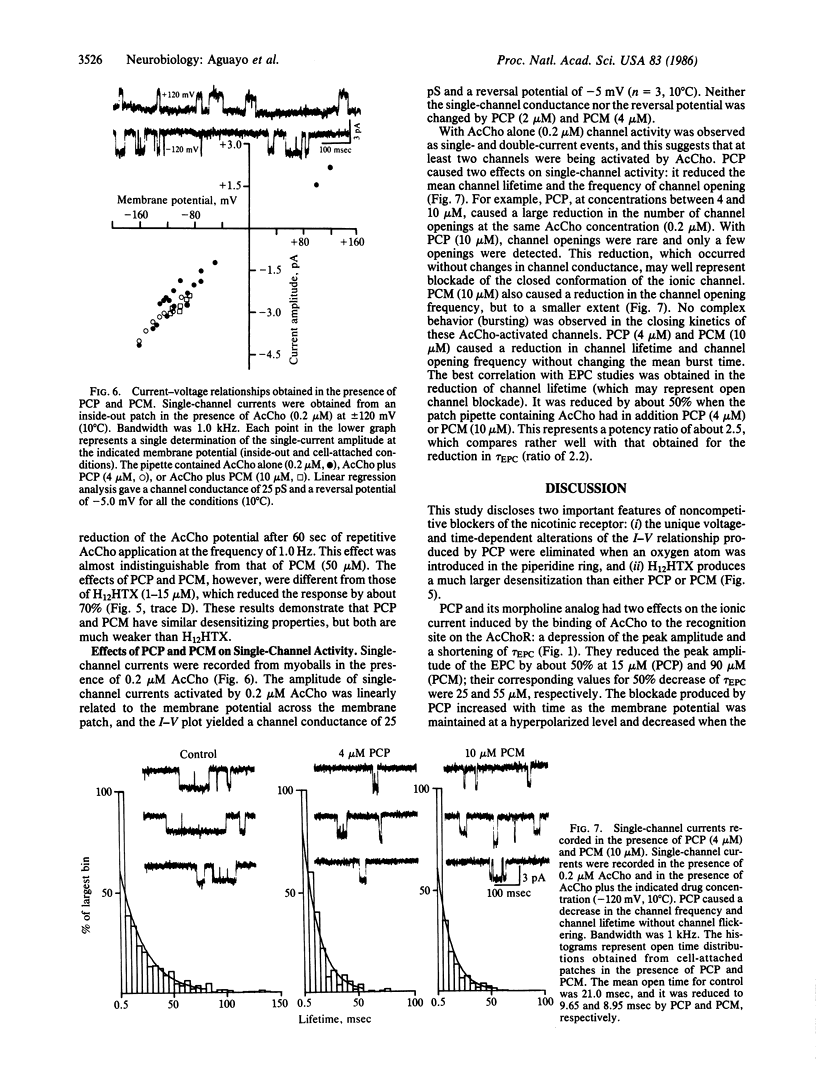
The actions of phencyclidine [1-(1-phenylcyclohexyl)piperidine, PCP] and its morpholine analog [1-(1-phenylcyclohexyl)morpholine, PCM] on ionic currents of nicotinic acetylcholine receptors were studied at the neuromuscular junction of frog skeletal muscle and on embryonic rat muscle cells in tissue culture. PCP and PCM reduced the peak amplitude and the decay time constant of the endplate current (EPC). PCP produced a voltage-dependent curvature and a time-dependent hysteresis loop at negative potentials (at potentials from -50 to -150 mV). In contrast, PCM caused a depression of EPC peak amplitude, but the current-voltage relationship (+60 to -150 mV) remained linear. When PCP-modified EPCs were elicited in trains at hyperpolarized potentials the amplitudes of successive events were progressively decreased and the magnitude of the decrease was dependent on the level of hyperpolarization. At positive potentials the process was reversed; the amplitude increased with successive stimulations. The EPC decayed exponentially in the presence of PCP and PCM, with a shortened time constant of decay that was less dependent on membrane potential than control. PCP and PCM caused only a 20% decrease of the amplitude of the iontophoretically evoked acetylcholine potential, which was significantly different from that induced by the desensitizing alkaloid perhydrohistrionicotoxin. Both PCP and PCM reduced by 50% the mean channel open time obtained from rat myoballs, giving a potency ratio for PCP to PCM of 2.5. This relative potency was correlated with that obtained for the reduction in the decay time constant of the EPC (ratio = 2.2). The effects of PCP on the peak amplitude of the EPC seem to be related to a conformational change of the acetylcholine receptor occurring before channel activation and not to a receptor desensitization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Ikeda S. R., Brookes N., Pascuzzo G. J., Rickett D. L., Albuquerque E. X. The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. II. Patch clamp studies. Mol Pharmacol. 1984 Jan;25(1):102–112. [PubMed] [Google Scholar]
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E. Sites of action of phencyclidine. II. Interaction with the ionic channel of the nicotinic receptor. Mol Pharmacol. 1980 Sep;18(2):167–178. [PubMed] [Google Scholar]
- Carp J. S., Aronstam R. S., Witkop B., Albuquerque E. X. Electrophysiological and biochemical studies on enhancement of desensitization by phenothiazine neuroleptics. Proc Natl Acad Sci U S A. 1983 Jan;80(1):310–314. doi: 10.1073/pnas.80.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi A. T., Miller E. R., Murphy D. L., Eldefrawi M. E. [3H]Phencyclidine interactions with the nicotinic acetylcholine receptor channel and its inhibition by psychotropic, antipsychotic, opiate, antidepressant, antibiotic, antiviral, and antiarrhythmic drugs. Mol Pharmacol. 1982 Jul;22(1):72–81. [PubMed] [Google Scholar]
- Eldefrawi M. E., Aronstam R. S., Bakry N. M., Eldefrawi A. T., Albuquerque E. X. Activation, inactivation, and desensitization of acetylcholine receptor channel complex detected by binding of perhydrohistrionicotoxin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2309–2313. doi: 10.1073/pnas.77.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Kuba K., Albuquerque E. X., Daly J., Barnard E. A. A study of the irreversible cholinesterase inhibitor, diisopropylfluorophosphate, on time course of end-plate currents in frog sartorius muscle. J Pharmacol Exp Ther. 1974 May;189(2):499–512. [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]


