Abstract
Follicular fluid is an important environment for oocyte development, yet current knowledge regarding its in vivo oxidant and antioxidant levels remains limited. Examining follicular fluid oxidants and antioxidants will improve understanding of their changes in vivo and contribute to optimization of in vitro maturation conditions. The aim of our study was to consider select markers, namely catalase (CAT) enzyme activity, total antioxidant capacity (TAC), and hydrogen peroxide (H2O2) in follicular fluid samples (n=503) originating from bovine antral follicles. We measured the dynamic changes in two relevant antioxidant measures and one reactive oxygen species (ROS) through stages of bovine follicular development and the estrous cycle.
CAT activity and H2O2 levels decreased significantly as follicle size increased, while TAC increased significantly as follicle size increased. Lower TAC and higher H2O2 in small follicles suggest increased ROS in the initial stages of folliculogenesis. Because CAT levels are highest in follicular fluid of small follicles in the setting of an overall low TAC, CAT may represent a dominant antioxidant defense in the initial stages of folliculogenesis. Future studies must focus on other reactive oxygen species and their various scavenger types during antral folliculogenesis.
Introduction
Reactive oxygen species (ROS) are powerful oxidants and physiological byproducts of metabolically active gametes and embryos (Agarwal and Allamaneni 2004). At low concentrations, ROS play important physiologic roles in vitro such as promoting the developmental competence of oocytes and regulating the rate of pre-implantation embryo development (Blondin et al. 1997; Yamashita et al. 1997; Combelles et al. 2009). However, ROS abundance can have deleterious effects on cellular function by inducing oxidative damage of intracellular components and inducing apoptosis (Guerin et al. 2001; Lopes et al. 1998; Yang et al. 1998). Oxygen radicals, such as hydrogen peroxide (H2O2) and the hydroxide radical (OH), comprise one group of highly reactive oxygen species (Sharma et al. 1999). In the follicular fluid of developing oocytes, enzymatic antioxidants, such as superoxide dismutase and catalase, work in concert with non-enzymatic antioxidants, such as vitamin C and taurine, presumably to counter the potentially harmful effects of ROS (Carbone et al. 2003; Sabatini et al. 1999; Shiotani et al. 1991).
The balance between ROS and antioxidants may have an important role in reproductive processes such as folliculogenesis. A Graafian follicle contains its own potential sources of ROS, including large numbers of macrophages, neutrophils, and metabolically active granulosa cells (Bertout et al. 2004). Current evidence suggests that ROS and/or antioxidants may be some of the factors influencing folliculogenesis, including the process of background follicular atresia (Valdez et al. 2005; Zhang et al. 2006). Previous reports have documented the presence of various antioxidant enzymes within follicular fluid, as well as the variation in oocyte antioxidant enzyme mRNA levels with increasing follicle size and either in vitro or in vivo maturation environments (Angelucci et al. 2006; El Mouatassim et al. 1999; Lonergan et al. 2003). In a recent study of bovine follicular fluid, Combelles et al found that levels of the antioxidant enzyme superoxide dismutase (SOD) decreased as the antral follicles matured from small follicles to larger follicles (Combelles et al. 2010). In another study using the porcine model, Basini et al. demonstrated that ROS markers did not increase as follicular size increased, which they proposed might be due to an effective increase in antioxidant enzyme activity (Basini et al. 2008). However, their finding could not be explained by an increase in catalase (CAT) activity alone and perhaps, an increase in follicular fluid estrogen levels may provide additional antioxidant support. Furthermore, there is no consensus in the literature regarding the effects of ROS and oxidative stress on clinical outcomes; conflicting reports exist with oxidative markers positively correlated with either in vitro fertilization and pregnancy rates (Attaran et al. 2000; Pasqualotto et al. 2004; Sabatini et al. 1999) or reduced embryo quality and diminished embryo formation (Das et al. 2006; Seino et al. 2002). Further insight into the oxidants and antioxidants of follicular fluid in vivo should thus enhance our understanding of the impact of these oxidative species on fertility outcomes.
Past reports tested many antioxidant supplementation protocols, but since there must be a balance of oxidant and antioxidant molecules in the follicular fluid, simply adding antioxidants may not be sufficient to combat ROS damage (Guerin et al. 2001). With enhanced knowledge of the native antioxidant capacity of follicular fluid, we may be better able to mimic in vivo conditions in vitro. As oxidative insults are likely to be higher during in vitro maturation (IVM) compared to in vivo maturation, defining the in vivo antioxidant profile of follicular fluid may aid in future development of antioxidant supplements for IVM culture systems and assisted reproductive technologies. Our current grasp of the antioxidant profile of follicular fluid is limited, notably in relation to follicle size and estrous stage and more broadly at times when the environment of the developing oocytes changes dynamically. To our knowledge, only the recent study by Combelles et al. (2010) has profiled any antioxidant levels in the bovine model, which has been established as a fitting model with which to study human folliculogenesis (Baerwald et al. 2003; Campbell et al. 2003; Ireland et al. 2000). However, additional antioxidant and oxidant parameters remain to be analyzed. Given that SOD protein and activity levels decreased with increased follicle size (Combelles et al., 2010) and SOD catalyzes the dismutation of superoxide into H2O2, it is now essential not only to examine CAT (in turn neutralizing H2O2 into harmless species) but also to measure H2O2 directly. Furthermore, SOD and CAT represent enzymatic antioxidants, and it remains unknown whether all types of antioxidant measures behave similarly during folliculogenesis, thus leading us to examine the total antioxidant capacity (TAC) together with CAT in the same follicular fluid samples. Our study tested the hypothesis that antioxidant levels, as measured by CAT and TAC levels, increased with progressive stages of bovine follicular development and that ROS production, as measured by H2O2, decreased with progressive stages of bovine follicular development. We further predicted differences in antioxidant and ROS levels in accordance with the stages of the estrous cycle.
Materials and methods
Collection of bovine antral follicles and follicular fluid
The bovine ovaries were collected from naturally cycling cows of approximately two and a half years of age at a local slaughterhouse (Champlain Beef Co., Whitehall, NY) with a permit issued by the USDA (United States Department of Agriculture). Ovaries were stored and transported to the laboratory in 0.9% NaCl, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B at 20°C. Ovarian pairs were staged for the phases of the estrous cycle based on morphological features of the corpus luteum and follicles as previously established (Ireland et al. 1980). Antral follicles were dissected, with grossly atretic ones avoided based on published morphological characteristics (Kruip and Dieleman, 1982). Dissected follicles were grouped into three size categories: small (2–5 mm), medium (6–8 mm), and large (>8 mm). These size ranges represent documented changes in the developmental competence of oocytes and key transitions in antral folliculogenesis (Lonergan et al., 1994; Lequarre et al., 2005).
Follicular fluid was aspirated from each follicle with an insulin syringe. Because of the limited volumes typically aspirated from small follicles, fluids were pooled within an ovary (from 4–10 follicles). Sample pooling was only performed for the small size category with follicular fluid from medium and large antral follicles handled and analyzed individually. Pooling of fluid in the small size category was only needed for the analyses of CAT and TAC but not of H2O2, for which follicular fluid was aspirated from individual follicles. Samples were stored on ice until centrifugation for 4.5 minutes at 5400 g. The supernatant was checked for the absence of any contaminating cells under the microscope and aliquots were stored at −80°C. Follicular fluid samples were collected, processed, and frozen within 2–4 hours of slaughter. For the H2O2 assay, fresh follicular fluid samples were analyzed without any freezing.
Measurement of antioxidant activity
Total antioxidant capacity (TAC) and catalase (CAT) activity were measured by assay kits obtained from Cayman Chemical (Ann Arbor, MI, USA). Assay plates were read using an ELISA plate reader (Bio-Tek Inc, VT, USA) at 750 nm for TAC and at 540 nm for CAT.
Catalase assay
Catalase is an antioxidant enzyme found in aerobically active cells that catalyzes the conversion of the ROS hydrogen peroxide to molecular oxygen and two molecules of water, according to the reaction H2O2 → O2 + 2 H2O. The enzyme also has peroxidatic activity in which it can transfer electrons with low molecular weight alcohols serving as electron donors, according to the equation H2O2 + AH2 → A + 2 H2O. The assay utilizes the peroxidatic function of the enzyme when methanol is used as the low molecular weight alcohol in the presence of sufficient amounts of hydrogen peroxide. The product of the reaction, formaldehyde, is measured spectrophotometrically when the chromogen Purpald (4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) binds to the aldehyde, becoming oxidized to give a purple color.
Follicular fluid samples were incubated at 37°C for 20 minutes, and then centrifuged with the subsequent supernatant diluted at 1:2 ratio with sample buffer. Shielded from light, 20μl of each sample was pipetted into a 96-well plate in duplicate and then incubated on a shaker at room temperature for 20 minutes with 100 μl assay buffer, 30 μl methanol and 20 μl hydrogen peroxide. Then 30 μl potassium hydroxide was added to terminate the reaction and the wells were incubated with 30 μl purpald for another 10 minutes at room temperature. The final incubation with 10 μl potassium periodate lasted for five minutes, after which point the plate was immediately placed in the plate reader and analyzed against the formaldehyde standard at 540 nm. The standards ranged from 0–75 μM formaldehyde. The concentration of formaldehyde was calculated in each sample from the linear regression equation of the standard curve.
Total antioxidant capacity
Total antioxidant capacity (TAC) includes both enzymatic antioxidants, such as catalase, and non-enzymatic antioxidants, such as ascorbic acid. The TAC assay measures both components by relying on the capacity of the antioxidants in the follicular fluid to prevent the oxidation of ABTS® (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) by metmyoglobin. The antioxidant capacity of the follicular fluid is compared to the antioxidant capacity of Trolox (a tocopherol analogue) and is therefore expressed as millimolar equivalents of Trolox.
Follicular fluid samples were incubated at 37°C for 20 minutes, and then centrifuged with the subsequent supernatant diluted at 1:10 ratio with sample buffer. Shielded from light, 10 μl of each sample was pipetted into a 96-well plate in duplicate with 10 μl of metmyoglobin, 150 μl ABTS®, and 40 μl of hydrogen peroxide. The plate was then incubated at room temperature on the shaker for five minutes. The plate was analyzed against the standard of Trolox activity at the end of the five-minute incubation on a plate reader at 750 nm. The standards ranged from 0–0.330 millimolar equivalents of Trolox. The total antioxidant capacities of the samples were calculated from the linear regression equation of the standard curve.
Measurement of Reactive Oxygen Species
Hydrogen peroxide (H2O2) assay
We used the Assay Designs’ Colorimetric Hydrogen Peroxide kit (Ann Arbor, MI). The kit is designed to measure low concentrations of H2O2 in biological matrices. The kit has a color reagent that contains a dye, xylenol orange, in an acidic solution with sorbitol and ammonium iron sulfate. The reaction produces purple color and the color intensity is in direct proportion to the concentration of H2O2 in the sample.
All reagents were allowed to warm to room temperature for at least 30 minutes, and the hydrogen peroxide color reagent was kept at 4°C. Standards contained 3,400, 1,700, 850,425, 212.5 and 106.25 ng/ml of hydrogen peroxide (converting to 100 mM, 50 mM, 25 mM, 12.5 mM, 6.25 mM and 3.125 mM, respectively. All standards and samples were run in duplicate. Samples in the small size category were diluted 1:10 with a 50mM sodium phosphate buffer (pH 6), while ones from medium and large follicles were diluted 1:4. The samples were read at an optical density of 550 nm. H2O2 concentrations were obtained from the linear regression equation of the standard curve.
Statistical analysis
A total of 503 samples were analyzed. Experimental data are presented as the median with the interquartile range (25th percentile, 75th percentile), as these parameters more accurately describe the data presented. Statistical analysis was performed using the Wilcoxon rank-sum test and the Kruskal-Wallis test, with significance level p = 0.05. Interaction analysis was performed to assess whether the relationship between each of the measured variables and estrous stage depended on follicle size. No significant interactions were found between CAT or TAC and estrous stage. When analyzing the relationship between H2O2 and estrous stage, a significant interaction was found between follicle size and estrous stage (P < 0.001). For this reason, the analysis of H2O2 and estrous stage was performed according to follicle size groups. A Spearman correlation analysis was also performed to test for a relationship between the TAC and CAT activity measured in the same follicular fluid samples.
Results
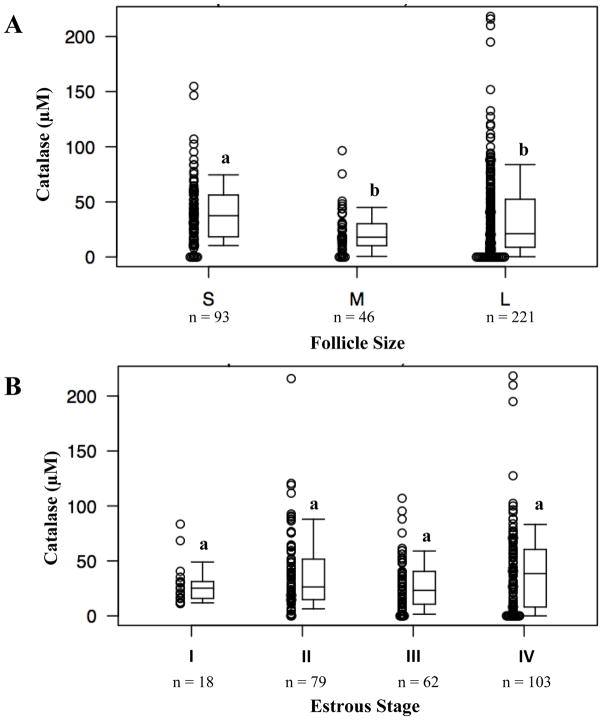
Catalase enzyme activity of follicular fluid in relation to follicle size and stages of the estrous cycle
Follicular fluid of the smallest follicle group contained the highest catalase enzyme activity (median 37.4 μM; interquartile range 18.4 μM, 56.2 μM). The small follicle group contained significantly higher catalase activity than both the medium group (P < 0.001) and the large group (P = 0.01) (Figure 1A). The medium (median 18.0 μM; 10.3 μM, 30.2 μM) and large follicle (median 21.2 μM; 8.8 μM, 52.4 μM) groups exhibited comparable levels of catalase activity. The median values and the plot do suggest, however, that despite the general trend mentioned, several samples from the large follicle group did have higher catalase values than any of the samples from the small or medium follicle groups.
Figure 1.
Catalase activity in follicular fluid collected from (A) different sized follicles and (B) different stages of estrous cycle. Different letters indicate significant differences (P < 0.05).
With respect to catalase enzyme activity by estrous stage, the median catalase activity generally trended upward. Catalase activity measured in stage I (median 25.2 μM; 16.0 μM, 31.2) trended upward across the stages toward the highest activity in stage IV (median 38.5 μM; 8.1 μM, 60.4 μM) (Figure 1B). However, these values did not reach statistical significance.
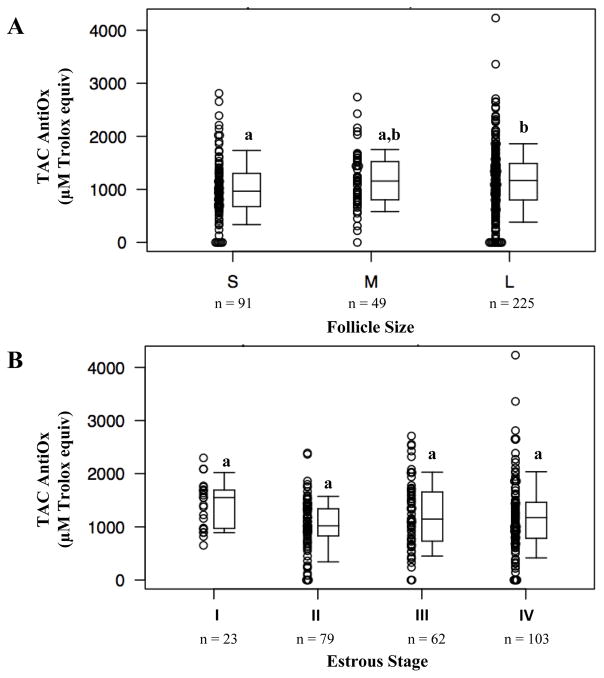
Total antioxidant capacity of follicular fluid in relation to follicle size and stages of the estrous cycle
When total antioxidant capacity was analyzed with respect to follicle size, total antioxidant capacity of follicular fluid increased as follicle size increased. Follicles of the small size category demonstrated a median of 966 μM Trolox equivalents (675 μM, 1302 μM Trolox equivalents). Large follicles (median 1168 μM Trolox equiv; 800 μM Trolox equiv, 1488 μM Trolox equiv) exhibited significantly higher total antioxidant capacity compared to the small follicles (P = 0.01; Figure 2A). Although total antioxidant capacity of medium follicles (median 1157 μM Trolox equiv; 804 μM Trolox equiv, 1522 μM Trolox equiv) also increased compared to the small follicle group, this difference did not reach statistical significance as it did for the large follicle group (P = 0.06). This was perhaps due to the limited number of medium-size follicles in the study.
Figure 2.
Total antioxidant capacity of follicular fluid collected from (A) different sized follicles and (B) different stages of estrous cycle. Different letters indicate significant differences (P < 0.05).
Pair-wise comparisons showed that estrous stage I (median 1549 μM Trolox equiv; 972 μM Trolox equiv, 1693 μM Trolox equiv) tended to have higher total antioxidant capacity than stages II (median 1019 μM Trolox equiv; 830 μM Trolox equiv, 1340 μM Trolox equiv), III (median 1146 μM Trolox equiv; 731 μM Trolox equiv, 1656 μM Trolox equiv) and IV (median 1172 μM Trolox equiv; 785 μM Trolox equiv, 1461 μM Trolox equiv) (P = 0.42). Stages I and II demonstrated the greatest difference in total antioxidant capacity (P =0.09), but all pair-wise comparisons between stages failed to reach statistical significance (Figure 2B).
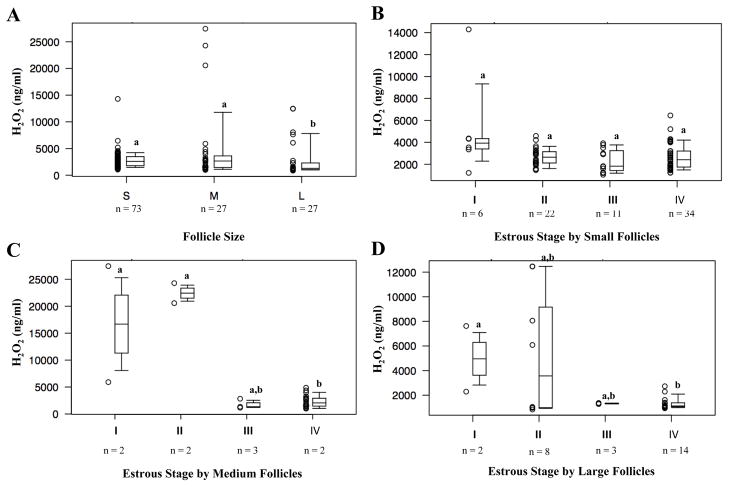
Hydrogen peroxide activity of follicular fluid in relation to follicle size and stages of the estrous cycle
Hydrogen peroxide levels decreased as follicles grew in size. Follicles of the small (median 2582 ng/ml 1786 ng/ml, 3485 ng/ml) and medium (median 2656 ng/ml; 1439 ng/ml, 3615 ng/ml) groups demonstrated similar H2O2 levels (P = 0.45). Large follicles demonstrated the lowest H2O2 activity (median 1283 ng/ml; 1054 ng/ml, 2291 ng/ml), such that significant differences existed between small and large follicle H2O2 levels (P < 0.001) and between medium and large follicle H2O2 levels (P = 0.01) (Figure 3A).
Figure 3.
H2O2 activity in follicular fluid collected from (A) different sized follicles. H2O2 activity in follicular fluid collected from different estrous stages, grouped by (B) small follicle size, (C) medium follicle size, and (D) large follicle size. Different letters indicate significant differences (P < 0.05).
As mentioned in the methods section, analysis of the relationship between H2O2 and estrous stage was performed by follicle size due to a significant interaction between these covariates. Examining H2O2 levels by estrous stage for small follicles, there does not appear to be significant evidence of a relationship between the two covariates (P = 0.14) (Figure 3B). For medium follicles, estrous stages I (median 16,676 ng/ml; 11,293 ng/ml, 22,059 ng/ml) and II (median 22,426 ng/ml; 21,498 ng/ml, 23,355 ng/ml) demonstrated significantly higher H2O2 levels than stages III (median 1342 ng/ml; 1253 ng/ml, 2083 ng/ml) and IV (median 2052 ng/ml; 1457 ng/ml, 2916 ng/ml) (P = 0.02) (Figure 3C). For large follicles, there did not appear to be significant evidence of relationship between H2O2 level and estrous stage (P = 0.79) (Figure 3D). There is a suggestion of an interaction in which medium follicle size has a more dramatic association between H2O2 and estrous stage, but that result is heavily influenced by only two observations with high H2O2 in each of estrous stages I and II in the median follicle size. If we overlook the questionable interaction between estrous stage and follicle size, we can then use a regression model with log transformed H2O2 as the outcome versus estrous stage, with follicle size as a covariate. In comparison to estrous stage I, stages III and IV have significantly lower covariate adjusted mean H2O2 (p=0.003 and 0.002, respectively). Stage II has a lower estimated mean than stage I, but the difference is not statistically significant (p=0.07). Stage II had a higher estimated mean than stages III and IV, but these differences were also of marginal significance (p=0.051 and 0.053, respectively). Stages III and IV had similar means (p=0.60). These results suggest that Stage I has the highest covariate-adjusted mean, with stages III and IV having the lowest means, and Stage II is in between. This result is consistent with the univariable result where no adjustment for follicle size was made.
Relationship between catalase activity and total antioxidant capacity of follicular fluid
Each follicular fluid sample was analyzed for both catalase enzyme activity and total antioxidant capacity. A Spearman correlation analysis showed the lack of association between these two parameters (0.05; p=0.35).
Discussion
The results of our study show that CAT activity is highest in follicular fluid of small follicles compared to medium and large size follicles. TAC was highest in the follicular fluid of large size follicles, while H2O2 levels were lowest in large compared to small and medium follicles. There were no statistically significant differences in CAT or TAC when comparing the different estrous stages. Analysis of covariate-adjusted mean H2O2 levels and estrous stage suggests that H2O2 levels decreased as the estrous cycle progressed.
Few investigators have studied individual antioxidant defenses in in vivo models of healthy subjects. Our CAT activity results conflict with a published study by Basini et al. (2008) that described CAT activity in porcine follicular fluid as being highest in the largest sized follicles. There may be a species-dependent difference in CAT expression or activity, as CAT expression varies in oocytes of different species (El Mouatassim et al. 1999). In this respect, the bovine model has been established as a fitting model with which to study human ovarian function and development due to similarities in physiology, including the wave model of folliculogenesis (Baerwald et al. 2003; Campbell et al. 2003; Ireland et al. 2000; Malhi et al. 2005). Combelles et al. recently published a study investigating levels of the antioxidant SOD in follicular fluid of differently sized bovine antral follicles (Combelles et al. 2010). They reported the highest SOD levels in small follicles compared with large follicles. Because SOD catalyzes the reaction that converts the superoxide anion radical to H2O2 and molecular oxygen, one would expect levels of H2O2 and the enzymes that scavenge H2O2 to be higher in smaller follicles as well. The two major enzymatic scavengers of H2O2 are CAT and glutathione peroxidase (GPx). While we did not measure GPx, our results (together with the work by Combelles et al., 2010) suggest a positive relationship between H2O2, SOD, and CAT levels in smaller follicles. Of note, Basini et al measured GPx in the follicular fluid of small, medium, and large porcine follicles and found significantly higher levels in small follicles compared to medium and large follicles (Basini et al. 2008).
Although CAT was highest in small follicles, TAC was lowest in small follicles and highest in large follicles. We had originally hypothesized that antioxidant capacity would increase during folliculogenesis; this hypothesis was based on the known increase in developmental competence with follicle size (Hagemann 1999; Lonergan et al. 1994; Machatkova et al. 2004) and the underlying assumption that increased antioxidants are beneficial during development. But as discussed below, direct evidence for a functional link between antioxidant and gamete quality is currently lacking. Further, TAC encompassesboth enzymatic and non-enzymatic antioxidants, and it is conceivable that CAT levels are high while other antioxidant components may be low in the small follicle (and vice versa for large follicles). Our findings buttress the need for future studies not to limit evaluation to total antioxidant capacity but rather to consider each antioxidant type (enzymatic and non-enzymatic).
To our knowledge, Basini et al is the only study documenting the levels of specific ROS in the follicular fluid of different size follicles. They found that H2O2 and hydroperoxide levels were highest in follicular fluid of small follicles and that these levels decreased as follicles grew, while the levels of the superoxide anion radical did not change with follicle size (Basini et al. 2008). Both Basini et al and our results support the notion that the levels of H2O2vary in the milieu of the oocyte as it progresses through its development, thus underscoring the need to evaluate the exact influences of H2O2 on oocyte development. This is particularly relevant since for instance, higher levels of H2O2 were associated with fragmented embryos and apoptosis (Yang et al. 1998), and the addition of exogenous ROS (including H2O2) to mouse oocytes also diminished viability and induced markers of aging (Goud et al. 2008). In light of the differential dynamics reported herein for select oxidant and antioxidant measures, there is a dire need to detail the metabolic activity and redox regulation of follicles at specific stages of development.
When analyzing oxidants and antioxidants according to estrous stage, it is important to note that both human and bovine follicular development occur in two to three waves during the estrous cycle. Stage I corresponds to days 1–4 of the estrous cycle, stage II to days 5–10, stage III to days 11–17, and stage IV to days 18–21 (Ireland et al. 2000). However, the waves of follicular development do not match these stages. While some subjects may have a 3-wave cycle, with waves beginning on days 2, 9, and 16 on average, others will have a 2-wave cycle, with waves beginning on days 3 and 10 on average (Ireland et al. 2000). This may explain why the relationships between CAT and TAC with estrous stages were unclear. Of note was an interaction between H2O2 levels and stages of the estrous cycle; additional studies that are based on in vivo monitoring and follicular fluid sampling are thus needed to verify this relationship. In this vein, future effort should distinguish between dominant, subordinate, healthy, and atretic follicles
Numerous past reports attempted to characterize follicular fluid ROS and antioxidants in relation to oocyte quality, embryo formation, and IVF outcomes. Oocytes developing within a follicular fluid of enhanced antioxidant capacity may be more likely to be fertilized than ones with lower levels of antioxidants. In a study of women undergoing IVF, Pasqualotto et al reported that oocytes with follicular fluid containing increased TAC levels resulted in higher pregnancy rates (Pasqualotto et al. 2004). In a more recent report, Pasqualotto et al demonstrated that higher follicular fluid levels of CAT and SOD correlated with better fertilization and cleavage rates for patients undergoing IVF (Pasqualotto et al. 2009). Elevated mean follicular fluid levels of selenium-dependent GPx correlated with improved oocyte fertilization rates for IVF patients (Paszkowski et al. 1995). Antioxidant enzymes have also been examined in the setting of age-related infertility, with the follicular fluid of older IVF patients demonstrating decreased levels of CAT and glutathione transferase than that of younger IVF patients (Carbone et al. 2003). However, there have been conflicting results among the studies conducted to date. Although CAT and glutathione transferase levels may decrease in older IVF patients’ follicular fluid, levels of SOD increase and appear inversely correlated with oocyte fertilization rates (Carbone et al. 2003; Sabatini et al. 1999). Sabatini et al postulates that an imbalance of the antioxidant enzymes in the follicular fluid of older IVF patients may result in increased ROS and poor IVF outcomes. Previous studies also reported improved IVF outcomes when ROS production is elevated. Attaran et al. (2000) describe significantly higher follicular fluid ROS for IVF patients that became pregnant compared to those that did not; and Pasqualotto et al. (2004) reported increased levels of lipid peroxidation in follicular fluid of oocytes that produced pregnancies versus those that did not. These observations suggest that there is a certain level of ROS that is beneficial for the maturation of oocytes. This idea is supported by the findings that even within a group of good quality oocytes, a subset of those oocytes with a low level of follicular fluid ROS developed in better quality embryos than the subset with ROS over a certain threshold value (Das et al. 2006). Our study also documents dynamic changes in three oxidative biomarkers during folliculogenesis. But given the current uncertainties on the exact functional significance of different oxidant and antioxidant levels, future work should expand on studies like ours that combine the analyses of oxidant and antioxidant measures. It is also essential to consider other enzymatic and non-enzymatic antioxidants as well as the various types of free radicals.
Our study has defined the dynamic changes of catalase, total antioxidant capacity, and hydrogen peroxide at different stages of follicular development and the estrous cycle in the bovine model. Much of the difficulty in drawing conclusions regarding the levels and effects of ROS and antioxidants in follicular fluid are due to not only the relative lack of reports on the subject, but also the inconsistency in model species and specific oxidants and antioxidants under study. It is thus necessary to assess further ROS and antioxidants in the context of follicle size, waves of follicular development, and the health and dominance status of the follicle. Future studies should also characterize the antioxidants relevant to the maturation and developmental competence of oocytes; these antioxidants may in turn prove instrumental to the improvement of in vitro maturation and the quality of resulting embryos.
Acknowledgments
This work was supported by funds from the Research Program Committee and the Centre for Reproductive Medicine, Cleveland Clinic, Cleveland, Ohio and grants from the Vermont Genetics Network (grant number P20 RR16462, INBRE Program, NIH/NCRR) and National Institute of Child Health and Development (R15 HD05645–01) (CMHC).
Footnotes
Publisher's Disclaimer: Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Reproduction, Fertility, and Development but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain. The definitive version is available at: http://www.publish.csiro.au/paper/RD10270.htm
References
- Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9:338–47. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- Angelucci S, Ciavardelli D, Di Giuseppe F, Eleuterio E, Sulpizio M, Tiboni GM, Giampietro F, Palumbo P, Di Ilio C. Proteome analysis of human follicular fluid. Biochim Biophys Acta. 2006;1764:1775–85. doi: 10.1016/j.bbapap.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45:314–20. [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80:116–22. doi: 10.1016/s0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- Basini G, Simona B, Santini SE, Grasselli F. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod Fertil Dev. 2008;20:269–74. doi: 10.1071/rd07147. [DOI] [PubMed] [Google Scholar]
- Bertout J, Mahutte NG, Preston SL, Behrman HR. Reactive oxygen species and ovarian function. In: Leung P, Adashi EY, editors. The Ovary. Elsevier Academic Press; San Diego: 2004. pp. 353–68. [Google Scholar]
- Blondin P, Coenen K, Sirard MA. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J Androl. 1997;18:454–60. [PubMed] [Google Scholar]
- Campbell BK, Souza C, et al. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod Suppl. 2003;61:429–43. [PubMed] [Google Scholar]
- Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–43. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–80. doi: 10.1016/s1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles CM, Holick EA, Paolella LJ, Walker DC, Wu Q. Profiling of superoxide dismutase isoenzymesin compartments of the developing bovine antral follicles. Reproduction. 2010;139:871–81. doi: 10.1530/REP-09-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, Chaudhury K. Reactive oxygen species level in follicular fluid--embryo quality marker in IVF? Hum Reprod. 2006;21:2403–7. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- El Mouatassim S, Guerin P, Menezo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod. 1999;5:720–5. doi: 10.1093/molehr/5.8.720. [DOI] [PubMed] [Google Scholar]
- Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008;44:1295–304. doi: 10.1016/j.freeradbiomed.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–89. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hagemann LJ. Influence of the dominant follicle on oocytes from subordinate follicles. Theriogenology. 1999;51:449–59. doi: 10.1016/S0093-691X(98)00239-8. [DOI] [PubMed] [Google Scholar]
- Ireland JJ, Mihm M, Austin E, Diskin MG, Roche JF. Historical perspective of turnover of dominant follicles during the bovine estrous cycle: key concepts, studies, advancements, and terms. J Dairy Sci. 2000;83:1648–58. doi: 10.3168/jds.S0022-0302(00)75033-8. [DOI] [PubMed] [Google Scholar]
- Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci. 1980;63:155–60. doi: 10.3168/jds.S0022-0302(80)82901-8. [DOI] [PubMed] [Google Scholar]
- Kruip TA, Dieleman SJ. Macroscopic evaluation of bovine follicles and its validation by micromorphological and steroid biochemical procedures. Reprod Nutri Dev. 1982;22:465–73. doi: 10.1051/rnd:19820403. [DOI] [PubMed] [Google Scholar]
- Lequarre AS, Vigneron C, Ribaucour F, Holm P, Donnay I, Dalbies-Tran R, Callesen H, Mermillod P. Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology. 2005;63:841–59. doi: 10.1016/j.theriogenology.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Gutierrez-Adan A, Rizos D, Pintado B, de la Fuente J, Boland MP. Relative messenger RNA abundance in bovine oocytes collected in vitro or in vivo before and 20 hr after the preovulatory luteinizing hormone surge. Mol Reprod Dev. 2003;66:297–305. doi: 10.1002/mrd.10357. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev. 1994;37:48–53. doi: 10.1002/mrd.1080370107. [DOI] [PubMed] [Google Scholar]
- Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900. doi: 10.1093/humrep/13.4.896. [DOI] [PubMed] [Google Scholar]
- Machatkova M, Krausova K, Jokesova E, Tomanek M. Developmental competence of bovine oocytes: effects of follicle size and the phase of follicular wave on in vitro embryo production. Theriogenology. 2004;61:329–35. doi: 10.1016/s0093-691x(03)00216-4. [DOI] [PubMed] [Google Scholar]
- Malhi PS, Adams GP, Singh J. Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol Reprod. 2005;73:45–53. doi: 10.1095/biolreprod.104.038745. [DOI] [PubMed] [Google Scholar]
- Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidativestress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–6. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Pasqualotto EB, Lara LV, Salvador M, Sobreiro BP, Borges E, Pasqualotto FF. The role of enzymatic antioxidants detected in the follicular fluid and semen of infertile couples undergoing assisted reproduction. Hum Fertil (Camb) 2009;12:166–71. doi: 10.1080/14647270903207941. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236:173–80. doi: 10.1016/0009-8981(95)98130-9. [DOI] [PubMed] [Google Scholar]
- Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. FertilSteril. 1999;72:1027–34. doi: 10.1016/s0015-0282(99)00411-2. [DOI] [PubMed] [Google Scholar]
- Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. 2002;77:1184–90. doi: 10.1016/s0015-0282(02)03103-5. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–7. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- Shiotani M, Noda Y, Narimoto K, Imai K, Mori T, Fujimoto K, Ogawa K. Immunohistochemical localization of superoxide dismutase in the human ovary. Hum Reprod. 1991;6:1349–53. doi: 10.1093/oxfordjournals.humrep.a137267. [DOI] [PubMed] [Google Scholar]
- Valdez KE, Cuneo SP, Turzillo AM. Regulation of apoptosis in the atresia of dominant bovine follicles of the first follicular wave following ovulation. Reproduction. 2005;130:71–81. doi: 10.1530/rep.1.00430. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yamazaki H, Kon Y, Watanabe T, Arikawa J, Miyoshi I, Kasai N, Kuwabara M. Progressive effect of alpha-phenyl-N-tert-butyl nitrone (PBN) on rat embryo development in vitro. Free Radic Biol Med. 1997;23:1073–7. doi: 10.1016/s0891-5849(97)00139-1. [DOI] [PubMed] [Google Scholar]
- Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13:451–8. doi: 10.1016/j.jsgi.2006.05.005. [DOI] [PubMed] [Google Scholar]