Abstract
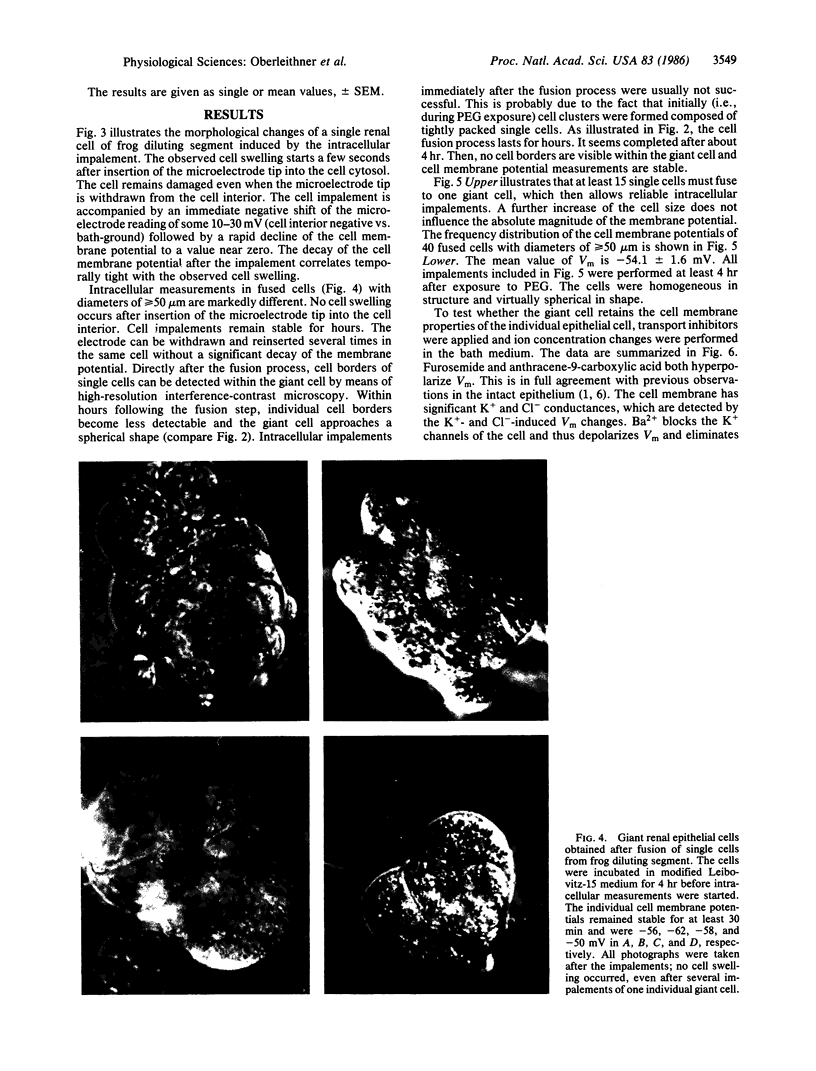
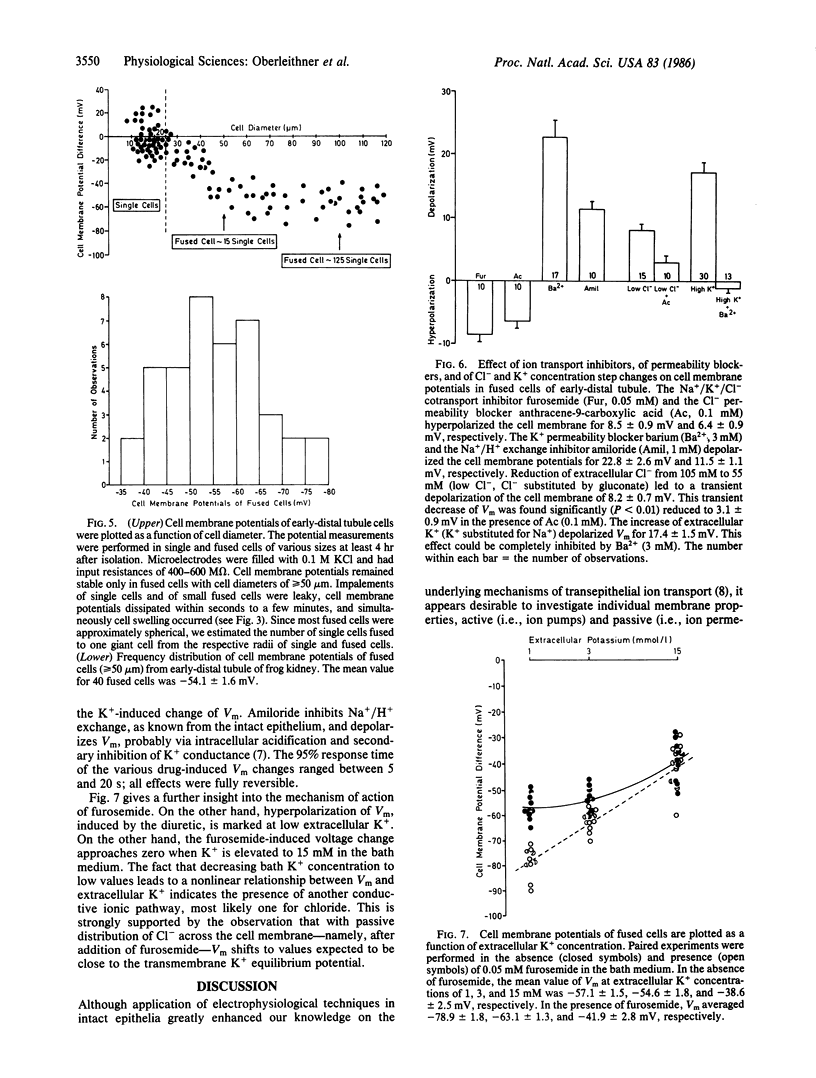
The investigation of epithelial ion transport at the cellular level by means of electrophysiological techniques is hampered by the small size of epithelial cells. Moreover, interpretation of experiments is complex due to poorly defined and highly variable paracellular leaks (shunt pathways). In search of a new experimental approach we developed a technique to isolate renal epithelial cells (diameter approximately equal to 10 micron) from diluting segments of the frog kidney and to fuse them to "giant" cells (diameter approximately equal to 100 micron). These cells generate membrane potentials of -54.1 +/- 1.6 mV (mean +/- SEM; n = 40). They are sensitive to the diuretic drugs furosemide and amiloride and to the K+- and Cl- -permeability blockers Ba2+ and anthracene-9-carboxylic acid. The experiments demonstrate membrane potential measurements in cells isolated from renal epithelium and fused to giant cells. The cells retain their specific membrane properties and could serve as a valuable experimental model in epithelial research.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R., Slayman C. L. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol. 1983;72(3):223–234. doi: 10.1007/BF01870589. [DOI] [PubMed] [Google Scholar]
- Bryant S. H., Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J Physiol. 1971 Dec;219(2):367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Transcellular cross-talk between epithelial cell membranes. Nature. 1982 Dec 23;300(5894):683–685. doi: 10.1038/300683a0. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Graf J., Gautam A., Boyer J. L. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Oberleithner H., Giebisch G. Relationship between cell volume and ion transport in the early distal tubule of the Amphiuma kidney. J Gen Physiol. 1985 Jul;86(1):31–58. doi: 10.1085/jgp.86.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y., Loewenstein W. R. Cell-to-cell passage of large molecules. Nature. 1966 Nov 5;212(5062):629–630. doi: 10.1038/212629a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Mechanism of distal tubular chloride transport in Amphiuma kidney. Am J Physiol. 1982 Apr;242(4):F331–F339. doi: 10.1152/ajprenal.1982.242.4.F331. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Potassium transport in the early distal tubule of Amphiuma kidney. Effects of potassium adaptation. Pflugers Arch. 1983 Mar 1;396(3):185–191. doi: 10.1007/BF00587854. [DOI] [PubMed] [Google Scholar]
- Oberleithner H. Intracellular pH in diluting segment of frog kidney. Pflugers Arch. 1985 Jul;404(3):244–251. doi: 10.1007/BF00581246. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Messner G., Wang W. Mechanism of hydrogen ion transport in the diluting segment of frog kidney. Pflugers Arch. 1984 Nov;402(3):272–280. doi: 10.1007/BF00585510. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Ritter M., Lang F., Guggino W. Anthracene-9-carboxylic acid inhibits renal chloride reabsorption. Pflugers Arch. 1983 Jul;398(2):172–174. doi: 10.1007/BF00581068. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977 Jun;69(6):879–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. II. Effects of ions and chelators on tissue dispersion. Exp Cell Res. 1973 Jan;76(1):25–30. doi: 10.1016/0014-4827(73)90414-x. [DOI] [PubMed] [Google Scholar]
- Westerwoudt R. J. Improved fusion methods. IV. Technical aspects. J Immunol Methods. 1985 Mar 18;77(2):181–196. doi: 10.1016/0022-1759(85)90031-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Vienken J. Electric field-induced cell-to-cell fusion. J Membr Biol. 1982;67(3):165–182. doi: 10.1007/BF01868659. [DOI] [PubMed] [Google Scholar]