Abstract
Background
White matter (WM) changes measured using diffusion tensor imaging (DTI) have been reported in Alzheimer’s disease (AD) and amnestic mild cognitive impairment (MCI), but changes in earlier pre-MCI stages have not been fully investigated.
Methods
In a cross-sectional analysis, older adults with MCI (n=28), older adults with cognitive complaints but without psychometric impairment (CC, n=29) and healthy controls (HC, n=35) were compared. Measures included whole-brain DTI, T1-weighted structural MRI, and neuropsychological assessment. Diffusion images were analyzed using Tract-Based Spatial Statistics (TBSS). Voxel-wise fractional anisotropy (FA) and mean, axial, and radial diffusivity (MD, DA, DR) were assessed and compared between groups. Significant tract clusters were extracted in order to perform further ROI comparisons. Brain volume was estimated using Freesurfer based on T1 structural images.
Results
The MCI group showed lower FA and higher RD than controls in bilateral parahippocampal WM. When comparing extracted diffusivity measurements from bilateral parahippocampal WM clusters, the CC group had values that were intermediate to the MCI and HC groups. Group difference in DTI measures remained significant after controlling for hippocampal atrophy. Across the entire sample, DTI indices in parahippocampal WM were correlated with memory function.
Conclusions
These findings are consistent with previous results showing changes in parahippocampal WM in AD and MCI compared to controls. The intermediate pattern found in the CC group suggests the potential of DTI to contribute to earlier detection of neurodegenerative changes during prodromal stages.
Keywords: Alzheimer’s disease, Diffusion tensor imaging, Mild cognitive impairment, MRI, Voxel-based method, White matter, Hippocampus, Memory, Fractional anisotropy, Diffusivity
1. Introduction
There has been an exponential growth in the number of published studies characterizing Alzheimer’s disease (AD) and its complex genetic and environmental determinants. In particular, progress in understanding basic disease mechanisms has lead to new therapeutic strategies currently undergoing clinical trials. To date results have been disappointing but there is a widespread perception that interventions need to be initiated earlier in the course of disease, prior to the onset of significant neurodegeneration. Therefore, a major focus of much recent research has been on methods that have potential to yield earlier detection and clinical diagnosis, ideally in the presymptomatic or early prodromal stages of AD. Amnestic mild cognitive impairment (MCI) has been widely studied as a prodromal form of dementia, conferring a 10-15% annual risk of converting to probable AD [1]. Although neuroimaging has been found to have a major role in the investigation of MCI and AD, few imaging studies have examined stages of prodromal AD earlier than MCI (pre-MCI). We previously described MCI-like gray matter changes on structural MRI in euthymic older adults, who, despite having marked cognitive complaints (CC), performed within normal limits relative to peers of same age and education on memory tests [2]. Based on these voxel-based morphometry (VBM) findings, it was suggested that older adults with cognitive complaints even when unaccompanied by deficits on formal testing, may be experiencing underlying neurodegenerative changes [2]. This finding has been confirmed and extended by other recent structural and spectroscopic MRI observations in a pre-MCI sample [3].
Thus far, conventional structural MRI has been mainly used to demonstrate AD progression related changes in gray matter of the brain [2-4]. This might be because white matter (WM) changes are occurring at a microscopic level, but have not accumulated enough to become obvious macroscopically, resulting in normally appearing WM on structural MRI [3, 5]. Unlike the T1-weighted structural MRI, diffusion tensor imaging (DTI) is a quantitative MRI technique that provides in vivo information about the three-dimensional diffusion of water molecules within the tissue [6]. Specifically, fractional anisotropy (FA) is a measure of anisotropic water diffusion, and reflects the degree of directionality of cellular structures within the WM fibers [7]. Loss of anisotropic diffusion could be related to abnormalities within the cellular microstructure. Mean diffusivity (MD) is a directionally averaged measure of the apparent diffusion coefficient and may help to better elucidate how WM integrity is changing [6]. Recent animal models suggested that the diffusivity measure can be further subdivided in to axial and radial components, which could provide information on axonal and myelin pathology selectively [8]. Therefore, recent DTI studies have also employed the axial diffusivity (DA) and radial diffusivity (DR), which quantify the magnitude of water diffusion parallel and perpendicular to the principal orientation of WM [9-15]. These may relate more closely to underlying pathology, because decreases in DA have correlated with axonal loss in some studies, whereas increases in DR are more characteristic of demyelination [16]. More importantly, it has been suggested that exploration of the full tensor behavior would be advantageous in studying neurodegeneration rather than assuming that a single metric (such as FA) is sufficiently sensitive to different pathological states [14].
DTI has been used to assess WM changes not detectable with conventional structural MRI in MCI and AD [9, 13, 14, 16-19], as well as normal aging [10-12, 20, 21]. However, findings of DTI in prodromal AD have been inconclusive [16]. There are extremely limited DTI studies in pre-MCI stages [22] and WM alterations in CC patients have not been investigated. In this study, we examined microstructural properties of WM underpinnings of memory complaints in older adults with normal memory test performance compared to individuals with MCI and healthy controls (HCs). An additional noteworthy feature of this study relative to prior research is the use of tract based spatial statistics (TBSS) [23], an automated voxel-wise analysis method to improve detection and localization of WM changes across individuals and groups. We hypothesized that the CC group would show intermediate changes in WM relative to the MCI and control groups, especially within episodic memory circuitry, given its role as the earliest symptom of AD. We also hypothesized that subjective and objective measures of memory would be directly related to WM integrity.
2. Material and Methods
2.1. Study Participants
Participants were 92 older adults enrolled in the Dartmouth Memory and Aging Study. The sample included 29 euthymic individuals with significant cognitive complaints despite cognitive test performance within the normal range (CC group), 28 patients with amnestic MCI (MCI group), and 35 healthy controls (HC group) without significant cognitive complaints or psychometric deficits. Further details regarding participant recruitment, selection criteria, and characterization are available in Saykin et al (2006) [2].
Participants provided written informed consent according to procedures approved by the Institutional Committee for the Protection of Human Subjects. Inclusion criteria were at least 60 years of age, right handed, fluent in English, and at least 12 years of formal education. Participants were required to have an informant who knew them well and could answer questions about their cognition and general health. Exclusion criteria included any medical, psychiatric, or neurologic condition (other than MCI) that could significantly affect brain structure or cognition, history of head trauma with loss of consciousness lasting more than 5 minutes, history of substance dependence, and factors contraindicating MRI. Non-amnestic forms of MCI [24] were excluded, although amnestic MCI with involvement of multiple domains was permitted.
2.2. Neuropsychological Assessment
Participants underwent a detailed neuropsychological evaluation, including measures of memory, attention, executive function, language, spatial ability, general intellectual ability, and psychomotor speed as well as standard dementia screens. Tests included Mini-Mental State Examination (MMSE) [25], Mattis Dementia Rating Scale-2 [26], California Verbal Learning Test (CVLT-II) [27], Boston Naming Test [28], selected subtests from the Delis–Kaplan Executive Function System (D-KEFS) [29], Wisconsin Card Sorting Test [30], selected subtests from the Wechsler Adult Intelligence Scale III and Wechsler Memory Scale III [31]. For the CVLT-II test, participants were randomly administered either the standard or alternate test forms to reduce practice effects engendered by multiple administrations of the same items. Form equivalency has been demonstrated by previous research and test forms were balanced across participant groups and/or balanced across participants within each group (χ2=1.262, p > .05).
Multiple inventories were employed to assess subjective cognitive ability, including the Memory Self-Rating Questionnaire, self and informant versions of the Neurobehavioral Function and Activities of Daily Living Rating Scale [32], self and informant versions of the Informant Questionnaire on Cognitive Decline in the Elderly [33], the four cognitive items from the Geriatric Depression Scale (GDS) [34], and 23 items from the Memory Assessment Questionnaire [35]. A Cognitive Complaint Index was calculated as the percentage of all items endorsed [2].
Group classifications (HC, CC, MCI) were based on results of the neuropsychological assessment, self and informant report indices, and the geriatric and neurologic evaluations. A multidisciplinary clinical consensus panel reviewed each case according to specific criteria, which is detailed in elsewhere [2].
2.3. MRI Acquisition
The MRI was acquired on a GE Signa 1.5T Horizon LX magnet with echo speed gradients using a standard head RF coil. DTI acquisition was conducted using a two-dimensional spin-echo sequence with an echo planar readout (SE-EPI-DTI) and a pair of diffusion weighting (DW) gradients positioned symmetrically around the 180° pulse [7]. The DTI parameters: TR/TE = 8000/78 ms, 36 axial slices with 3mm thickness, 128 × 128 image matrix, 24cm × 24cm field of view. The amplitude of the total diffusion gradient was 20mT/m and was applied in 12 non-collinear directions uniformly distributed in three-dimensional space (minimum energy scheme) [36]. The effective diffusion weighting was b=800 sec/mm2. Two image-volumes with no diffusion weighting (b=0 sec/mm2) were also acquired at the beginning of each DTI scan. Acquisition of all b=800 sec/mm2 and b=0 sec/mm2 images was repeated 4 times. The duration of the DTI scan was about 7min. In addition, a T1-weighted three-dimensional spoiled gradient echo (SPGR) acquisition with TR/TE = 25/3ms, flip angle 40 degrees, 1 NEX, a slice thickness of 1.5 mm (no skip) and in-plane resolution of 0.9375 mm was performed for anatomic reference.
2.4. Imaging analysis
The DTI data were analyzed in the frame of the FSL package (FMRIB Center, Oxford, United Kingdom). Preprocessing included correction for motion and eddy current effects in DTI images. Subsequently, FMRIB’s Diffusion Toolbox [37] was used to fit the tensor model and to compute the FA, MD, DA and DR maps. After that, TBSS analysis was utilized [23]. TBSS addresses many concerns regarding previous methods of whole brain voxel-by-voxel analyses of diffusion-weighted data, including reliable registration of subjects to a common space, choice of smoothing kernel, and partial volume effects [9-14, 16, 17]. All individual FA maps were nonlinearly registered to the template and then affine-transformed into standard Montreal Neurological Institute (MNI) space. A mean WM skeleton was generated based on the mean FA image of all subjects. Each subject’s aligned FA image was projected onto the mean FA skeleton, and voxel-wise group comparisons of FA values were conducted along the skeleton. Voxel-wise analysis of MD, DA and DR maps was conducted in the same manner using the same FA skeleton.
To determine degree of brain atrophy, each participant’s cortical and subcortical tissue volume and intracranial volume (ICV) were estimated using automatic parcellation as implemented in the FreeSurfer software package [38] based on T1-weighted (SPGR) anatomical images as described in our previous publications [4, 39].
2.5. Statistical analysis
All skeletonized DTI maps were included in voxel-wise group statistical analyses using a generalized linear model approach with age and gender as covariates. Inference on these statistics was carried out using the “randomise” program within FSL, which performs permutation testing (5000 permutations) that does not rely on a Gaussian distribution [40]. With this approach, voxel-wise differences among groups applying two-sample t tests were assessed. Correction for multiple comparisons was achieved through a combination of individual voxel probability and cluster size thresholding using Monte Carlo simulation for Type I error control (α<0.05) [41-43]. As estimated by the simulation, only clusters with an individual voxel threshold of p<0.01, yielding a brain-wise probability at p<0.05 of finding such a cluster under the null hypothesis, were considered significant at p<0.01 (corrected) [42, 43].
For exploratory ROI analyses, mean DTI indices values for each subject were extracted from clusters showing significant between-group differences in FA. MANOVA (multivariate analysis of variance) was performed using the Statistical Package for Social Sciences (SPSS, v. 17.0) to assess differences in DTI indices between groups. A series of covariance analyses was used to assess the relationships between neuropsychological performance and mean DTI indices. For demographic and cognitive variables comparison, ANOVAs were employed with Tukey’s post-hoc or χ2 tests for categorical variables. Partial correlations controlling for participants’ age and gender were performed to investigate relationships between cognitive indices, and areas across the multiple tensor parameters showing group differences. To investigate if atrophy influenced DTI results, all analyses were repeated adjusting for tissue volumes.
3. Results
Subject characteristics
The three groups of participants did not differ significantly with respect to age and education (Table 1), although the mean age was slightly higher for the CC and MCI groups than for the HC participants. Male-to-female ratio among the three groups was not significantly different by χ2 test but also was not perfectly balanced. Thus, Age and gender were entered as covariates in all statistical analyses of DTI [10, 12, 43]. Assessment of cognitive performance was based on age, education, and gender-adjusted scores, as applicable [2]. As expected, MCI patients had significantly lower mean MMSE, DRS-2 total scores and CVLT-II scores than the CC and HC groups (see Table 1). The CC and HC groups did not differ from each other in terms of performance on the MMSE, DRS-2 and CVLT-II scores. Although significant differences among all three groups were found on the WMS-III Logical Memory immediate and delay scores (MCI < CC < HC), memory performance of CC group is still considered within the normal range [2, 44].
Table 1.
Sample Characteristics
| HC (n=35) | CC (n=29) | MCI (n=28) | F/χ2 | p value | Post-hoc comparison | |
|---|---|---|---|---|---|---|
| Age (yrs) | 71.6 (5.2) | 73.4 (6.3) | 74.3 (5.8) | 1.639 | 0.200 | NS |
| Education (yrs) | 16.7 (2.7) | 16.6 (3.0) | 16.6 (3.0) | 0.002 | 0.998 | NS |
| Gender (M/F) | 10:25 | 11:18 | 15:13 | 4.107 | 0.128 | NS |
| MMSE | 28.9 (1.2) | 28.8 (1.1) | 27.0 (1.9) | 15.840 | p<1×10-5 | HC>MCI(p<1×10-5); CC>MCI (p<1×10-5) |
| DRStot | 141.4 (1.8) | 141.1 (2.3) | 137.8 (3.1) | 20.643 | p<1×10-5 | HC>MCI(p<1×10-5); CC>MCI (p<1×10-5) |
| CVLT-IItot | 50.1 (7.8) | 47.7 (9.6) | 34.7 (8.2) | 28.191 | p<1×10-5 | HC>MCI(p<1×10-5); CC>MCI (p<1×10-5) |
| CVLT-II_SD | 11.5 (2.4) | 10.7 (3.3) | 6.1 (2.8) | 31.663 | p<1×10-5 | HC>MCI(p<1×10-5); CC>MCI (p<1×10-5) |
| CVLT-II_LD | 12.3 (2.5) | 10.9 (3.3) | 6.6 (3.1) | 28.409 | p<1×10-5 | HC>MCI(p<1×10-5); CC>MCI (p<1×10-5) |
| WMS-III_LM | 52.2 (6.4) | 46.0 (8.1) | 37.4 (9.8) | 22.974 | p<1×10-5 | HC>CC(p<0.05); HC>MCI (p<1×10-5); CC>MCI(p<0.001) |
| WMS-III_LMD | 34.1 (4.7) | 28.9 (6.2) | 22.8 (7.4) | 23.316 | p<1×10-5 | HC>CC(p<0.01); HC>MCI (p<1×10-5); CC>MCI(p<0.005) |
Note: Demographic and cognitive characteristics include mean (standard deviation) for each group.
HC = healthy control; CC = cognitive complaint; MCI = mild cognitive impairment; NS = not significant; MMSE = Mini-Mental State Examination (max 30); DRStot = Dementia Rating Scale-2 total scores (max 144); CVLT-IItot = California Verbal Learning Test-II total learning raw scores (max 80); CVLT-II_SD = California Verbal Learning Test-II short delay raw scores (max. 16); CVLT-II_LD = California Verbal Learning Test-II long delay raw scores (max. 16);WMS-III_LM = Wechsler Memory Scale-III, logical memory immediate recall raw scores (max. 75); WMS-III_LMD = Wechsler Memory Scale-III, logical memory delay recall raw scores (max. 50).
Whole brain DTI
At the threshold of p<0.01 (corrected), voxel-wise TBSS analysis revealed lower FA only in bilateral parahippocampal WM in the MCI group compared to HCs (Fig. 1) (cluster size, left: 252 mm3; right 176 mm3). Higher DR corresponded to lower FA in right parahippocampal WM in MCI patients compared to the HC group. Relative to the CC group, participants with MCI showed significantly lower FA in bilateral parahippocampal WM with slightly larger clusters (left: 451 mm3; right 337 mm3). No region showed higher FA in MCI group compared to HC or CC group. Moreover, voxel-based analysis of DTI indices did not reveal any difference between CC and HC groups.
Figure 1. Voxel-wise DTI comparison using tract-based spatial statistics analysis.
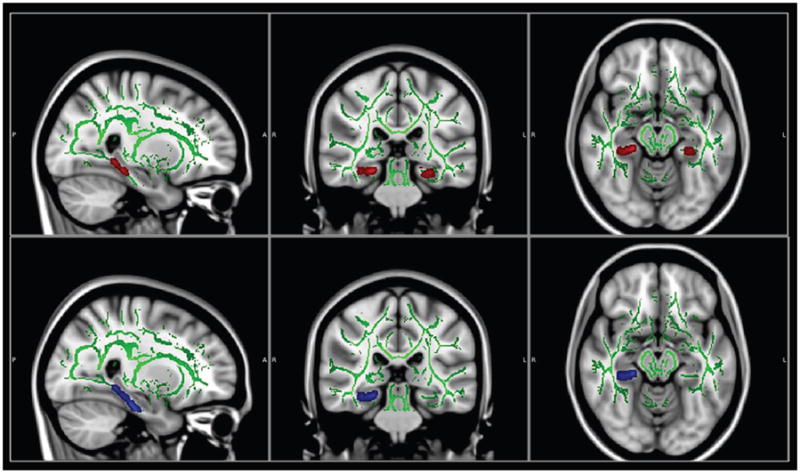
The brain images showing underlying standard Montreal Neurological Institute (MNI) atlas MNI152 1-mm brain template and white matter skeleton derived from tract-based spatial statistics (TBSS) analysis (shown in green). Red color indicates tracts with reduced fractional anisotropy (FA) in bilateral parahippocampal white matter in patients with MCI vs. controls; Blue color indicates region with increased radial diffusivity (DR) in right parahippocampal white matter in MCI vs. controls. Only clusters surviving correction for multiple comparisons of voxel-wise whole brain analysis are shown on brain images (p<0.01). Statistical maps were dilated from the TBSS skeleton for visualization purposes.
Regional analysis of DTI
We used the significant bilateral parahippocampal clusters from the FA contrast between MCI and HC as ROIs for subsequent analyses. In addition to FA changes shown in TBSS, significant differences between MCI and HC were also found in DR of left parahippocampal WM (p<0.05), and in all three diffusivity indices (MD, DR and DA) for right parahippocampal WM (p<0.05), whereas MCI and CC only differed in FA on these ROIs bilaterally (p<0.05). Consistent with study hypotheses, the CC group demonstrated intermediate changes of all diffusivity measures, with values falling between those of the MCI and HC groups (Fig. 2A and 2B), except FA of left parahippocampal ROI. Moreover, a significant difference between the CC and HC groups was found in MD of right parahippocampal ROI and a similar trend was also found in DR and DA in the same region.
Figure 2. Group comparison using regions of interest (ROIs) analysis on DTI indices.
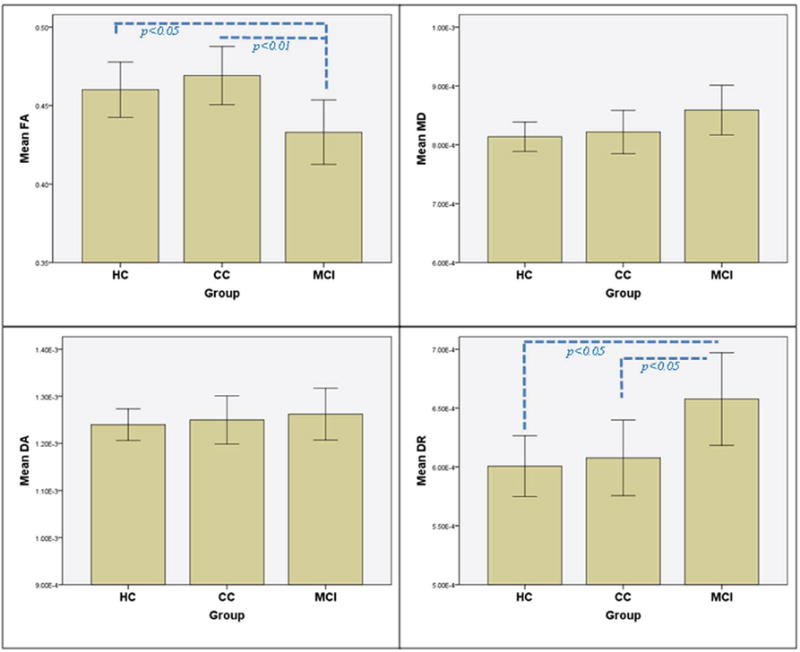

Group comparison of DTI indices in (A) left parahippocampal white matter and (B) right parahippocampal white matter. Age and sex adjusted means (± SE). (FA = fractional anisotropy; MD = mean diffusivity; DR = radial diffusivity; DA = axial diffusivity).
Relationship between DTI and memory/cognition
Cognitive scores showed modest but significant positive correlations with FA in the overall sample (see Table 2). Most correlations with the MMSE, DRS-2, CVLT-II and WMS-III scores were found for bilateral parahippocampal WM. Conversely, DR of the left and MD, DR, DA of the right parahippocampal WM were negatively correlated with most of these neuropsychological measures. Specifically, lower FA of the left parahippocampal WM and higher MD and DR of the right parahippocampal WM were associated with lower neuropsychological performance.
Table 2.
Partial correlation coefficients relating regional DTI indices and neuropsychological measures (controlled for age, gender and years of education)
| Left Parahippocampal WM | Right Parahippocampal WM | |||||||
|---|---|---|---|---|---|---|---|---|
| FA | MD | DR | DA | FA | MD | DR | DA | |
| MMSE | 0.20* | -0.06 | -0.11 | 0.02 | 0.20* | -0.11 | -0.14 | -0.05 |
| DRStot | 0.15 | -0.08 | -0.13 | 0.01 | 0.23* | -0.27** | -0.29** | -0.21* |
| CVLT-IItot | 0.27** | -0.12 | -0.19* | -0.01 | 0.20* | -0.22* | -0.23* | -0.20* |
| CVLT-II_SD | 0.29** | -0.16 | -0.23* | -0.04 | 0.10 | -0.25* | -0.25* | -0.22* |
| CVLT-II_LD | 0.25* | -0.15 | -0.21* | -0.04 | 0.11 | -0.23* | -0.23* | -0.21* |
| WMS-III_LM | 0.24* | -0.18 | -0.24* | -0.06 | 0.19* | -0.20* | -0.21* | -0.17 |
| WMS-III_LMD | 0.21* | -0.16 | -0.22* | -0.06 | 0.14 | -0.19* | -0.18 | -0.19 |
Note: symbol
indicates p<0.05;
indicates p<0.01;
WM = White Matter; FA = fractional anisotropy; MD = mean diffusivity; DR = radial diffusivity; DA = axial diffusivity; MMSE = Mini-Mental State Examination (max 30); DRStot = Dementia Rating Scale-2 total scores (max 144); CVLT-IItot = California Verbal Learning Test-II total learning raw scores (max 80); CVLT-II_SD = California Verbal Learning Test-II short delay raw scores (max. 16); CVLT-II_LD = California Verbal Learning Test-II long delay raw scores (max. 16);WMS-III_LM = Wechsler Memory Scale-III, Logical Memory immediate recall raw scores (max. 75); WMS-III_LMD = Wechsler Memory Scale-III, Logical Memory delay recall raw scores (max. 50).
Additional analyses
We investigated the relationship between brain atrophy measured using FreeSurfer and DTI changes across groups. After controlling for age, sex, education and ICV, significantly smaller volume was found only in bilateral hippocampi in MCI patients as compared to either HC or CC groups (p<0.01). Consistent with previous report [2], CCs showed intermediate hippocampal volume bilaterally, although the difference between HC and CC was not statistically significant. No significant partial correlation was found between hippocampal volume and DTI measures of parahippocampal WM in the overall sample. We repeated the group comparison of DTI indices using ICV normalized hippocampal volume as an additional covariate to further test if DTI alone adds incremental information. Although the effect size was slightly attenuated, all group differences in DTI indices in the original analysis remained significant indicating that the DTI indices contribute independent information regarding stage-specific group differences. Furthermore, in the view of a recent report of the relationship between education and WM microstructure in aging and AD [45], we also repeated TBSS analyses using years of education as additional covariate and found no significant effect of education on final results in this sample.
4. Discussion
In this study, TBSS analysis of DTI measures demonstrated lower FA and concordant higher DR in parahippocampal WM in the MCI group reflecting regional abnormalities in WM microstructure and integrity. These results are generally consistent with previous DTI studies in MCI and AD [1, 13, 14, 17-19]. The novel aspect of the present report was the inclusion of a group of individuals with marked cognitive complaints but psychometric performance within normal limits. This CC group demonstrated intermediate DTI diffusivity values in parahippocampal WM falling between the MCI and HC control groups. This profile of results suggests that DTI can contribute new information related to WM changes at pre-MCI stages of probable AD.
Parahippocampal WM is comprised of multiple important fiber systems, including the perforant pathway and fibers projecting from the amygdala to the parahippocampal region [13, 14]. Recent DTI results suggest that damage to the parahippocampal WM, in addition to atrophic changes in the hippocampus and entorhinal cortex, may contribute to memory dysfunction in MCI and AD [13, 14, 17, 19]. Various observations predict that axonal tract degeneration in early AD would include parahippocampal WM extending to, and continuing along, the posterior cingulum bundle [14]. Emerging evidence indicates that disconnection of medial temporal lobe fiber pathways represents a very early event in the course of AD and that demyelination may represent one contributing mechanism [11]. Higher DR with concomitant lower FA in the MCI group has shown apparent in the parahippocampal WM, suggesting that the pathology in this region includes some form of myelin degradation [9, 13]. In addition, we also found higher DA and MD in CC and MCI patients, particularly in right parahippocampal WM.
Nevertheless, the interpretation of differences in the measured diffusion tensor is complex and should be performed with care [6, 14, 15]. Whereas DTI is an established method to illustrate diffusional properties of white matter, the biological underpinning of tensor changes remains incompletely understood [6, 14, 15]. It is widely assumed, for instance, that phenomena such as demyelination and axonal loss may cause a scenario in which FA reductions are driven by increased DR and constant DA [8, 14, 46]. On the other hand, if changes in diffusion along the direction of the semi-major axis of the tensor ellipsoid (measured as DA) were proportional to those of the semi-minor axes (DR), then the FA, which is a function of this ratio, would remain relatively unchanged [14]. It is conceivable, that contributions of other neurobiological processes involved in white matter tract degeneration such as destruction of neurofibrils and glial alterations can give rise to diffusion tensor behaviors that are not fully captured by FA changes [14].
In this study, we did not observe a difference in FA between the CC and HC groups; however, there was a significantly higher MD of right parahippocampal WM in CC group relative to controls, while regional DR and DA showed similar trends. Moreover, the DTI indices were found in association with declarative memory performance. It has been reported that high MD values in the hippocampal formation of healthy elderly individuals predict memory decline [20]. Rogalski and colleagues [19] also found that MCI demonstrated a significant increase in MD, but no difference in FA, both in the parahippocampal WM region and in fibers modeled to pass through the parahippocampal region.
In consistence with our previous VBM reports [2, 4, 39], the present results demonstrate that compared to controls, MCI patients have volume loss in the hippocampus. Although the relation between DTI measures and more classical imaging indices of pathology, including brain volume, is not fully understood, a recent study in AD suggests that alteration of DTI measures, including effects in the parahippocampal WM, is independent of gray matter degeneration as measured by hippocampal volume [13]. Our results showing diffusion alteration in parahippocampal WM in prodromal AD after controlling for hippocampal volume are in accord with this finding. These results suggest a pattern of general emerging parenchymal loss and degradation (smaller volume and higher MD) in individuals with CC compared to healthy older people. However, the microstructural organization of remaining fibers, as determined by measures of anisotropic diffusion, is not significantly different from that of controls. Thus, diffusivity measures might be more sensitive than FA predicting earliest changes of WM microstructure in prodromal AD [19]. One can also speculate that there may be greater changes in the absolute dimensions of the diffusion ellipsoid rather than in its shape at very early stage of AD [14]. This prediction would translate to concordant and more sensitive results for DA, DR and MD when compared with FA [14].
The present study has several limitations. First, this cohort is highly educated and therefore may not be broadly representative of patients with prodromal AD. It has been hypothesized that WM microstructure may contribute to brain reserve capacity in humans[47]. Individuals with higher education and cognitive reserve might be more sensitive to subtle decline in cognitive function and therefore may present themselves for research participation with higher frequency than samples with lower education. Second, several recent reports indicated an influence of AD risk factors such as APOE epsilon 4 allele status and family history of dementia on WM integrity [11, 21]. We did not find statistically significant effects of these variables but it should be noted that the present study did not have sufficient power to detect relatively small effects of these variables or interactions among these factors. Future DTI studies of WM integrity in pre- and prodromal AD samples should continue to address genetic and other AD risk factors.
In conclusion, our results, together with other recent findings, suggest that DTI may be able to serve as an additional stage-related biomarker that can contribute information above and beyond established medial temporal atrophy and cognitive measures. Finally, the present study confirmed and extended previous VBM findings that cognitive complaints in older adults may indicate underlying neurodegenerative changes even when unaccompanied by deficits on formal testing [2, 3]. The CC group may represent a pre-MCI stage and may provide an earlier therapeutic opportunity than mild cognitive impairment [3]. However, the rate to which older adults with cognitive complaints progress to AD or other types of dementia has been somewhat controversial and may relate to ascertainment methodology and sample specific characteristics. We will continue to follow this cohort to examine longitudinal changes. The field is shifting toward a biomarker based assessment of risk in detection of AD including amyloid PET, CSF analytes and structural MRI [48]. The present study contributes to the body of work suggesting a potential role for DTI yet additional research is needed to demonstrate the sensitivity and specificity of early WM changes.
Highlights.
MCI patients show abnormalities in parahippocampal white matter (WM) tracts. Elders with cognitive complaints show intermediate changes related to MCI and control. Alteration in WM in prodromal AD is independent of gray matter degeneration.
Acknowledgments
This study was supported in part by grants from National Institutes of Health, NIA R01 AG19771 and Indiana Economic Development Corporation, IEDC #87884 (to AJS); National Institutes of Health, NIA P30 AG10133-18S1 Core Supplement to Drs. B. Ghetti and AJS; Dr. Wang receives research support from Siemens AG [PI] and UL1 RR025761 [PI of an Indiana CTSI pilot project];
Disclosures statement Dr. Wang receives research support from Siemens AG [PI] and UL1 RR025761 [PI of an Indiana CTSI pilot project].
J. West reports no disclosures.
Dr. Flashman served as a consultant to Solvay Pharmaceuticals and Cerebrio; and receives research support from New Hampshire Hospital, Welch Allyn Inc. [Site PI], the NIH [site PI, co-investigator], Department of Defense USAMRAA [co-investigator], NINDS [co-investigator], and CDC [co-investigator].
Dr. Wishart’s receives support from the NIH (NS045830, NS056228), the Congressionally Directed Medical Research Program of the Department of Defense, the National Multiple Sclerosis Society, the Dartmouth Center for Clinical and Translational Science, and investigator-initiated grants from Bayer HealthCare Pharmaceuticals and Teva Pharmaceutical Industries. She was a co-investigator on an investigator-initiated grant from EMD Serono Inc. and served on a scientific advisory board for Bayer HealthCare Pharmaceuticals. She is a site PI on two investigator-initiated research grants from Genzyme Corporation and is a co-investigator on a grant from the NIH (AG19771) and a National MS Society Center Award.
Dr. Santulli serves on the speaker’s bureau of Novartis, Forest, Eli Lilly, Pfizer and Eisai. He receives funding support from the Alzheimer’s Disease Neuroimaging Initiative [Site PI at Dartmouth] in addition to the Memory and Aging Study [co-investigator].
Dr. Rabin receives support from the NIH/NIA (1SC2AG039235-01A1 [PI]) and the Professional Staff Congress of the City University of New York (PSCREG-41-224 [PI]).
Dr. Pare reports no disclosures.
Dr Arfanakis receives support from the NIH (NIBIB R21 EB006525) and the Alzheimer’s Association (New Investigator Research Grant)
Dr. Saykin serves as Editor-in-Chief of Brain Imaging and Behavior; has served as a consultant to Baxter International Inc., Bristol-Myers Squibb, and Pfizer Inc.; and receives research support from Eli Lilly and Company, Siemens AG, Welch Allyn Inc., the NIH (R01 CA101318 [PI], R01 AG19771 [PI], RC2 AG036535 [Core Leader], P30 AG10133–18S1 [Core Leader], and U01 AG032984 [Site PI and Chair, Genetics Working Group]), the Indiana Economic Development Corporation (IEDC #87884), and the Foundation for the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao LL, Mueller SG, Buckley ST, Peek K, Raptentsetseng S, Elman J, Yaffe K, Miller BL, Kramer JH, Madison C, Mungas D, Schuff N, Weiner MW. Evidence of neurodegeneration in brains of older adults who do not yet fulfill MCI criteria. Neurobiol Aging. 2010;31:368–377. doi: 10.1016/j.neurobiolaging.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR, Jr, Weiner MW, Saykin AJ. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31:1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang PJ, Saykin AJ, Flashman LA, Wishart HA, Rabin LA, Santulli RB, McHugh TL, MacDonald JW, Mamourian AC. Regionally specific atrophy of the corpus callosum in AD, MCI and cognitive complaints. Neurobiology of Aging. 2006;27:1613–1617. doi: 10.1016/j.neurobiolaging.2005.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 9.Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junqué C, Solé-Padullés C, Peña-Gómez C, Bargalló N, Molinuevo JL, Bartrés-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer’s disease. Neuroimage. 2010;52:1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. Neuroimage. 2010;52:1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Salat DH, Tuch DS, van der Kouwe AJW, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiology of Aging. 2010;31:244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133:529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 16.Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, Kivipelto M, Hallikainen M, Vanninen R, Soininen H. Diffusion tensor imaging and Tract-Based Spatial Statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Kantarci K, Avula R, Senjem ML, Samikoglu AR, Zhang B, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, Ferman TJ, Boeve BF, Petersen RC, Jack CR., Jr Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology. 2010;74:1814–1821. doi: 10.1212/WNL.0b013e3181e0f7cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogalski EJ, Murphy CM, deToledo-Morrell L, Shah RC, Moseley ME, Bammer R, Stebbins GT. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment: a diffusion tensor imaging study. Behav Neurol. 2009;21:51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlesimo GA, Cherubini A, Caltagirone C, Spalletta G. Hippocampal mean diffusivity and memory in healthy elderly individuals: a cross-sectional study. Neurology. 2010;74:194–200. doi: 10.1212/WNL.0b013e3181cb3e39. [DOI] [PubMed] [Google Scholar]
- 21.Smith CD, Chebrolu H, Andersen AH, Powell DA, Lovell MA, Xiong S, Gold BT. White matter diffusion alterations in normal women at risk of Alzheimer’s disease. Neurobiology of Aging. 2010;31:1122–1131. doi: 10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenset V, Bjornerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tonnessen P, Gjerstad L, Fladby T. Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini Mental State”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Jurica P, Leitten C, Mattis S. Dementia Rating Scale-2. Psychological Assessment Resources, Inc.; Lutz, FL: 2001. [Google Scholar]
- 27.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition: Adult Version Manual. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 28.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. Third Edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- 29.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 30.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test Manual Revised and Expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- 31.Wechsler D. Wechsler Memory Scale-Third Edition WMS-III Administration and Scoring Manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 32.Saykin AJ. Neurobehavioral function and activities of daily living rating scale (NBFADL-63 item version) Dartmouth Medical School; Hanover: 1992. [Google Scholar]
- 33.Jorm AF, Jacomb PA. An informant questionnaire on cognitive decline in the eldery (IQCODE): socio-deomographic correlates reliability, validity and some norms. Psychological Medicine. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 34.Yesavage JA, Brink TL, Lose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of geriatric depression rating scale: A preliminary report. J Psychiat Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 35.Santulli R, Saykin A, Rabin L, Wishart H, Flashman L, Pare N, Nutter-Upham K, Pixley H. Differential sensitivity of cognitive complaints associated with amnestic MCI: Analysis of patient and informant reports. Alzheimer’s Association International Conference on the Prevention of Dementia; Washington, DC. June 18-21, 2005; 2005. [Google Scholar]
- 36.Hasan KM, Parker DL, Alexander AL. Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imaging. 2001;13:769–780. doi: 10.1002/jmri.1107. [DOI] [PubMed] [Google Scholar]
- 37.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 39.Shen L, Saykin AJ, Kim S, Firpi HA, West JD, Risacher SL, McDonald BC, McHugh TL, Wishart HA, Flashman LA. Comparison of manual and automated determination of hippocampal volumes in MCI and early AD. Brain Imaging Behav. 2010;4:86–95. doi: 10.1007/s11682-010-9088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward BD. Simultaneous Inference for fMRI Data, Biophysics Research Institute. Medical College of Wisconsin; Milwaukee: 1997. [Google Scholar]
- 42.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, Hamer HM, Oertel WH, Rosenow F, Knake S. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage. 2011;54:2557–2562. doi: 10.1016/j.neuroimage.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 44.Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, Santulli RB. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009;16:357–376. doi: 10.1080/13825580902825220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teipel SJ, Meindl T, Wagner M, Kohl T, Burger K, Reiser MF, Herpertz S, Moller HJ, Hampel H. White matter microstructure in relation to education in aging and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2009;17:571–583. doi: 10.3233/JAD-2009-1077. [DOI] [PubMed] [Google Scholar]
- 46.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 47.Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, Herpertz SC, Moller HJ, Hampel H. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49:2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 48.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


