Abstract
We demonstrate the operation of a digital microfluidic lab-on-a-chip system utilizing Electro Wetting on Dielectrics (EWOD) as the actuation principle and a High Fundamental Frequency (HFF; 50 MHz) quartz crystal microbalance (QCM) resonator as a mass-sensitive sensor. In a first experiment we have tested the reversible formation of a phosphor-lipid monolayer of phospholipid vesicles out of an aqueous buffer suspension onto a bio-functionalized integrated QCM sensor. A binding of bio-molecules results in an altered mass load of the resonant sensor and a shift of the resonance frequency can be measured. In the second part of the experiment, the formation of a protein multilayer composed of the biomolecule streptavidin and biotinylated immunoglobulin G was monitored. Additionally, the macroscopic contact angle was optically measured in order to verify the bio-specific binding and to test the implications onto the balance of the surface tensions. Using these sample applications, we were able to demonstrate and to verify the feasibility of integrating a mass-sensitive QCM sensor into a digital microfluidic chip.
Keywords: QCM, Mass sensor, Functionalized surface, Self assembled monolayer, Electro wetting on dielectrics
1. Introduction
The realization of a lab-on-a-chip, which is designed to perform and online monitor (bio-)chemical reactions by processing microliter volumes of different reagents and reaction products, is one of the major goals within microfluidics. For this purpose, the fluids can either be driven as a continuous flow through a fluidic channels or the fluid can be actuated and manipulated in terms of individually addressable discrete droplets. The latter holds the advantage of more degrees of freedom and a higher flexibility to react on real-time measurement results. Furthermore, measurements can be performed on precisely controlled sample droplets out of a microfluidic stream saving the rest of the sample fluid for further operations. Essential for this so called digital microfluidics is the actuation principle selected for single droplet manipulations. It defines the requirements for the design of the actuation elements as well as the specifications for the integration of the sensor elements into the microfluidic chip.
We discuss our work on a lab on a chip system including Electro Wetting on Dielectrics (EWOD) as an actuation mechanism for digital microfluidics, see Section 1.1. Specifically, we consider a coated quartz crystal microbalance (QCM) as a mass sensitive resonant chemical sensor (Section 1.2). Our aim was to investigate the suitability of a QCM sensor in such a digital microfluidics platform. Using a QCM with a chemical interface in a digital microfluidics setup could provide the opportunity to sensitively record the sensor's response after the sample droplet has been removed from the sensor where specifically bound target molecules remain in the interface. The response due to the latter could then be measured without spurious effects due to liquid loading. As will be discussed below, this seeming advantage could not be exploited as anticipated. Due to the different fabrication technologies, a hybrid technology had to be devised. We report on the challenges associated with this hybrid technologies and the performance obtained with the first series of prototype devices. As a particular requirement for the utilization of digital microfluidics, hydrophobic surfaces have to be used. This requirement also imposes restrictions on the chemical interface of the QCM sensor, which was in our case constituted by a self-assembled monolayer (SAM) the hydrophobic properties of which are investigated as well. The setup displaying the integration of the QCM is presented in Section 2. Measurements and results are shown in Section 3. Conclusions of the observed results and the prospective outlook are presented in Section 4.
1.1. Electro wetting on dielectrics
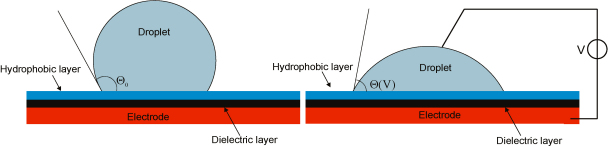
Digital microfluidics deals with single liquid droplets which are individually addressable and can be manipulated in a programmable way. A common actuation mechanism for these manipulations is known as electro-wetting on dielectrics. In open or covered EWOD systems the droplet is resting on a single plate or is squeezed between two plates, respectively. In both cases, the droplet adopts the shape with the minimal total surface energy. The different contributions to the total energy, e.g., surface tensions γ, electric potential differences, surfactants as well as gravitation change the actual droplet shape [1]. At the contact line between the liquid droplet and the solid surface a third phase is present, namely the ambient fluid which encapsulates the droplet and can consist of a liquid or gaseous phase. For small droplets with a high surface to volume ratio, gravitation can be neglected. Thus, the shape can be approximated by a sphere which is cut off by the surfaces of the contacting plates. The tangent to the approximated sphere in a point of the contact line includes the so called contact angle with the solid surface, see Fig. 1.
Fig. 1.
In the left part a droplet resting on an electrode covered with a dielectric layer with a hydrophobic surface displays a contact angle θ0. In the right part an applied voltage between the droplet and the electrode lowers the contact angle.
In other words, the contact angle θ of a droplet (denoted by the subscript L) in contact with a hydrophobic surface (S), surrounded by an ambient fluid (G), is predetermined through the balance of the surface tensions, given by the Young's equation:
| (1) |
The application of a voltage V between two electrodes, which are in contact with the droplet, changes the charge distribution at the droplet surface. This lowers the effective surface energy of the solid/liquid interface. The relation between the charge distributions at the interfaces, consisting of the charges at the electrode surface and a double layer formed out of counter ions in the droplet, and the applied voltage can be described as the double layer capacitance.
The applied potential difference drops at the double layer and causes hydrolysis at too high voltages. In order to avoid the hydrolysis a dielectric layer isolates the electrode from the droplet. The surfaces charge density is now proportional to the specific capacitance per area c of the isolating layer and the applied voltage. Additionally, the potential difference drops at the dielectric layer, which reduces the electric field strength within the droplet to a negligible level. The capacitive energy stored at the solid to liquid interface decreases the surface energy and is proportional to the specific capacitance c and the square of the applied voltage V, see Eq. (2).
| (2) |
The Lippmann–Young equation (3) describes the decrease of the contact angle θ (CA) starting from a CA at zero applied potential θ0 due to an applied voltage. The change of the contact angle is depicted in Fig. 1.
| (3) |
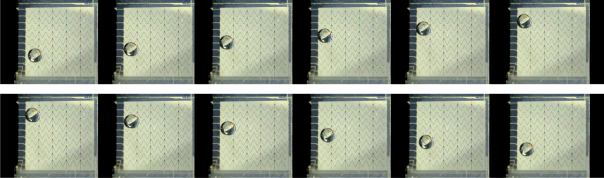
If the voltages are applied to individually addressable electrode areas located under the droplet, the resulting charge distribution on the surface is inhomogeneous. Thus, the surface tensions are unbalanced along the contact line which implies a force pulling the contact line outwards. If the surface of the contacting solids is hydrophobic to a certain extent [2], the droplet moves until a new balance of forces is reached, i.e., until the overlap of the droplet with the actuated electrode is maximized. With a sequence of actuation steps a consecutive transportation can be achieved, see Fig. 2. Different actuation sequences and electrode designs can be used for EWOD manipulations, e.g., creation, transport, mixing, divisions and accurate handling of multiple small volume droplets [3–5] on a common electrode array. This offers the possibility of performing different (bio)-chemical reactions on the same chip by simply changing the sequence of droplet manipulations.
Fig. 2.
Automated droplet actuation across several electrode lines of the EWOD base plate. The saw tooth design ensures the overlap of the droplet with at least two neighboring electrodes.
1.2. Sensor principle
In this paper we show the feasibility of integrating a quartz crystal microbalance (QCM) into a digital microfluidic chip. The QCM is utilized by many research groups as a mass sensitive sensor. The experimental aspects of the use of a QCM are discussed in [6]. Among other applications it can be used for detecting the specific binding of bio-molecules [7]. According to the Sauerbrey relation [8], a change in surface mass deposition Δm on the sensor surface, e.g., due to biochemical binding of molecules, results in a corresponding shift of the fundamental resonance frequency Δf of the thickness shear mode. The Sauerbrey relation is given below (4), where ρ denotes the mass density (2.648 g/cm3) and μ the shear modulus (29.47 GPa) of the quartz crystal.
| (4) |
In addition to this, the viscosity ηL and the density ρL of the liquid do also contribute to the shift of the resonance frequency [8]. Kanazawa and Gordon investigated that dependency and stated the relation:
| (5) |
A crucial factor for the performance of QCM systems is the fundamental resonance frequency f0 of the piezoelectric sensor due to the quadratic dependency of the frequency shift. Assuming a high resonance frequency, the same shift of the resonance frequency is achieved by a lower bonded mass. Thus, the use of a quartz crystal with a high fundamental frequency results in a highly sensitive mass sensor, which is able to detect the binding of a smaller amount of molecules.
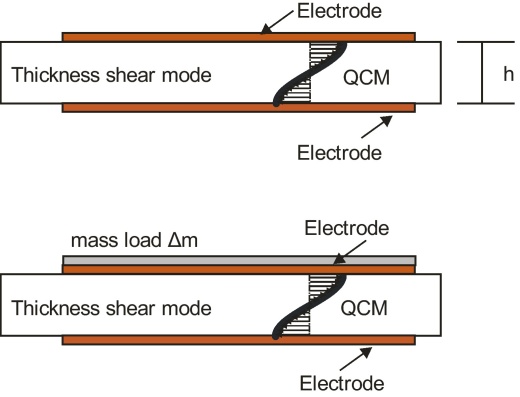
Following the resonance condition (6), the fabrication of TSM resonators with higher values of f0 is basically achieved by reducing the plate thickness h of the sensor, which is illustrated in Fig. 3.
| (6) |
The transverse shear waves induced from the quartz crystal propagate in the fluid and are damped by the fluid. At a distance δ from the crystal surface the oscillation amplitude is vanishing. This penetration depth is reciprocal to the oscillation frequency [9].
| (7) |
A higher oscillation frequency reduces the volume above the sensor which acts as an unwanted mass load.
Fig. 3.
The drawing in the upper part shows a QCM which resonates in the thickness shear mode. The displacements over thickness are indicated by the arrows. The thickness h of the quartz resonator is crucial for the fundamental resonance frequency. In the lower part an additional mass load adheres to the sensor which results in a shift of the resonance frequency.
For practical purposes, the minimum thickness of the quartz crystal is constrained by mechanical stability issues. Most applications utilize either 5 MHz or 10 MHz resonators, with a corresponding plate thickness of 0.33 mm or 0.17 mm, which provides a sufficient mechanical stability. Thus, these sensors can easily be clamped into the wall of a fluidic chamber, with the drawback of being less sensitive to changes in mass deposition and a higher penetration depth.
Occasionally, sensors with resonance frequencies between 20 MHz and 30 MHz are used (e.g., [10,11]). These sensors are smaller in diameter (typically about 8 mm) and already rather thin and fragile.
An alternative approach to realize quartz crystals with higher fundamental frequencies is to form quartz resonators with an “inverted mesa” structure [12]. These quartz disks with a diameter and thickness of about 5 mm and 0.1 mm, respectively, have a membrane of reduced thickness only in their small central circular area of about 5 mm2. The surrounding thicker material provides a better mechanical stability, which is independent from the membrane thickness. The thin center can have a thickness as low as 8.3 μm, which results in a high fundamental frequency (HFF) of up to f0 = 200 MHz. In setups which use high fundamental frequency quartz sensors in continuous (micro-)fluidic systems much effort is needed to seal and contact the sensor.
The consecutive and reversible formation of multi layers of molecules onto the surface of a quartz crystal was shown in [12]. These multi layers consist of phospholipids which are able to form a link with a monolayer of 1-octadecanethiol and the bio-molecule Streptavidin which is able to bind on biotin carrying phospholipids. In that contribution, the reagents were provided in a continuous flow.
In our contribution the sample liquid is provided to the QCM sensor element in terms of single droplets, which allows for an individual control and manipulation prior and posterior to the measurement. The functionality of transporting the droplets to and away from the sensor spot as well as measuring a biochemical reaction on the sensor surface has been integrated into a digital microfluidic platform. An AT cut quartz which resonates in a thickness shear mode with a fundamental resonance frequency of f0 ≈ 50 MHz was utilized.
2. Integration of the QCM
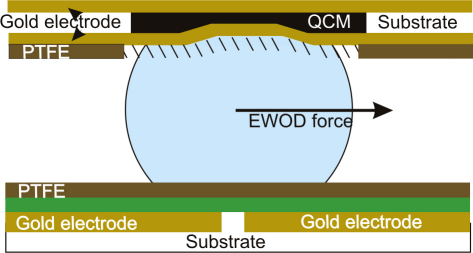
The microfluidic chip is designed as a closed system consisting of a base and a cover plate. The cover plate has to contain all the structured electrodes necessary for the EWOD actuations. Thus, the QCM, which is integrated into the base plate, is electronically separated from the top plate. Also, the actuation sequences for the droplet manipulations and the AC signal for the impedance measurements are not applied at the same time to rule out any interference. In order to integrate a sensor into a digital microfluidic platform the sensor has to display a hydrophobic surface. This hydrophobic surface allows for the movement of the droplet to and away from the sensor and can be an intrinsic property of the sensor or can be added by a coating with a hydrophobic material or by a surface treatment which enhances intrinsic hydrophobic properties. For an illustration of the microfluidic platform see Fig. 4. The functionality of the sensor depends on the ability to specifically bind bio-molecules. This functionality is provided by a hydrophobic self assembled monolayer (SAM), which is described in Section 2.2.
Fig. 4.
Assembled chip displaying a base plate suitable for EWOD actuations and a cover plate into which a QCM sensor coated by a hydrophobic self assembled monolayer of 1-octadecanethiol is integrated. A droplet of PBS is transported to and off the sensor site.
The contact area of the droplet has to match the membrane area of the HFF QCM. The two different contact angles θ1 and θ2, between the droplet and the QCM surface or between the droplet and the top plate surface, respectively, define the radius r of the droplet and the maximal plate distance h at a given droplet volume.
| (8) |
| (9) |
The droplet is confined between the base and the cover plate positioned in a 2 mm distance, as illustrated in Fig. 4 and is transported by means of EWOD actuation to and off the sensor field.
As a second step, the QCM has to be bonded as planar as possible into the base plate in order to avoid an obstacle for the droplet movement. The transverse shear mode of the QCM is stimulated by an electric field applied across the quartz crystal, which requires electric contacts on both sides of the active quartz crystal membrane. In order to meet all these requirements two alternative designs of the base plate were tested. The first design utilizes a HFF quartz crystal which was integrated as planar as possible into a circuit board. Onto both sides of the quartz crystal electric chromium/gold contacts were deposited. One side of the quartz crystal was in direct electric contact with the copper layer of the circuit board. The other side which is intended to be wetted by the droplet was electrically contacted by a gold wire bond; see left part of Fig. 5.
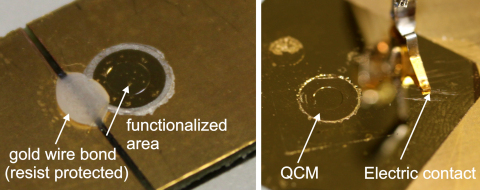
Fig. 5.
Left part: Photograph of a gold covered and functionalized QCM, which was bonded on a gold plated circuit board. Right part: A QCM was integrated in a glass plate. The both sides of the chip were covered with gold and the active side was bio-functionalized.
In the second design a hole of the same diameter as the quartz crystal was drilled into a glass substrate. The QCM was glued from the backside into that hole. The QCM was contacted on both sides by physical vapor deposited chromium/gold electrodes, which also covered the whole surface of the substrate; see right part of Fig. 5.
2.1. Design of the electrowetting platform
The microfluidic platform is realized as a covered EWOD system which consists of a base and a cover plate. The cover plate consists of a glass substrate onto which an electrode array (a 3 nm chromium adhesion layer plus a conductive 50 nm gold layer) was photo-lithographically structured. In order to obtain EWOD forces exceeding the minimal actuation forces of PBS on PTFE the applied voltage has to be at minimum 36 V. For the calculation of the minimal voltage the Eq. (10) suggested by [13] was used assuming a small hysteresis angle α. The hysteresis angle α represents the “pinning” of the contact line up to a certain unbalance of the surface tensions.
| (10) |
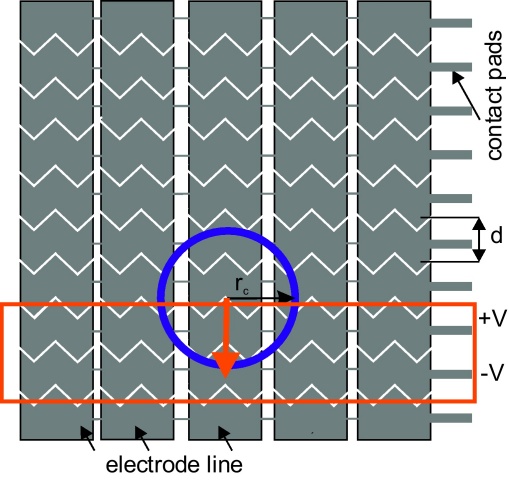
The dielectric layer which was used to isolate the electrode array consists of a 150 nm thick Al2O3 layer and a hydrophobic top coating of 1.6 μm Polytetrafluorethylen (Teflon AF, obtained from DuPont, PTFE). The breakdown voltage of the realized isolation layer exceeds 100 V. The maximal resulting reduction of the surface energy was 0.11 J m−2 which provides the electro capillary forces needed for dragging the droplet across more sticking surfaces. The width of each electrode (1 mm) was less than the half contact radius of the droplet separated from each other by a saw-tooth like 40 μm broad separation line. This design ensures an overlap of the droplet with at least three electrodes at an arbitrary position, see Fig. 6. An applied voltage between two adjacent electrodes neighboring the droplet induces a bipolar charge distribution in the droplet. The overlap area of the droplet with the two actuated electrodes has a reduced surface energy due to the applied voltage. Thus, the droplet relocates to the center of these two electrodes. Switching the actuation voltage to the next pair of electrodes pulls the droplet towards those. As a result, a consecutive movement of the droplet is achieved by actuating only ungrounded coplanar electrodes which gives the designer of the chip the freedom to vary the electrode configuration of the second plate into which the sensor is integrated.
Fig. 6.
Scheme of the electrode array suitable for EWOD actuation. The width d of the electrode is less than half of the contact radius rc of the droplet (indicated by the blue circle). This ensures that the droplet overlaps with at least three electrodes. Applying a voltage difference on two adjacent electrodes lowers the surface energy in the overlap region (indicated by the red box). Thus, droplet movement across the electrode lines (red arrow) can be achieved. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.2. Functionalization of the QCM
As described in Section 1.1, for a reliable EWOD actuation the coating of all surfaces contacting the droplet has to be hydrophobic with a low hysteresis angle. The first step of integrating a QCM sensor is to cover the surface with a hydrophobic layer which on the one hand allows for actuation, and on the other hand enables the binding of the bio-molecules of interest. This layer was formed by a self assembled monolayer (SAM) of 1-octadecanethiol, obtained from Sigma Aldrich. The functionalized surface of the gold covered glass substrate showed hydrophobic properties with a contact angle of 101 ± 1° and a low hysteresis angle α. First tests confirmed the feasibility of the surface tension driven transportation.
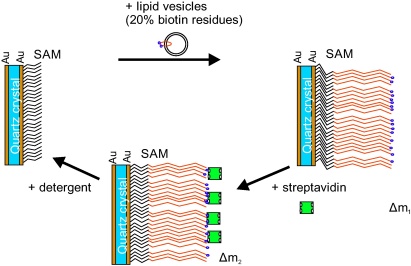
Furthermore, it enables the formation of a phosphor-lipid monolayer out of an aqueous buffer suspension, which serves as a sample reaction for bio-chemical bindings in general and can be measured by a corresponding change of the resonance frequency of the QCM. A solution of phosphor-lipids which carried biotin residues on 20% of the phospholipid head groups in a phosphate-buffered saline (PBS) were prepared as described in [14]. The biotin residues form a link with the bio-molecule streptavidin, which can be measured as a second additional mass load. The formation of this bio-molecular multilayer onto a gold covered surface is schematically depicted in Fig. 7.
Fig. 7.
Scheme of the formation of multiple layers of bio-molecules onto a gold covered surface. The self assembled monolayer (SAM) enables the binding of phospholipids which results is a change of the mass deposition Δm1. A second bio-film is formed by exposing the protein streptavidin to biotin residues of the phospholipids Δm2. By washing the surface with the detergent octyl-d-glucopyranoside the complete bio-film down to the SAM is removed.
3. Measurements
3.1. Test of the integrated QCM
A series of phosphate buffered saline (PBS) droplets of approximately 5 μl volume were transported to and away from the QCM sensor. Initial reference spectra were taken at ambient temperature of 24.1 °C to determine the fundamental frequency and the corresponding quality factor Q = f0/ΔfFWHM in an unloaded condition and loaded with the PBS droplet. In both cases the sensors surface was covered by the self assembled monolayer.
The results of these initial reference spectra of different devices are listed in Table 1.
Table 1.
The fundamental frequency and the quality factor Q of different fabricated QCM devices.
| Device number | Electric contact | Unloaded |
Loaded with PBS droplet |
||
|---|---|---|---|---|---|
| f0/Mhz | Q | f0/MHz | Q | ||
| 1 | PVD gold contact | 50.986249 | 2549 | 50.961249 | 1020 |
| 2 | Bonded/1 | Not recorded | 50.777582 | 394 | |
| 3 | Bonded/2 | 50.983244 | 3288 | 50.948350 | 1298 |
| 4 | Bonded/3 | 50.894244 | 686 | 50.779073 | 163 |
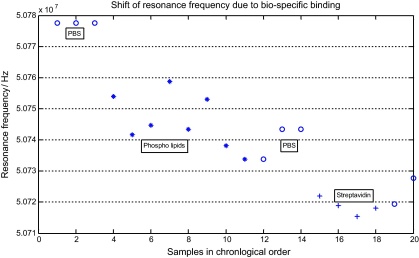
The results of these initial measurements on the PBS droplets are indicated as the first group of “o” in Fig. 8. Subsequently, a series of PBS droplets containing biotin carrying phosphor lipids (“*” in Fig. 8) was directed over the QCM sensor. In agreement with the theory, a shift to lower resonance frequencies of the QCM was determined via impedance measurements and a stable level was reached after 8 min indicating the formation of a phospholipid monolayer, which carries biotin residues on 20% of the phospholipid head groups [14]. Measurements on pure PBS droplets (second group of “o” in Fig. 8) confirmed the stability of the formed monolayer. A multilayer formation was achieved by directing a series of streptavidin enriched PBS droplets (“+” in Fig. 8) to the QCM sensor spot. The streptavidin molecules form a bio-specific link with the Biotin-presenting phospholipid coating of the QCM [15]. An additional decrease of ∼20 kHz in the resonance frequency of the QCM was determined; see Fig. 8. After the first three measurements in a time interval of 3 min the signal did not decrease further. Finally, two PBS droplets (third group of “o” in Fig. 8) were placed on top of the sensor in order to confirm the multilayer formation as the origin of the frequency shift and to rule out shifts due to a different viscosity of the dissolution. Each new layer of biomolecules resulted in a damping of the sensor signal which lowered the Q-factor to approximately one third of the initial value.
Fig. 8.
Results obtained from droplet samples moved to the QCM-sensor. At the beginning the fundamental resonance frequency was determined by measuring three times the resonance frequency of the QCM in contact with droplets of phosphate buffered saline (PBS; o). As a second step PBS droplets containing phosphor lipids were transported to the sensor site. The formation of a bio-molecule layer is detected by a decrease of the resonance frequency (*). Measurements on three additional PBS droplets confirmed the mass deposition as the origin of frequency shift. An additional exposure of the sensor with PBS droplets containing streptavidin (+) resulted in a second drop of the resonance frequency indicating the formation of a second molecule layer.
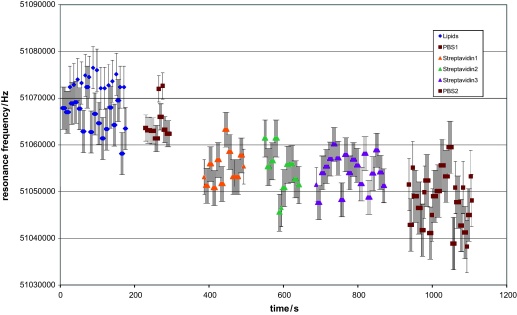
The few obtained resonance frequencies, depicted in Fig. 8, showed a large fluctuation between different droplets of the same chemical constitution. In order to show if the droplet manipulations or if the method of the QCM integration is responsible for the large variance, many measurements were performed on the same droplet in contact with the QCM sensor. The binding load of the sensor should not be altered during these measurements because of the identical composition of the used droplets. For a first measurement, the resonance frequency of the integrated gold covered QCM sensor loaded with a PBS droplet was determined. The measurement results showed a standard variance of 390 Hz. A second measurement was performed on a series of droplets which allowed for the binding of streptavidin onto a layer of phosphor-lipids. The initial droplet containing phosphor-lipids was first replaced by a PBS droplet, then by a series of three droplets containing streptavidin and finally by a PBS droplet. The resonance frequency was determined several times for each of these droplets which are depicted in Fig. 9. The variance within one block of measurement data, corresponding with the same droplet, was in the range of 2.8–4 kHz which is quite high. On the other hand no difference between the single droplets of the same composition could be seen. Thus, we conclude that the exchange of the droplet by capillary forces does not change the binding status of the QCM surface. The anticipated increase of sensitivity due to unloading the sensor by dragging the droplet away could not be found. Because a liquid film remained on the sensor site which alters the effective mass of the sensor and did increase the variations in the measured resonance frequencies even further.
Fig. 9.
Results obtained from droplet samples moved to the QCM-sensor. Each block of measurement data is taken from the same droplet. Once more the reduction in the resonance frequency corresponds with a binding of molecules. No change in the measured resonance frequency and their variances between the droplets of the same composition could be seen (Streptavidin 1–3). Thus, the high variations are a direct result of the applied integration method. Measurements on two PBS droplets before and after the binding of streptavidin molecules confirm the bio-chemical binding.
After directing the first of the streptavidin enriched PBS droplets to the sensor a decrease in the contact angle was observed. This indicated that the binding of the bio-molecules corresponds with a higher surface energy which resulted in a sticking of the droplet. In the case of a positive detection of bindings the hydrophobic properties of the functionalized QCM get lost. Therefore, we were not able to drag the droplet away from the QCM by means of electrowetting actuation and had to remove the droplet manually.
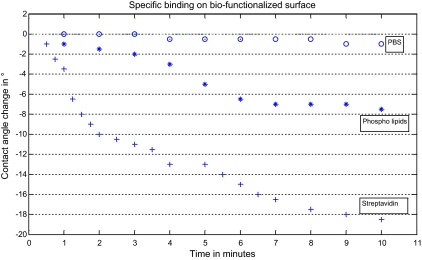
In order to quantify the sticking problem which is associated with the binding of bio-molecules we prepared a flat gold surface which was covered with the self assembled monolayer of 1-octadecanethiol. Onto this monolayer droplets of 5 μl volume of different compositions where deposited and the contact angle was measured with the optical contact angle meter G10 from Krüss GmbH, Germany. The changes of the contact angle of a PBS droplet without additional constituents due to evaporation or other non-binding interactions of the buffer with the SAM were measured over time. These measurement results are indicated with “o” in Fig. 10. The next measurement series examines the influence of the bio-specific binding of the phosphor lipids onto the contact angle. The measured contact angles of a single PBS droplet containing phospholipids are indicated with “*” in Fig. 10 and a decrease of 8° of the contact angle within a period of 10 min was measured.
Fig. 10.
The decrease of the contact angle of a PBS droplet enriched with phospholipids on the SAM surface is indicated (*). The decrease in the contact angle origins from the change of the surface composition due to the bio-chemical binding.
The droplet was removed by rolling of the surface and on the same spot a PBS droplet enriched with streptavidin was deposited. Results of contact angle measurement on this droplet are indicated with “+” in Fig. 10. The formation of a multilayer reduced the contact angle by 18° within 10 min. Thus, the origin of the droplet sticking can clearly be associated with the change of the surface energy due to the binding of the bio-molecules. Onto this new bio-film surface a droplet of PBS containing streptavidin (+) was deposited and the change of the contact angle measured over time. All changes are measured in relation to their initial contact angle. The contact angle of a pure PBS droplet (o) was measured in order to indicate the changes due to evaporation or other non-binding interactions of the buffer with the SAM.
4. Conclusion
From the recorded data of table one it can be seen that the loading with the PBS droplet strongly reduces the quality factor. The advantage of digital microfluidics is that the QCM can be wetted by bio-molecule containing liquids in order to perform chemical bonding. Following this, the sensor spot can be de-wetted by transporting the droplet away in order to perform undamped measurements. The quality factors as well as the statistical spread do strongly depend on the actual fixation for the quartz crystal into the base plate. The same integration and bonding method can result in very different resonating spectra, e.g., device 3 and 4 of Table 1. All devices fabricated by the two different methods showed large standard deviations. Thus, we were not able to reproduce the much lower deviations as stated in [12]. We expect that a stress free fixation of the quartz crystal would deliver less scattering of measurement results.
Both a chemical and a physical solution for the “sticking droplet” problem are currently being investigated. The chemical approach of either washing away the binding molecules or the new formation of a hydrophobic surface is feasible but has to be adapted to the restrictions of digital microfluidics. As a first test we brought a droplet detergent of octyl-d-glucopyranoside to the surface coated with phospholipids. The binding tail was removed and the original resonance frequency restored. The detergent itself was hydrophilic which rules out transportation by means of EWOD. The physical approach deals with a replacement of the sample droplet by a buffer droplet. This remains at the energetically favorable sensor spot and can be replaced by another sample or buffer droplet. The latter solution holds the advantage of keeping the surface of the functionalized area and the bio-molecules wetted but would exclude the measurements of the fundamental frequency of the undamped sensor.
We presented the integration of a functionalized QCM sensor with high fundamental frequency into a platform designed for EWOD actuations. The frequency shift of the resonance frequency, indicating the binding of bio-molecules out of single droplets to the functionalized surface, was detected. Thus, the applicability of functionalized QCM sensors in digital microfluidics was shown. The drawback of this detection method is that after the first binding of lipids the hydrophobic layer gets more and more hydrophilic. Therefore, the QCM sensor principle has to be applied to a different bio-chemical system where the hydrophobic properties are conserved during the detection process.
Acknowledgements
This work was supported by the Austrian Science Fund FWF under contract number L442-N14.
Biographies

Thomas Lederer obtained his Mag, (M.Sc.) degree in physics at the University of Vienna, Austria, in 2005. After his diploma in the field of Quantum Optics and Quantum Communications he prolonged his research at this topic for half a year. He is currently doing his Ph.D. at the Institute for Microelectronics and Microsensors at the Johannes Kepler University Linz, Austria, working in the field of microfluidics and microactuation.

Brigitte Paula Stehrer was born in Linz, Austria in 1981. She obtained her degree Diploma Engineer in technical physics from Johannes Kepler University in Linz. Since 2008 she is a Ph.D. student at the department of Soft Matter Physics in the same university. Her current research work has been focused on QCM biosensors and her research interests include biosensing and microfluidic systems.

Siegfried Bauer (M’99-SM’02) received the Master and Ph.D. degrees in physics from the Technical University in Karlsruhe in 1986 and 1990, respectively. In 1992 he joined the Heinrich Hertz Institute for Communication Engineering in Berlin, Germany. In 1996 he earned the Habilitation Degree from the University of Potsdam. In 1997 he became a Professor of Experimental Physics at the Johannes Kepler University in Linz, Austria. Since 2002 he has been head of the Soft Matter Physics Department. Dr. Bauer's research is devoted to functional soft matter and its application to flexible and stretchable sustainable electronics and energy harvesting.

Bernhard Jakoby obtained his Dipl.-Ing. (M.Sc.) in Communication Engineering and his doctoral (Ph.D.) degree in electrical engineering from the Vienna University of Technology (VUT), Austria, in 1991 and 1994, respectively. In 2001 he obtained a venia legendi for Theoretical Electrical Engineering from the VUT. From 1996 to 1999 he held the position of a Research Associate and later Assistant Professor at the Delft University of Technology. From 1999 to 2001 he was with the Automotive Electronics Division of the Robert Bosch GmbH, Germany, where he was conducting development projects in the field of automotive liquid sensors. In 2001 he joined the newly formed Industrial Sensor Systems group of the VUT as an Associate Professor. In 2005 he was appointed Full Professor of Microelectronics at the Johannes Kepler University Linz, Austria. He is currently working in the field of liquid sensors and monitoring systems.

Wolfgang Hilber obtained his Dipl.-Ing. (M.Sc.) in physics and his doctoral (Ph.D.) degree in technical sciences at the Johannes Kepler University Linz, Austria, in 1993 and 1997, respectively. After some years of collaboration in research projects at the Carinthian Tech Research (CTR GmbH) in Villach, Austria, and at the Johannes Kepler University Linz, Austria, in the field of in-line process control for semiconductor manufacturing processes, he joined the R&D division of E+E Electronics GmbH, Austria, in 2000. There he conducted development projects in the field of thin-film sensor technology. He is currently (since 2006) working as an assistant at the Institute for Microelectronics and Microsensors at the Johannes Kepler University Linz in the field of microsensors and microfluidics.
References
- 1.Shapiro B., Moon H., Garrell R.L., Kim C.-J. Equilibrium behaviour of sessile drops under surface tension, applied external fields, and material variations. J. Appl. Phys. 2003;93:3. [Google Scholar]
- 2.Zeng J., Korsmeyer T. Principles of droplet electrohydrodynamics for lab-on-a-chip. Lab Chip. 2004;4:265–277. doi: 10.1039/b403082f. [DOI] [PubMed] [Google Scholar]
- 3.Lederer T., Hilber W., Jakoby B. Proceedings MME. 2009. Optimization of 2-D cross-reference EWOD platform. [Google Scholar]
- 4.Paik P., Pamula V., Fair R.B. Rapid droplet mixers for digital microfluidic systems. Lab Chip. 2003:253–259. doi: 10.1039/b307628h. [DOI] [PubMed] [Google Scholar]
- 5.Cho S.K., Moon H., Kim C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003;12:1. [Google Scholar]
- 6.Bruckenstein S., Shay M. Experimental aspects of use of the quartz crystal microbalance in solution. Electrochim. Acta. 1985;30(10):1295–1300. [Google Scholar]
- 7.Sota H., Yoshimine H., Whittier R.F., Gotoh M., Shiohara Y., Hasegawa Y., Okahata Y. A versatile planar QCM-based sensor design for nonlabeling biomolecule detection. Anal. Chem. 2002;74(15):3592–3598. doi: 10.1021/ac025526b. [DOI] [PubMed] [Google Scholar]
- 8.Sauerbrey G.Z. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift für Physik. 1959;155:206–222. [Google Scholar]
- 9.Landau L.D., Lifshitz E.M. Pergamon Press; 1959. Fluid Mechanics. [Google Scholar]
- 10.Kanazawa K.K., Gordon J. The oscillation frequency of a quartz resonator in contact with a liquid. Anal. Chim. Acta. 1985;175:99–105. [Google Scholar]
- 11.Okahata Y., Niikura K., Furusawa H., Hisao M. A highly sensitive 27 MHz quartz-crystal microbalance as a device for kinetic measurements of molecular recognition on DNA strands. Anal. Sci. 2000;11:1113–1119. [Google Scholar]
- 12.Sagmeister B.P., Graz I.M., Schwödiauer R., Gruber H., Bauer S. User-friendly, miniature biosensor flow cell for fragile high fundamental frequency quartz crystal resonators. Biosens. Bioelectron. 2009;24:2643–2648. doi: 10.1016/j.bios.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Berthier J., Dubois Ph, Clementz Ph, Claustre P., Peponnet C., Fouillet Y. Actuation potentials and capillary forces in electrowetting based microsystems. Sens. Actuators A-Phys. 2007;134(2):471–479. [Google Scholar]
- 14.Lingler S., Rubinstein I., Knoll W., Offenhäusser A. Fusion of small unilamellar lipid vesicles to alkanethiol and thiolipid self-assembled monolayers on gold. Langmuir. 1987;13:7085–7091. [Google Scholar]
- 15.Kalb E., Frey S., Tamm L.K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta. 1992;1103:307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]