Abstract
In intact blood vessels, many vasodilators act by stimulating the release from endothelium of factor(s) that relax vascular smooth muscle and stimulate increases in cGMP. To investigate how endothelium regulates cGMP production in vascular smooth muscle, bovine aortic endothelial cells and rat aortic smooth muscle cells were cultured both separately and together in cocultures for 48 hr. Nitroprusside (1 mM) increased intracellular cGMP concentration 30-fold in smooth muscle cells (from a basal level of 103 +/- 54 fmol/mg of cell protein to 2920 +/- 1800 fmol/mg) but only 2-fold in endothelial cells (from 41 +/- 7 fmol/mg to 93 +/- 23 fmol/mg). When endothelial and smooth muscle cells were cocultured as a mixed cell population (1:1 cell ratio), both basal and nitroprusside-stimulated cGMP levels were significantly increased (550 +/- 250 and 13,240 +/- 9950 fmol/mg of total cell protein, respectively). The calcium ionophore A23187 (10 microM) caused no increase in cGMP concentration in either cell type cultured alone but produced a 6-fold increase in cocultures. Neither aspirin nor 5,8,11,14-icosatetraynoic acid influenced these results. No changes in cAMP levels were detected. Using cocultures in which one cell type was grown on microcarrier beads, we have shown that cGMP increased only in vascular smooth muscle cells and was not dependent upon the formation of junctions between endothelium and smooth muscle cells. In long-term (48-hr) mixed-cell cocultures, but not in short-term microcarrier cocultures, amplification of the nitroprusside-induced increase in cGMP was observed. These results show that responses associated with endothelium-dependent relaxation can be reconstituted in cultured endothelial and vascular smooth muscle cells and that endothelium generates a humoral factor(s) that stimulates accumulation of smooth muscle cGMP and has a longer-term effect that amplifies guanylate cyclase stimulation by nitroprusside, a drug acting directly upon smooth muscle to stimulate formation of the cyclic nucleotide. Cultured cells provide a valuable model system for the study of endothelium-vascular smooth muscle interactions.
Full text
PDF


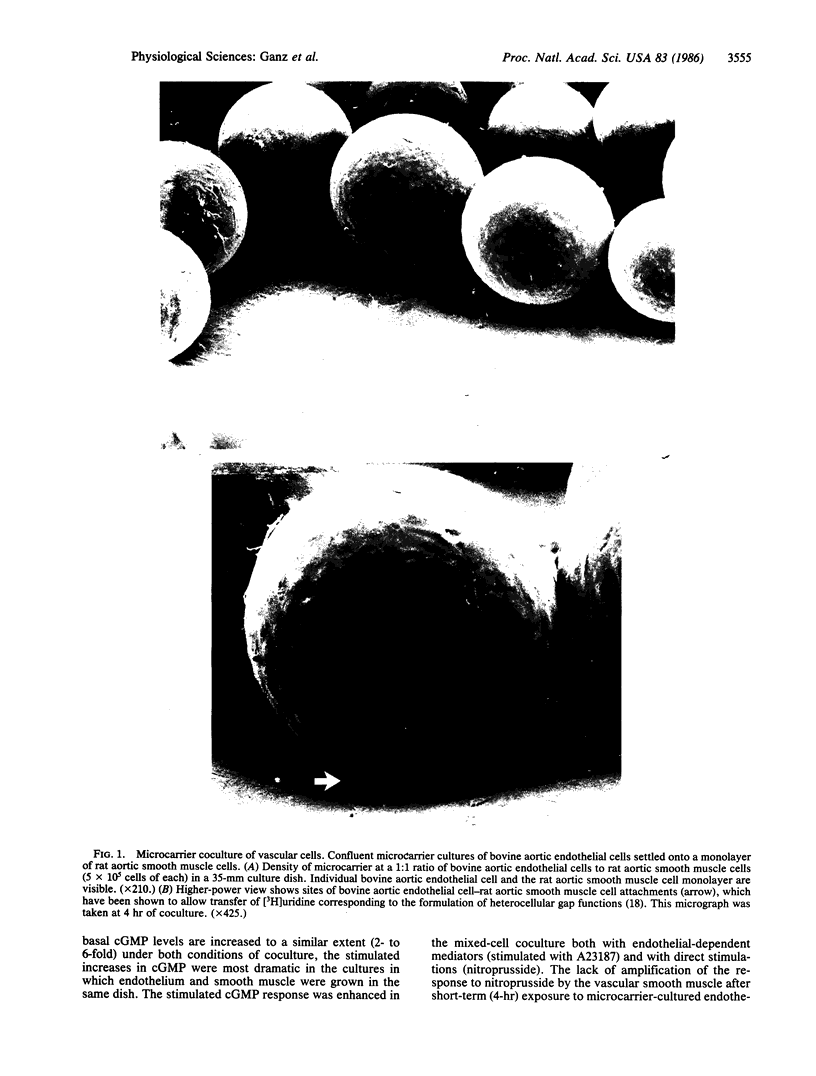

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. A., Alexander R. W., Ekstein L. S., Atkinson W. J., Gimbrone M. A., Jr Angiotensin increases cytosolic free calcium in cultured vascular smooth muscle cells. Hypertension. 1985 May-Jun;7(3 Pt 2):I105–I109. doi: 10.1161/01.hyp.7.3_pt_2.i105. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Ganz P., Diehl P. S. Reversible microcarrier-mediated junctional communication between endothelial and smooth muscle cell monolayers: an in vitro model of vascular cell interactions. Lab Invest. 1985 Dec;53(6):710–718. [PubMed] [Google Scholar]
- Davies P. F., Kerr C. Co-cultivation of vascular endothelial and smooth muscle cells using microcarrier techniques. Exp Cell Res. 1982 Oct;141(2):455–459. doi: 10.1016/0014-4827(82)90234-8. [DOI] [PubMed] [Google Scholar]
- Davies P. F. Microcarrier culture of vascular endothelial cells on solid plastic beads. Exp Cell Res. 1981 Aug;134(2):367–376. doi: 10.1016/0014-4827(81)90436-5. [DOI] [PubMed] [Google Scholar]
- Diamond J., Chu E. B. Possible role for cyclic GMP in endothelium-dependent relaxation of rabbit aorta by acetylcholine. Comparison with nitroglycerin. Res Commun Chem Pathol Pharmacol. 1983 Sep;41(3):369–381. [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gajdusek C., DiCorleto P., Ross R., Schwartz S. M. An endothelial cell-derived growth factor. J Cell Biol. 1980 May;85(2):467–472. doi: 10.1083/jcb.85.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz P., Gaspar J., Colucci W. S., Barry W. H., Mudge G. H., Alexander R. W. Effects of prostacyclin on coronary hemodynamics at rest and in response to cold pressor testing in patients with angina pectoris. Am J Cardiol. 1984 Jun 1;53(11):1500–1504. doi: 10.1016/0002-9149(84)90567-8. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Peach M. J. Calcium- and endothelial-mediated vascular smooth muscle relaxation in rabbit aorta. Hypertension. 1982 May-Jun;4(3 Pt 2):19–25. [PubMed] [Google Scholar]
- Singer H. A., Saye J. A., Peach M. J. Effects of cytochrome P-450 inhibitors on endothelium-dependent relaxation in rabbit aorta. Blood Vessels. 1984;21(5):223–230. doi: 10.1159/000158515. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Matsubara T., Nishizuka Y. Inhibitory action of guanosine 3', 5'-monophosphate on thrombin-induced phosphatidylinositol turnover and protein phosphorylation in human platelets. Biochem Biophys Res Commun. 1981 Jul 16;101(1):61–67. doi: 10.1016/s0006-291x(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J., Leusen I. Role of the endothelium in the vasodilator response of rat thoracic aorta to histamine. Eur J Pharmacol. 1983 Jan 28;87(1):113–120. doi: 10.1016/0014-2999(83)90056-0. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]





