Abstract
Mitochondria are key cytoplasmic organelles, responsible for generating cellular energy, regulating intracellular calcium levels, altering the reduction-oxidation potential of cells, and regulating cell death. Increasing evidence suggests that mitochondria play a central role in aging and in neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and Freidriech ataxia. Further, several lines of evidence suggest that mitochondrial dysfunction is an early event in most late-onset neurodegenerative diseases. Biochemical and animal model studies of inherited neurodegenerative diseases have revealed that mutant proteins of these diseases are associated with mitochondria. Mutant proteins are reported to block the transport of nuclear-encoded mitochondrial proteins to mitochondria, interact with mitochondrial proteins and disrupt the electron transport chain, induce free radicals, cause mitochondrial dysfunction, and, ultimately, damage neurons. This article discusses critical issues of mitochondria causing dysfunction in aging and neurodegenerative diseases, and discusses the potential of developing mitochondrial medicine, particularly mitochondrially targeted antioxidants, to treat aging and neurodegenerative diseases.
Keywords: Amyloid beta, Alzheimer’s disease, Amyotrophic lateral sclerosis, Amyloid precursor protein, Adenosine triphosphate, Caloric restricted, Electron transport chain, FRDA, Freidriech ataxia, Hydrogen peroxide, Huntington’s disease, Mitochondrial DNA, Peroxisome proliferator activated receptor–coactivator, Superoxide radical, Oxidative phosphorylation, Parkinson’s disease, Reactive oxygen species, SS peptide
Introduction
Increasing evidence suggests that mitochondria are involved in aging (Wallace 2005a; Beal 2005; Reddy 2006a, Reddy 2007), age-related neurodegenerative diseases (Wallace 1999; Swerdlow and Khan 2004; Hirai et al. 2001; Wallace 2005a; Beal 2005; Reddy and Beal 2005; Lin and Beal 2006; Martin 2006; Abeliovich and Beal 2006; Trushina and McMurray 2007; Fukui and Moraes 2007; Kim et al. 2007, Swerdlow 2007b), cancer (Wallace 2005a; Kroemer 2006; Galluzzi et al. 2006), and diabetes (Moreira et al. 2007; Rabol et al. 2006; Rolo and Palmeira 2006; Simmons 2006) (see Table 1). Genetic mutations are reported to cause a small proportion of neurodegenerative diseases, including Parkinson’s disease (PD), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS). Causal factors are still unknown for the vast majority of late-onset cases of AD, PD and ALS. In addition, pathomechanisms of disease processes and selective neuronal death in these diseases are not clearly understood. It has been established that aging is a major risk factor for developing several neurodegenerative diseases (Beal 2005; Wallace 2005a; Reddy 2006a; Reddy 2007). However, the precise connection between aging and neurodegenerative diseases is unclear. Recently, several studies and reviews have reported that age-related, mitochondrially generated reactive oxygen species (ROS) is a factor in the development and progression of late-onset neurodegenerative diseases (Swerdlow and Khan 2004; Beal 2005; Wallace 2005a; Reddy 2006a; Schapira 2006; Lin and Beal 2006; Reddy and Beal 2008). If this proves to be the case, one possible means to delay the progression of these diseases may be to treat mitochondria before symptoms appear.
Table 1.
Involvement of mitochondrial dysfunction in aging, cancer, diabetes, and neurodegenerative disorders
| Neurodegenerative disease |
Mutations in Gene or gene product affected by mitochondrial dysfunction |
|---|---|
| Aging | DNA mutations in the mitochondrial genome are the major contributing factors of aging and senescence. Accumulation of somatic mitochondrial DNA defects is responsible for ROS production, and oxidative damage in aged tissues. Over-expression of mitochondrially targeted antioxidants such as catalase, manganese superoxide dismutase, and methionine sulfoxide reductase decease oxidative damage, and extend healthy lifespan |
| Cancer | Cancer cells accumulate mitochondrial DNA defects leading to deficient mitochondrial respiration and ATP generation. Cancer cells up-regulate rate limiting processes and enzymes of glycolysis. Cancer cells adapt to decreased oxygen tension that is characteristic of most solid tumors as the pre-malignant lesion grows progressively |
| Diabetes | Hyperglycemia causes pathological consequences of type 1 and 2 diabetes. ROS production is increased in hyperglycemia, and mitochondrial ROS disrupt glucose stimulated insulin secretion by pancreatic beta cells because pancreatic beta cells are susceptible to oxidative damage |
| ALS | SOD1 is a cytosolic ROS scavenging enzyme. Mutant SOD1 aggregates have also been found in mitochondrial outermembrane, intermembrane space, and matrix. Mutant SOD1 induces free radicals and mitochondrial dysfunction in ALS patients. Over-expression of wild-type SOD1 plays a major role in causing late-onset sporadic ALS |
| Friedreich ataxia | Frataxin (gene product of Friedreich ataxia) is a mitochondrial protein responsible for heme biosynthesis and, formation of iron-sulfur clusters. Mutations in frataxin facilitate accumulation of iron in mitochondria, induce free radicals, and cause mitochondrial dysfunction |
| Late-onset AD | Mutations in mtDNA subunits and control region of mitochondrial genome are involved in late-onset AD |
| Familial AD | APP: Amyloid beta monomers and oligomers were found in mitochondrial membranes and matrix in APP transgenic mice, and neuroblastoma cells expressing mutant APP. Glycolysated APP is found in mitochondrial membranes in the brain cortical mitochondria of AD patients, and correlated with ApoE4 genotype. Amyloid beta binds to mitochondrial matrix protein, Aβ-induced alcohol dehydrogenase. Mitochondria APP and amyloid beta induce free radicals, decrease cytochrome oxidase activity, and inhibit ATP production |
| Presenilins (PS1 and PS2) were found in the mitochondrial membranes along with γ-secretase complex proteins APH and nicastrin | |
| ApoE4: N-terminal ApoE4 is associated with mitochondria, and cause mitochondrial oxidative damage | |
| HD | Mutant huntingtin (gene product of HD) binds to outer mitochondrial membrane and induces free radical production, and may interrupt with calcium uptake. Mitochondrial movement is interrupted in mitochondria from HD neurons |
| Late-onset PD | Mutations in mitochondrial gene, 12SrRNA found in late-onset PD, and causes mitochondrial dysfunction |
| Familial PD | Alpha synuclein is a cytoplasmic protein, primary component of Lewy Bodies. Over-expression of wild-type or mutant α-synuclein impairs mitochondrial function |
| DJ1 is a redox sensor protein, localized to mitochondrial intermembrane space and matrix | |
| PINK 1 is nuclear encoded mitochondrial kinase protein, and overexpression of PINK1 causes reduced mitochondrial membrane potential | |
| Parkin is a gene product of Ubiquitin E3 ligase and is associated with outer mitochondrial membrane, and induces free radical production | |
| LRRK2: Found to be associated with outer membrane and may induce free radicals | |
| OMI/HTRA2: Pro-apoptotic serine protease found in the mitochondrial intermembrane space |
Recent advances in molecular, cellular, biochemical, and animal-model studies of inherited neurodegenerative diseases revealed that mutant proteins––such as amyloid beta (Aβ) in AD; mutant huntingtin in HD; mutant SOD1 in ALS; mutant parkin, mutant DJ1, and mutant α-synuclein in PD; and frataxin in Freidriech ataxia (FRDA)––are associated with mitochondria, leading to increased production of free radicals, low production of cellular ATP, and ultimately cell death (Lin and Beal 2006). It is now known that mitochondrial dysfunction is a common cellular change in the disease process of several inherited neurodegenerative diseases. The involvement of mitochondrial dysfunction in both inherited and late-onset neurodegenerative diseases points to the need to develop therapies to treat mitochondria in age-related neurodegenerative diseases. This article discusses structural and functional changes that have been found in mitochondria in aged individuals and in patients with age-related neurodegenerative diseases. This article also outlines the potential of mitochondrial medicine to treat aging and neurodegenerative diseases.
Mitochondrial Synthesis, Lifespan, and Decay
Mitochondria, which are cytoplasmic organelles, are the result of a symbiotic association between a glycolytic protoeukaryotic cell and oxidative bacterium about 1.5 billion years ago (Reddy and Beal 2005). Mitochondria are present virtually in all eukaryotic cells, including neurons. The half-life of neuronal mitochondria is about one month (Reddy 2007). However, the decay of old mitochondria and the synthesis of new mitochondria are active in all cells, including neurons. Because of continuous mitochondrial recycling, the function of mitochondria is well maintained in neurons (Chang and Reynolds 2006). The precise mechanisms by which the mitochondrial synthesis happens are not completely understood. There is evidence to suggest that mitochondrial DNA (mtDNA) divide, facilitate mitochondrial replication, and complete the synthesis of new mitochondria. Mitochondria also increase from preexisting mitochondria due to mitochondrial fission, which occurs in all eukaryotic cells, including neurons (Chang and Reynolds 2006; Reddy 2007).
A mitochondrion contains 2–10 copies of mtDNA (Reddy and Beal 2005). The mtDNA copy number, and the number of mitochondria per cell, is purely dependent on cell type, and energy demand in the cell. For example, the number of mitochondrial DNA in fertilized human oocytes is about 250,000, while for unfertilized oocytes, the mean mitochondrial DNA number is 164,000 (Van Blerkom 2008). In pyramidal neurons from hippocampus and cortex, the copy number of mitochondrial DNA, and the number of mitochondria are expected to be high because energy demand is high in those neurons.
In an oxidative stress state or when cells are exposed to mitochondrial toxins, mitochondria divide rapidly due to increased free radical production and the activation of mitochondrial fission proteins (Reddy 2007). The newly synthesized mitochondria in a diseased state may be generally heterogeneous (both defective and functionally active). This fact was supported by a recent AD study by Hirai et al. (2001). They found increased oxidative damage in AD patients, a striking and significant increase in mtDNA in cell body mitochondria in the pyramidal neurons, and cytochrome oxidase in their neuronal cytoplasm, suggesting that oxidative stress may be a key factor for increased mitochondrial turnover and the production of defective mitochondria (Hirai et al. 2001).
It is well established that mitochondria in the cell body are transported down the axons and dendrites to serve cellular energy demands (Reddy and Beal 2008). If mitochondria localized in the cell body are damaged or are otherwise degraded, such as by aging or by mutant proteins, these defective mitochondria may be transported to synaptic terminals via natural mitochondrial trafficking, where they produce low levels of ATP due to their degradation. Synaptic terminals are sites of high-energy demand. Therefore, increased transport of mitochondria to synaptic terminals may be critical and necessary to deliver mitochondria. Older synaptic mitochondria may exhibit greater damage from oxidative stress than cell-body mitochondria (Reddy and Beal 2008), and this increased damage may affect neurotransmission and may ultimately be responsible for clinical symptoms in neurodegenerative diseases.
Mitochondrial Structure and Function
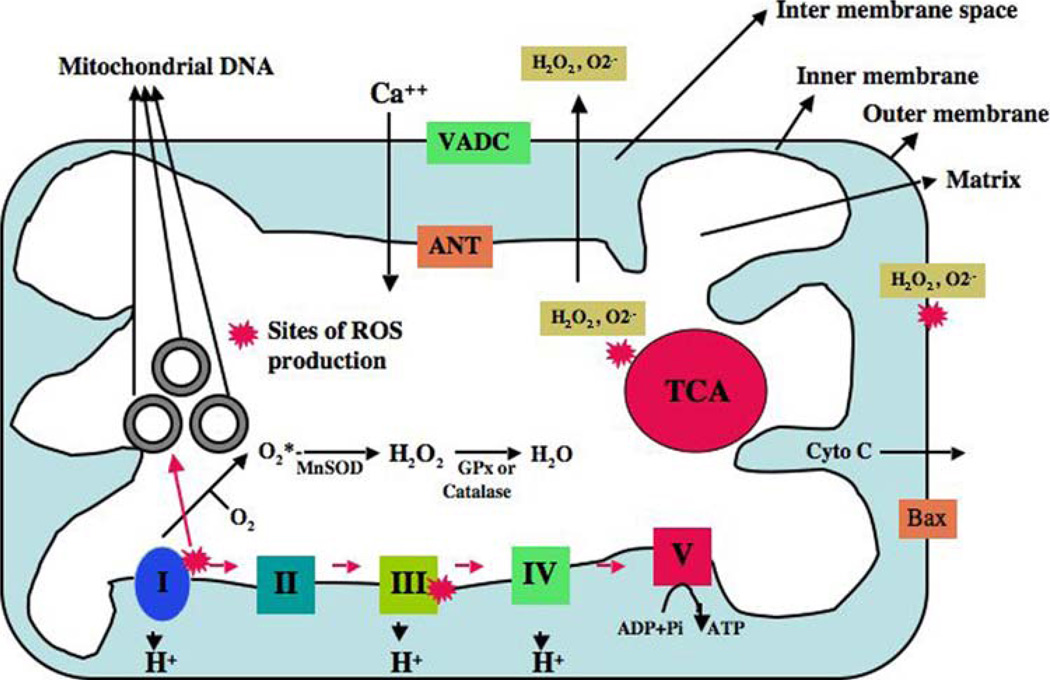
Mitochondria are the power houses of cells, providing energy for several cellular functions, including intracellular calcium regulation, ATP production, the release of proteins that activate the caspase family of proteases, and the alteration of the reduction-oxidation potential of cells and free radical scavenging. Mitochondria are compartmentalized with two lipid membranes: the inner mitochondrial membrane and the outer mitochondrial membrane (Reddy 2007). The inner mitochondrial membrane houses the mitochondrial respiratory chain and provides a highly efficient barrier to the flow of ions. The inner mitochondrial membrane covers the mitochondrial matrix, which contains tricarboxylic acid and beta-oxidation. The outer mitochondrial membrane is basically porous and allows the passage of low molecular weight substances between the cytosol and the intermembrane space (see Fig. 1).
Fig. 1.
Structure of mitochondria and sites of free radical generation. A mitochondrion is compartmentalized with two lipid membranes: the inner mitochondrial membrane and the outer mitochondrial membrane. The inner mitochondrial membrane houses the mitochondrial respiratory chain and provides a highly efficient barrier to ionic flow. Each mitochondrion contains 2–10 copies of a mitochondrial genome. In the ETC complexes I and III leak electrons to oxygen, producing primarily superoxide radicals. Superoxide radicals are dismutated by manganese superoxide dismuase and produce H2O2. In addition, ETC involves H2O2 reducing to H2O and O2 by catalase or glutathione peroxidase accepting electrons donated by NADH and FADH2 and then yielding energy to generate ATP from adenosine diphosphate and inorganic phosphate. Free radicals are also generated by tricarboxylic acid in the matrix. These radicals are carried to the cytoplasm via voltage-dependent anion channels, and may involve oxidation DNA and proteins in the cytoplasm
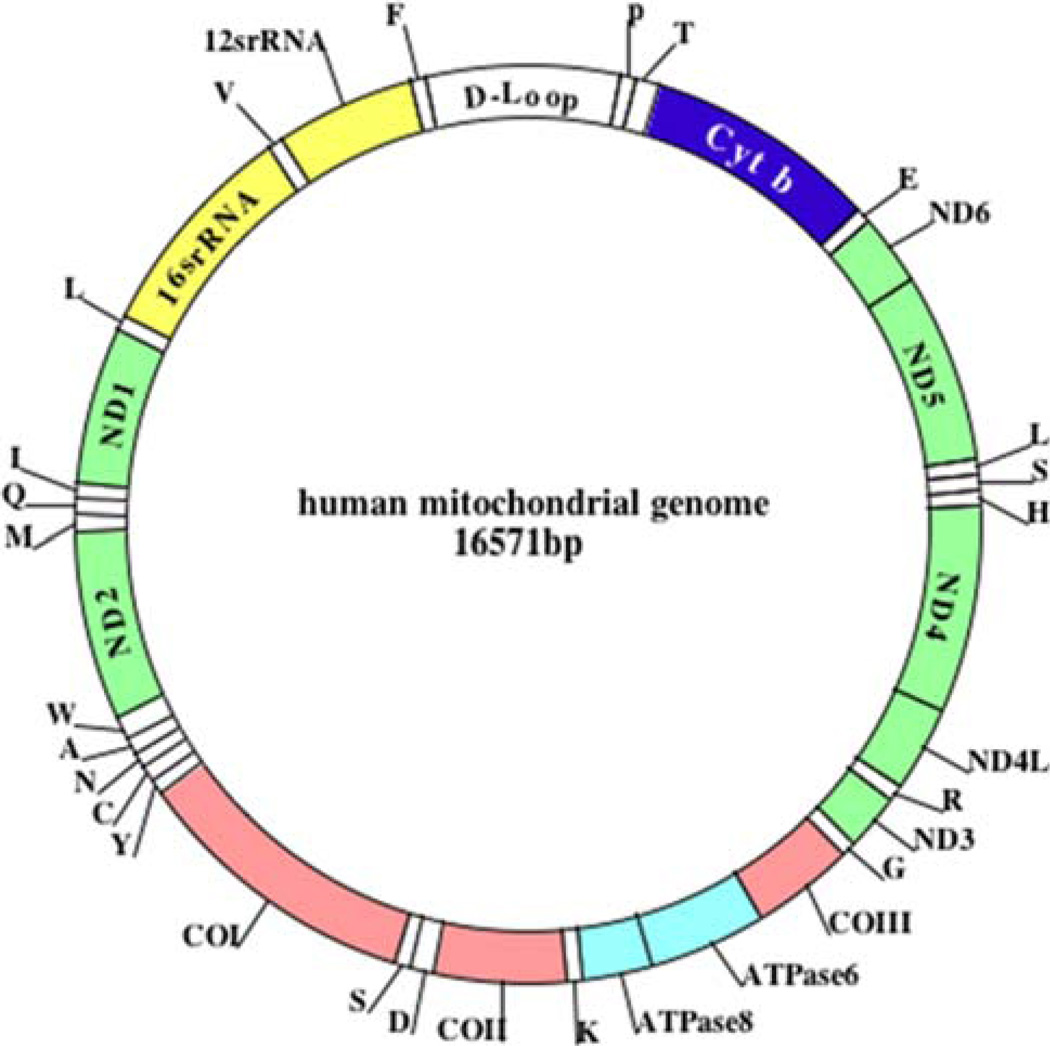
Mitochondria are controlled by both nuclear and mitochondrial genomes. MtDNA consists of a 16.5 kb double-stranded circular DNA molecule that is maternally inherited (Anderson et al. 1981) (Fig. 2). MtDNA has 2 strands: a guanine-rich heavy strand (or outer strand) and cytosine-rich light strand (or inner strand). The electron transport chain (ETC) comprises 13 polypeptide genes. MtDNA also encodes the 12S and 16S rRNA genes, and the 22 tRNA genes, which are required for mitochondrial protein synthesis. MtDNA encodes 7 subunits (ND1, 2, 3, 4, 4L, 5, and 6) of the 46 subunits constituting complex I, one of 11 subunits of complex III, 3 of 13 subunits of complex IV, and 2 of 17 subunits of complex V (Reddy and Beal 2005). Nuclear genes encode the remaining mitochondrial proteins (other than ETC), metabolic enzymes, DNA and RNA polymerases, ribosomal proteins, and mtDNA regulatory factors, such as the mitochondrial transcription factor A. Nuclear mitochondrial proteins are synthesized in the cytoplasm and are subsequently transported into mitochondria. The import of the majority of mitochondrial proteins is accomplished by membrane-spanning, multi-subunit translocators of the outer and inner mitochondrial membranes.
Fig. 2.
The structure of human mitochondrial DNA. As shown, mitochondrial DNA is circular, and it has 2 strands––a guanine-rich outer strand and cytosine-rich inner strand. The mitochondrial DNA is encoded by 13 polypeptide chains, which encode all essential components of ETC. The mitochondrial DNA also encodes the 22 tRNAs (which are indicated as capital letters outside of outer mitochondrial strand), 12S and 16S rRNA. D-loop represents the control region of mitochondrial DNA. The somatic mtDNA mutations have also been reported to be elevated in Parkinson’s disease (Ikebe et al. 1990; Ozawa et al. 1990), Huntington’s disease (Horton et al. 1995), AD (Corral-Debrinski et al. 1994), livers of alcoholics (Fromenty et al. 1995), ovaries of post-menopausal women (Fromenty et al. 1995), and reduced mobility sperm (Kao et al. 1995)
Mitochondrial ATP is generated via oxidative phosphorylation (OXPHOS). OXPHOS is dependent on 5-subunit polypeptide complexes (I–V) located within the inner mitochondrial membrane (Fig. 1). These polypeptide complexes utilize flavins, nicotinamides, cytochromes, iron-sulfur clusters and copper centers to transfer electrons in a series of oxidation reduction steps. Through this succession of oxido-reduction reactions, electrons pass along the ETC complexes and generate an electrochemical gradient by fueling the extrusion of protons from the matrix across the inner mitochondrial membrane complexes. ATP is then generated by the dissipation of this proton gradient through complex V. Mitochondria are critical in the metabolism of all the mammalian cells, including brain neurons. Abnormalities in mitochondrial structure and function may lead to age-related neurodegenerative diseases (Reddy 2007).
Reactive Oxygen Species Production and Mitochondrial Structural Changes
Mitochondrial structure and shape are maintained by the balancing of 2 opposing processes of mitochondria: mitochondrial fission and mitochondrial fusion. Mitochondrial fusion is the integration of 2 mitochondria. Fusion allows mitochondria within a cell to support each other. Mitochondrial fusion protects cells from the detrimental effects of mtDNA mutations by allowing functional complementation of mtDNA gene products. Mitochondrial fusion changes the shape of mitochondria to suit a particular developmental function (Chan 2006). Mitochondrial fusion lacking cells show decreased mitochondrial function. (Chan 2006). It is possible that mitochondrial fusion is minimal at synaptic terminals because isolated mitochondria are localized at synaptic terminals. The absence of mitochondrial fusion may lead to increased production of ROS and low ATP, and may ultimately lead to decreased neurotransmission, particularly in a diseased state.
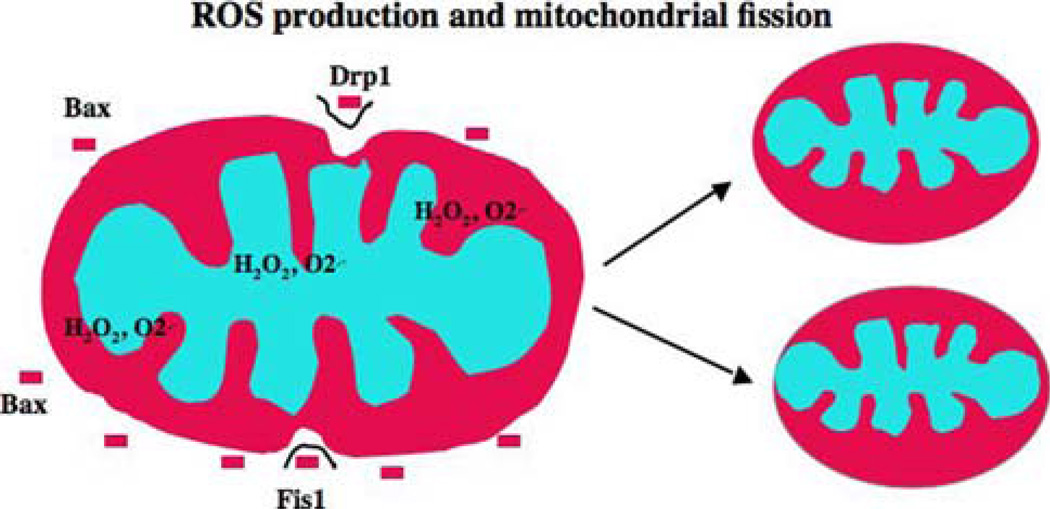
Mitochondrial fission is the fragmentation of mitochondria. Mitochondrial fission plays an important role in apoptosis (Bossy-Wetzel 2003). Mitochondrial fission is activated by Drp1 and Fis1 molecules. Drp1 and Fis1 molecules are activated when mitochondrial ROS levels are increased. Decreased levels of Drp1 and Fis1 reduce apoptosis. Mitochondrial fusion is reduced following the induction of apoptosis, and the over-expression of mitofusins can reduce apoptosis.
The production of ROS is an important physiological by-product of the ETC chain. During the transfer of electrons to molecular oxygen, 0.4% to 5% of electrons in the respiratory chain lose their way and participate in the formation of superoxide radicals (Reddy 2006a; Vinogradov and Grivennikova 2005). The generated from this loss of electrons ultimately activates the mitochondrial permeability transition pore and destroys the cell by apoptosis. The production of mitochondrial occurs primarily at discrete points in the ETC at complexes I and III, and in components of tricarboxylic acid, including α-ketoglutarate dehydrogenase (Fig. 1). is also generated by the outer mitochondrial membrane (Reddy 2007).
Several lines of evidence suggest that increased mitochondrial ROS is responsible for changes in mitochondrial morphology, including mitochondrial fission (Fig. 3): (1) Benard et al. (2007) found that when mammalian cells were treated with rotenone, mitochondrial ROS production increased in these cells and inhibited cellular ATP production, with these changes ultimately leading to mitochondrial fission and decay (Benard et al. 2007). (2) Yoon et al. (2006) studied mitochondrial fission and the high glucose-induced overproduction of ROS. The mitochondria underwent rapid fragmentation with a concomitant increase in ROS after exposure to high glucose concentrations, suggesting that the dynamic change of mitochondrial morphology in high-glucose conditions contributes to ROS overproduction and that mitochondrial fission and fusion may be previously unrecognized targets for the control of acute and chronic ROS production in hyperglycemia-associated disorders (Yoon et al. 2006). (3) Barsoum et al. (2006) recently found that mitochondria undergo profound mitochondrial fission and apoptosis in neurodegenerative diseases (Barsoum et al. 2006). (4) Kim et al. (2007) found that SIRT1 is upregulated in mouse models of AD and ALS, and in primary neurons challenged with neurotoxic insults. In cell-based models of tauopathies and ALS, SIRT1 and resveratrol promoted neuronal survival. In the inducible p25 tau transgenic mice, resveratrol reduced neurodegeneration in the hippocampus, prevented learning impairment, and decreased the acetylation of the known SIRT1 substrates PGC-1α and p53. Furthermore, injection of SIRT1 lentivirus in the hippocampus of p25 transgenic mice conferred significant protection against neurodegeneration (Kim et al. 2007). It is also possible that in late-onset neurodegenerative diseases, an increase in age-related ROS production may cause mitochondrial fragmentation, which may lead to mitochondrial dysfunction and neuronal cell death.
Fig. 3.
Production of reactive oxygen species and mitochondrial fission. Several mitochondrial toxins, including Aβ peptide, nitric oxide, and rotenone, induce the generation of mitochondrial reactive oxygen species (ROS). In addition, conditions such as hyperglycemia and aging may induce free radicals. The increased production of ROS activates fission molecules, including dynamin-related protein 1 (Drp1) and fission 1 (Fis1), which may lead to mitochondrial fission. Fis 1 protein is localized in the outer mitochondrial membrane, and Drp1 is localized mostly in the cytoplasm, and a fraction of Drp 1 is localized in outer mitochondrial membrane. Drp 1 punctates spots on mitochondria, and these punctate constriction spots lead to mitochondrial fission. The level of mitochondrial fission depends on the free radical production and the activity of Drp1 and Fis1
Aging and Mitochondrial Dysfunction
Mitochondrial dysfunction has been implicated in aging and age-related neurodegenerative diseases (Fig. 4) (Lin and Beal 2006; Reddy 2007; Swerdlow 2007a). Germ-line DNA changes in mtDNA cause mitochondrial diseases (Copeland 2008; Howell et al. 2005; Reddy and Beal 2005) (Table 1 and Fig. 2). Somatic DNA changes may contribute to aging and age-related diseases, including cancer, diabetes, and neurodegenerative diseases (Reddy and Beal 2005; Wallace 2005a, b; Reddy 2007). In aging, mitochondrial dysfunction is caused by an accumulation of mtDNA mutations and an increase in ROS production.
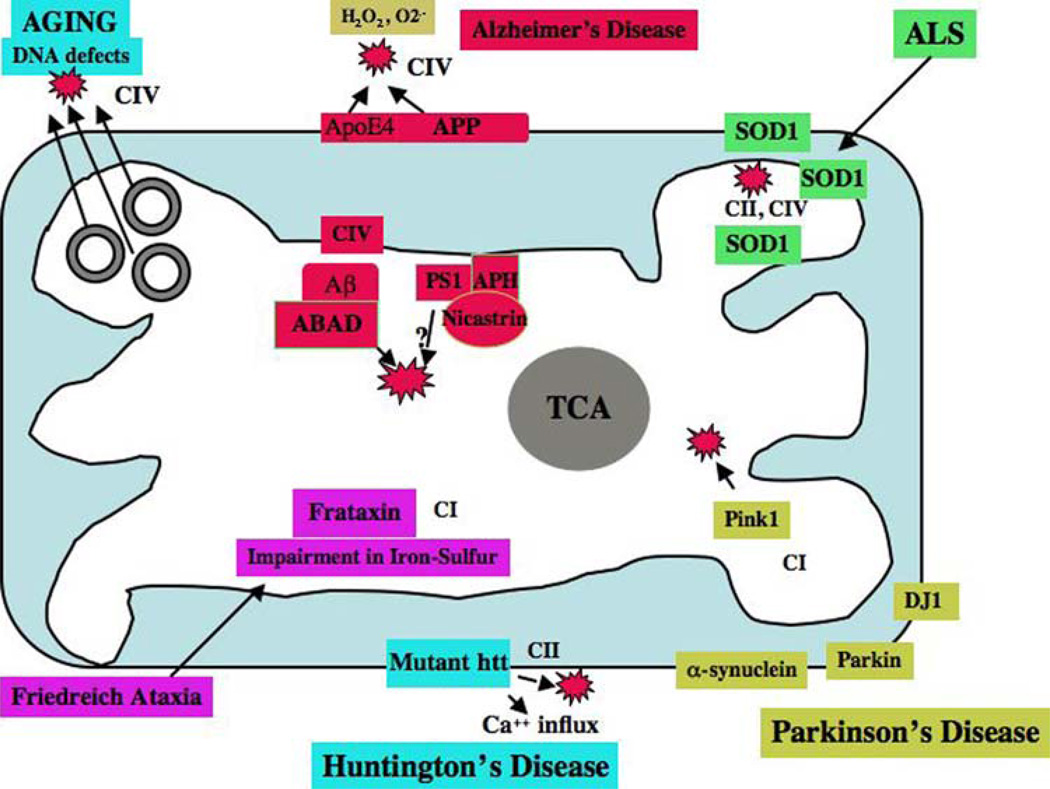
Fig. 4.
Interaction of proteins in neurodegenerative diseases and mitochondria. The accumulation of mitochondrial DNA mutations may induce ROS production and cause oxidative damage in aged tissues. In AD, age-related production of ROS and decreased ATP levels may contribute to the production of Aβ peptides. Aβ peptides enter mitochondria, induce free radicals, decrease cytochrome oxidase activity, and inhibit ATP generation. In AD brains, APP is transported to outer mitochondrial membranes, blocks the import of nuclear cytochrome oxidase proteins to mitochondria, and may be responsible for decreased cytochrome oxidase activity. In AD neurons (from AD patients, AD transgenic mice, APP cells), Aβ is found in the mitochondrial matrix and binds to ABAD, produces free radicals, and causes mitochondrial dysfunction. The N-terminal portion of ApoE4 is associated with mitochondria, induces free radicals, and causes oxidative damage. Gamma secretase complex proteins, such as presenilins, APH, and nicastrin, were found in the mitochondria and may contribute to Aβ production and free radical generation. In HD neurons, mutant Htt binds to the outer mitochondrial membrane and induces free radical production. H2O2may also interrupt with calcium uptake. In PD neurons, mutant proteins of α-synuclein, parkin, PINK1, and DJ1 are associated with mitochondria and cause mitochondrial dysfunction. Complex I activity is inhibited in PD neurons. In ALS, mutant SOD1 is localized in the inner and outer mitochondrial membranes and matrix, and induces free radical production and oxidative damage. Impairment of complexes II and IV are associated with ALS. Frataxin (a gene product in Freidriech ataxia) is a mitochondrial protein responsible for heme biosynthesis and the formation of iron-sulfur clusters. In Freidriech ataxia, mutant frataxin facilitates the accumulation of iron in mitochondria and induces free radicals
Mitochondrial ETC is responsible for the transfer of electrons from NADH or FADH to electron acceptors until the final transfer of electrons to oxygen leads to the production of H2O2. However, these biochemical events lead to 0.4–5% of electron leakage and, subsequently, leads to the production of ROS (Richter et al. 1988; Vinogradov and Grivennikova 2005). mtDNA is localized close to the sites of ROS production and may be vulnerable to DNA damage. Oxidized guanosine levels are higher in mtDNA than in nuclear DNA. mtDNA mutations that reduce the accuracy of electron transfer may increase the production of ROS and decrease ATP production. An increase in the production of ROS may further damage mtDNA, and vice versa.
In studies of aging and mtDNA, researchers found that several tissues from aged individuals have lower mitochondrial function than those from younger individuals (Cooper et al. 1992). Both mtDNA point mutations and deletions are highly prevalent in aged cells, and 8-hydroxy- 2-deoxyguanosine appears to be highly prevalent in aged tissues (Kujoth et al. 2006). To determine the role of mitochondrial mutations in aging, two research groups developed mouse lines containing a point mutation in the proofreading region of a DNA polymerase gene, the catalytic subunit of mtDNA polymerases (Trifunovic et al. 2004; Kujoth et al. 2005). The mutant DNA polymerase-γ exhibits normal DNA polymerase activity but lacks the exonuclease activity necessary for proofreading. Mice that carried homozygous mutations showed several folds of mtDNA point mutations in different tissues. These homozygous mutated mice had reduced lifespans and exhibited early onset of age-associated features, including weight loss, reduction in subcutaneous fat, hair loss, curvature of the spine, and osteoporosis, suggesting that mtDNA changes are critical in the aging process. From these aging studies, it appears that mitochondrially generated ROS (includingH2O2 and superoxide radicals) is a critical factor in aging (Trifunovic et al. 2004; Kujoth et al. 2005). If mitochondrially generated ROS production can be controlled, it may be possible to delay aging (i.e., extend a healthy lifespan). Several aging transgenic mice studies revealed that mitochondrially targeted antioxidants play an important role in decreasing free radical production and oxidative damage and aging phenotypes (Van Remmen et al. 2004; Ran et al. 2004; Chen et al. 2004; Schriner et al. 2005). Overall, these aging mice studies suggest that mtDNA mutations are critically involved in producing aging phenotypes, and further, mitochondrially generated free radicals are important factors in determining aging and longevity.
Alzheimer’s Disease
AD is a late-onset mental illness that is characterized by the loss of memory and an impairment of multiple cognitive function (Hardy and Selkoe 2002; Mattson 2004; LaFerla et al. 2007; Reddy and Beal 2008). It is estimated that by the year 2050, 50% of people worldwide (approximately 370 million people) who are 85 years of age or older will be afflicted with AD (Reddy and McWeeney 2006). With such a large, aged population poised to be afflicted, AD has become a major health concern that must be reckoned with. Early detection, prevention, and therapeutic interventions are urgently needed for this devastating illness.
The pathological features of AD are the presence of extracellular protein deposits (Aβ plaques) and intracellular neurofibrillary tangles, particularly in the brain regions related to learning, memory, and cognition (Selkoe 2001; Mattson 2004; Reddy and Beal 2008). Recent biochemical and cellular studies of postmortem brains from AD patients and AD transgenic mice revealed that mitochondrial oxidative damage and synaptic dysfunction are the earliest cellular events in AD development and progression.
Mutations in amyloid precursor protein (APP), presenilin 1 (PS1), and presinilin 2 (PS2) have been identified as causal factors in a small protion of total AD cases, but for the vast majority of AD cases, causal factors still unknown. Apolipoprotein (a cholesterol regulator gene) with genotype 4 (ApoE4) (Saunders et al. 1993) and SOLR1 (neuronal sortilin-related receptor 1 gene) are reported to involve in late onset AD (Rogaeva et al. 2007). Early detection, prevention, and therapeutic interventions are urgently needed for AD.
Alzheimer’s Disease and Mitochondrial Dysfunction
Molecular mechanisms that cause AD are still not clearly understood, particularly those mechanisms involved in early molecular changes in the disease process. Oxidative damage has been reported to be an early event in AD pathogenesis (Swerdlow and Khan 2004; Reddy and Beal 2005; Nunomura et al. 2006; Lin and Beal 2006; Reddy 2006a; Sultana et al. 2006; Ding et al. 2007). Oxidative damage is estimated to occur in the AD brain before the onset of Aβ aggregates, tau pathologies, synaptic dysfunction, and inflammation of brain (Reddy et al. 2004; Manczak et al. 2004; Nunomura et al. 2006; Lin and Beal 2006). Oxidative damage has been found in the brain, platelets, and fibroblast mitochondria of AD patients. Decreased levels of mitochondrial enzymes––including pyruvate dehydrogenase complex, α-ketoglutarate dehydrogenase complex, and cytochrome oxidase––have been reported (Parker et al. 1990; Gibson et al. 1998). Markers of oxidative damage have been found in lesions in both the brains of both AD patients (Smith et al. 1996; Bozner et al. 1997; Maurer et al. 2000; Manczak et al. 2004; Devi et al. 2006; Mohmmad Abdul et al. 2006) AD transgenic mice (Smith et al. 1998; Anandatheerthavarada et al. 2003; Reddy et al. 2004; Li et al. 2004; Manczak et al. 2006). These findings suggest that oxidative damage is an early, critical event in AD development and progression.
In the last decade, Swerdlow et al. have developed cybrids of mitochondria from sporadic AD patients and control subjects and studied mitochondrial function in those cybrids (Swerdlow et al. 1997; Trimmer et al. 2004; Khan et al. 2000). These studies reported increased ROS production and decreased cytochrome oxidase activity in mitochondrial cybrids prepared from sporadic AD patients compared to cybrids from control subjects (Swerdlow et al. 1997). Further, they also found increased mitochondrial abnormalities including mitochondrial swelling in AD cybrids compared to cybrids prepared from control subjects (Trimmer et al. 2004; Khan et al. 2000). These findings further confirm the presence of mitochondrial dysfunction in AD, and also suggest that cybrids are good models to study the mitochondrial function in neurodegenerative diseases.
Recent cellular, biochemical, and animal-model studies revealed that mutant proteins of AD, including presenilins, APP, Aβ, and ApoE4, are associated with mitochondria and cause oxidative damage and mitochondrial dysfunction in AD (Fig. 4) (Lustbader et al. 2004; Crouch et al. 2005; Caspersen et al. 2005; Manczak et al. 2006; Devi et al. 2006; Schmidt et al. 2007; Ankarcrona and Hultenby 2002; Hansson et al. 2004; Behbahani et al. 2006; Chang et al. 2005).
Amyloid Beta Induced Mitochondrial Dysfunction
Ever since the Aβ peptide was discovered in the brain, substantial research has focused on understanding Aβ toxicity in brain samples from patients with AD. The 4 kDa Aβ peptide, a cleaved product of APP, cleaves via sequential proteolysis of aspartyl beta secretase and presenilin-dependent γ secretase. APP is synthesized in the cell bodies of neurons and is anterogradely transported to nerve terminals via axons in the neurons. Several studies have recently found Aβ in mitochondrial membranes, supporting the hypothesis that Aβ enters mitochondria (Fig. 4) (Crouch et al. 2005; Caspersen et al. 2005; Manczak et al. 2006; Devi et al. 2006). Further, a time-course investigation of Aβ and free radical production revealed that H2O2 production significantly correlates with soluble Aβ but not insoluble Aβ, suggesting that in AD progression, soluble Aβ enters mitochondria and induces free radicals (Manczak et al. 2006). Further, Lustbader et al. (2004) recently demonstrated a direct link between Aβ and the mitochondrial matrix protein Aβ binding alcohol dehydrogenase in APP transgenic mice (Lustbader et al. 2004). In additional studies of neurons from these APP mice, the neurons showed increased free radicals, decreased mitochondrial complex IV activity, and decreased ATP levels (Takuma et al. 2005; Manczak et al. 2006). Collectively, these studies suggest that Aβ enters mitochondria and interacts with mitochondrial proteins, disrupts the ETC, and generates ROS, and that free radicals derived from molecular oxygen in the mitochondria inhibit the generation of cellular ATP (Reddy and Beal 2008).
Presenilins Induced Mitochondrial Dysfunction
It is well documented that presenilins play a major role in mitochondrial dysfunction and calcium homeostasis in AD (Guo et al. 1997, 1999; Keller et al. 1998; Chan et al. 2002; Tamagno et al. 2008; Mattson 2007). Several recent studies of presenilins and mitochondria found that presenilins and other γ-secretase complex proteins are localized to mitochondria (Fig. 4) (Ankarcrona and Hultenby 2002; Hansson 2004). Further, presenilins have been found to be part of a complex that involves FKBP38 and the anti-apoptotic protein Bcl2 (Wang et al. 2005a, b). FKBP38 is a regulator of Bcl2, localizes to mitochondria, and inhibits apoptosis by targeting Bcl2 to the mitochondrial membranes (Shirane and Nakayama 2003). Presenilins promote apoptosis by degrading FKBP38 and Bcl2 proteins in the endoplasmic reticulum and Golgi.
Recently, Behbahani et al. (2006) investigated the impact of presinilins and γ-secretase on mitochondrial function using mouse embryonic fibroblasts derived from wild-type, PS1−/−, PS2−/−, and PS double knock-out embryos. Measurements of mitochondrial membrane potential (DeltaPsim) showed a higher percentage of fully functional mitochondria in PS1−/− and PSwt compared to the percentage in PS2−/− and PSKO cells. This finding was evident in investigations of whole cell preparations and isolated mitochondria. Interestingly, pre-treatment of isolated mitochondria with the γ-secretase inhibitor L-685,458 resulted in a decreased population of mitochondria with a high percentage of DeltaPsim in PSwt and PS1−/− cells, indicating that PS2/γ-secretase activity can modify DeltaPsim. PS2−/− cells showed a significantly lower basal respiratory rate as compared to other cell lines. These findings point toward a specific role for PS2/γ-secretase in the proper functioning of mitochondria and indicate an interplay between PS1 and PS2 in mitochondrial functionality (Behbahani et al. 2006).
Tau and Mitochondrial Dysfunction
It has been proposed that Aβ aggregates and hyperphosphorylated tau may block the transport of cell organelles, including mitochondria, to nerve terminals and synapses (where ATP demands are high) (Reddy and Beal 2005). Several lines of evidence support this hypothesis: (1) Cell biology and biochemical experiments using various neuronal and non-neuronal cells expressing mutant tau showed that tau is capable of reducing net anterograde transport of vesicles and cell organelles by blocking the microtubule tracks. Thus, a misregulation of tau could cause the starvation of synapses and enhanced oxidative stress, long before tau detaches from microtubules and aggregates into AD neurofibrillary tangles. Furthermore, mitochondrial trafficking (or movement) decreased, and severe ATP depletion was observed in the nerve terminals and synapses (Mandelkow et al. 2004; Thies and Mandelkow 2007). A recent study revealed that tau-induced synaptic decay could be relieved by the activation of the kinase MARK2 (microtubule-associated protein/microtubule affinity regulating kinase 2)/Par-1 (protease-activated receptor 1), which remove tau from the microtubule tracks and reverse the transport block leads to the rescue of dendritic spines, synapses, mitochondrial transport, and ATP levels, suggesting that tau is involved in the decrease of mitochondrial ATP at dendritic spines and synapses (Thies and Mandelkow 2007). (2) Stamer et al. (2002) studied the effect of tau protein on trafficking of vesicles and organelles in primary cortical neurons, retinal ganglion cells, and neuroblastoma cells. They found that tau inhibits the kinesin-dependent transport of peroxisomes, neurofilaments, and Golgi-derived vesicles into neurites, and that the loss of peroxisomes makes cells vulnerable to oxidative stress and leads to degeneration. In particular, they found that tau inhibits the transport of APP into axons and dendrites, causing impaired axonal transport (Stamer et al. 2002). (3) In another study, Perry et al. (1999) demonstrated that in AD, activated extracellular receptor kinase increases in the same vulnerable neurons that are the sites of oxidative damage and abnormal phosphorylation. These findings suggest that the dysregulation of extracellular receptor kinase, likely resulting from oxidative stress, could play an important role in the increased phosphorylation of cytoskeletal proteins observed in AD (Perry et al. 1999).
ApoE4 and Mitochondrial Dysfunction
Increasing evidence suggests that the ApoE4 genotype is involved in mitochondrial oxidative damage in AD patients (Fig. 4). (1) Recently, Devi et al. (2006) found that non-glycosylated, full-length, C-terminal-truncated APP accumulates exclusively in the protein import channels of mitochondria of human AD brains but not in those channels, in age-matched controls. The accumulation of APP across mitochondrial import channels, which varied with the severity of AD, inhibited the entry of nuclear-encoded cytochrome c oxidase subunits IV and Vb proteins. This inhibition was associated with decreased cytochrome c oxidase activity and increased levels of H2O2. Analysis of the apolipoprotein genotype revealed that brain specimens from AD subjects with the E3/E4 alleles had the highest content of mitochondrial APP and that this mitochondrial APP directly correlated with mitochondrial dysfunction. (2) To determine the relationship between ApoE4 and mitochondrial association, Chang et al. (2005) expressed ApoE4 with C- or N-terminal truncations or mutations in transfected Neuro-2a cells. They found that the N-terminal of ApoE4 (amino acids 1–272) was neurotoxic, but the full-length ApoE4 (1–299) and ApoE4 (1–240) were not, suggesting that the lipid-binding region (241–272) mediates neurotoxicity. Immunofluorescence staining showed that ApoE4 (1–272) formed filamentous inclusions containing phosphorylated tau in some cells and interacted with mitochondria in others, leading to mitochondrial dysfunction, as determined by MitoTracker staining and flow cytometry. Thus, the lipid- and receptor-binding regions in ApoE4 fragments acted together and caused mitochondrial dysfunction and neurotoxicity, which may be important in AD pathogenesis (Chang et al. 2005).
Parkinson’s Disease
PD is a degenerative disorder, characterized by muscle rigidity, tremor, a slowing of physical movement, and in extreme cases, a loss of physical movement. The morphological and pathological changes of PD are the loss of dopaminergic neurons in the pars compacta region of the substantia nigra and the presence of cytoplasmic inclusions, or Lewy bodies, containing α-synuclein (Gandhi and wood 2005; Abeliovich and Beal 2006; Martin 2006; Thomas and Beal 2007). PD is both chronic and progressive. It is now well established that both genetic and environmental toxins are involved in the development of PD.
In the last decade, genetic discoveries in PD revealed that DNA mutations are linked to PD: α-synuclein (autosomal-dominant form of PD) (Polymeropoulos et al. 1997), Parkin (autosomal recessive juvenile form of PD) (Kitada et al. 1998), DJ1 (an autosomal-recessive, early-onset form of PD) (Bonifati et al. 2003), PTEN- induced kinase 1 (autosomal recessive form of PD) (Valente et al. 2004), OMI/HTRA2 (Strauss et al. 2005), leucine rich repeat kinase (LRRK2) (late-onset, autosomal-dominant form of PD) (Zimprich et al. 2004), and ubiquitin carboxy-terminal esterase L1 (Leroy et al. 1998).
The exposure of the environmental toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), to humans results in permanent parkinsonian syndrome (Langston et al. 1983). Thus, MPTP is used as a model for PD as it can rapidly induce parkinsonian symptoms in human beings and other animals of any age. Other toxin-based models employ PCBs, paraquat (a herbicide) in combination with maneb (a fungicide) and rotenone (an insecticide), and specific organochlorine pesticides, including dieldrin and lindane. MPTP administered to laboratory animals was found to destroy the substantia nigra and to induce the formation of Lewy bodies in aged chimpanzees. Other studies found that MPTP is converted into the active neurotoxic metabolite N-mentyl-4-phenylpyridinium ion and accumulates via a dopamine transporter into dopamergic neurons where N-mentyl-4-phenylpyridinium inhibits complex I. The chronic administration of rotenone (ETC complex I inhibitor) also produced an animal model that reproduces the loss of dopaminergic nigral neurons, parkinsonism phenotype, and α-synuclein inclusions that have been seen in human PD.
PD and Mitochondrial Dysfunction
Mutations in mtDNA are known to cause PD. A point mutation in the mitochondrial 12SrRNA has been found in PD family, and the G11778A mutation in the complex I gene (ND4) has been found in a PD family associated with Leber’s optic neuropathy (Simon et al. 1999). Mutations in nuclear-encoded mtDNA polymerase-γ gene impair mtDNA replication and result in multiple mtDNA deletions, typically causing chronic, progressive external opthalmoplegia and myopathy. In those diseases, polymerase-γ gene mutations co-segregate with parkinsonism.
The inhibition of complex 1, which is found in a PD patients (Gandhi and Wood 2005; Thomas and Beal 2007), induces the generation of free radicals that cause oxidative stress and depletion of ATP (Fig. 3) (Gandhi and Wood 2005). Elevated levels of lipid peroxidation markers (4-hydroxynonenal and malondialdehyde) and protein nitration have been found in the substantia nigra and Lewy bodies (Andersen 2004). Reduced levels of glutathione and oxidized glutathione, which act as antioxidants, are the earliest markers of nigral cell loss in the PD brain (Sian et al. 1994). Furthermore, a reduction of complex 1 activity by 30% has been founded in brain, muscle, and platelets of idiopathic PD patients (Schapira et al. 1990; Parker et al. 1989). Support for a primary role of oxidative stress in PD progression has emerged from the study of rare familial forms of PD.
A variety of missense, truncating, splice site, and deletion mutations have been identified in the gene DJ-1 (Bonifati et al. 2003), which causes the autosomal recessive form of parkinsonism. The precise function of DJ-1 is unclear, but the over-expression of DJ-1 appears to protect cells against mitochondrial complex 1 inhibitors and oxidative damage induced by H2O2. This effect appears to be abrogated by DJ-1 mutations (Canet-Avilés et al. 2004) or by DJ-1 knockdown using siRNA (Taira et al. 2004). DJ-1 may act directly as an antioxidant because it can be oxidized at the cysteine residue C106. Moreover, it has been demonstrated that endogenous DJ-1 is localized to the mitochondrial matrix and the mitochondrial intermembrane space, in addition to its cytoplasmic pool (Zhang et al. 2005). Interestingly, a quantitative proteomic study of the substantia nigra of mice treated with MPTP revealed a significant increase in the protein DJ-1 in mitochondrial fraction of the substantia nigra (Jin et al. 2005). Together, this evidence suggests that DJ-1 may play an important role in neuroprotection against oxidative damage caused by mitochondrial toxins (Fig. 4).
Missense and truncating mutations of the PINK1 gene were found to cause autosomal recessive PD. Further analysis of the PINK1 protein revealed that PINK 1 has a highly conserved kinase domain and a mitochondrial-targeting motif. The presence of the N-terminal mitochondrial-targeting domain, combined with transfected cells, is interesting (Beilina et al. 2005). As with the other genes that cause autosomal recessive parkinsonism, PINK1 has been suggested to have neuroprotective properties against a variety of cellular stresses, a function that is lost by the mutation G309D, which has been identified in certain PD families (Valente et al. 2004). These studies suggest that mitochondria may play a large role in PD.
Alpha Synuclein, PD, and Mitochondrial Dysfunction
Alpha synuclein is a presynaptic protein and mainly expressed in neocortex, hippocampus, substantia nigra, thalamus, and cerebellum. It is also found in glial cells. Genetic mutations in α-synuclein were found in rare forms of familial PD (Polymeropoulos et al. 1997). Over-expression or gene duplication of wild-type α-synuclein may produce Lewy body pathology and PD phenotype.
Recently, Devi et al. (2008) investigated the connection between α-synuclein and mitochondrial interaction. They found that 32 amino acids of N-terminal human α-synuclein contain cryptic mitochondrial targeting signal, and it is important for targeting of α-synuclein into mitochondria. Mitochondrial imported α-synuclein is mainly associated with the inner mitochondrial membrane. Mitochondrial wild-type α-synuclein caused reduced mitochondrial complex I activity and increased production of ROS in dopaminergic neurons. Further, these changes occurred at an early on in dopaminergic neurons expressing familial α-synuclein A53T mutation. Mitochondria of PD-affected regions including substantia nigra and striatum but not the cerebellum from PD subjects showed significant accumulation of α-synuclein and decreased complex I activity. Analysis of mitochondria from PD brain and α-synuclein expressing dopaminergic neuronal cultures using blue native gel electrophoresis and immunocapture technique showed the association of α-synuclein with complex I proteins. Based on these findings, Devi et al. concluded that mitochondrial α-synuclein may interact with ETC complex I proteins and impair mitochondrial function (Devi et al. 2008).
Leucine-rich Repeat Kinase 2 (LRRK2), PD, and Mitochondrial Dysfunction
Recently, genetic mutations have been found in LRRK2 gene, and have been identified as the cause of the late-onset, autosomal-dominant Park8 type of PD (Farrer et al. 2005; Nichols et al. 2005). A small proportion (1–2%) of sporadic PD patients are also associated with genetic mutations in LRRK2 gene (Gilks et al. 2005).
LRRK2 is expressed in the striatum and frontal cortex, whereas the dopamine neurons themselves are devoid of LRRK2 messenger RNA (Galter et al. 2006). LRRK2 seems to play a role in synaptic vesicle functions, including neurite outgrowth. LRRK2 resides diffusely throughout the cytoplasm, and also associates with the mitochondrial outer membrane (West et al. 2005). DNA sequence analysis indicates that LRRK2 comprises several domains, including a leucine-rich repeat domain, a guanosine triphosphatase domain Ras of complex proteins, C-terminal of Roc domain, a MLK-like domain, and a C-terminal WD40 domain (Paisan-Ruiz et al. 2004; Zimprich et al. 2004). The kinase activity may be the link between LRRK2 and its role in PD pathogenesis, and disease-causing mutations in LRRK2 gene affects cell viability due to mitochondria-dependent cell death (Thomas and Beal 2007).
Huntington’s Disease
HD is an autosomal, dominantly inherited disorder characterized phenotypically by chorea, involuntary movements, dystonia, intellectual impairment, and emotional disturbances (Vonsattel et al. 1985; Folstein 1990; Bates 2005). HD is prevalent in persons of Caucasian origin, occurring in 4–10 per 100,000 Caucasians. Knowledge of the neuropathological events that lead to HD may provide insights into the disease mechanisms of other CAG repeat disorders.
HD is caused by a genetic mutation that results in an expanded polyglutamine encoding repeat (CAG)n, within exon 1 of the HD gene. In persons affected with HD, the number of CAG repeats ranges from 37–121 whereas in unaffected persons, it ranges from only 6–35 (Reddy et al. 1999). Huntingtin (Htt), the product of HD gene, is a 350 kDa protein, ubiquitously expressed and primarily localized in the cytoplasm of neurons (Reddy et al. 1998; Reddy et al. 1999; Bates 2005). However, a portion of N-terminally extended polyglutamine Htt is translocated into the cell nucleus. Both mutant and normal Htt are expressed ubiquitously. However, the selective and premature death of striatal projection neurons has been reported in HD patients and HD transgenic mice (Vonsattel et al. 1985; Folstein 1990; Reddy et al. 1998; Reddy and Tagle 1999; Hodgson et al. 1999; Schilling et al. 1999; Van Raamsdonk et al. 2007). Causes of this selective neuronal loss in HD are still not clearly understood. Further, how the mutant Htt causes the progression of HD is still unclear. To explain the causes of disease progression in HD, several mechanisms have been proposed, including axonal trafficking, protein-protein interaction, transcriptional dysregulation, and mitochondrial oxidative damage (Browne and Beal 2004; Browne and Beal 2006; Li and Li 2006; Trushina et al. 2004; Trushina and McMurray 2007; Orr et al. 2008).
HD and Mitochondrial Dysfunction
HD patients showed significant weight loss, suggesting that bio-energetic defects might be implicated in HD pathogenesis (Trushina and McMurray 2007). Further, imaging the basal ganglia of HD patients with positron emission tomography revealed a marked reduction in glucose metabolism. In addition, biochemical studies of mitochondria in striatal neurons from late-stage HD patients revealed reduced activity of several components of OXPHOS, including complexes II, III, and IV of the ETC (Fig. 4) (Browne et al. 1997; Tabrizin et al. 1999; Browne and Beal 2004; Fukui and Moraes 2007).
Recent studies of HD knock-in striatal cells and lymphoblasts from HD patients revealed that expanded CAG repeats are associated with low mitochondrial ATP and decreased mitochondrial ADP-uptake, suggesting that HD mutation is associated with mitochondrial defects (Seong et al. 2005). Biochemical studies of HD mice and HD cell lines revealed that calcium-induced mitochondrial permeability is a major factor in HD pathogenesis (Panov et al. 2002, 2005). Further, recent mitochondrial trafficking studies of HD neurons revealed that mutant Htt aggregates impair mitochondrial movement in HD cortical neurons (Chang et al. 2006).
It has been proposed that the abnormal transcriptional regulation of nuclear-encoded mitochondrial genes may be involved in HD pathogenesis. Indeed, mutant Htt binds to several key transcription factors—including TATA binding proteins (Huang et al. 1998; Perez et al. 1998), Sp1 (Shimohata et al. 2000), and nuclear scaffold protein NAKAP (Sayer et al. 2005). This mutant Htt interaction may interfere with the gene expression, activity. and transcriptional regulation of HD neurons. This possibility is supported by recent studies of PGC1α (potent suppressor of ROS) in HD (Cui et al. 2006; St-Pierre et al. 2006; Weydt et al. 2006). PGC1α was found to be decreased in HD postmortem brains, in cell lines expressing mutant Htt, and in HD mouse models, suggesting that the mutant Htt promotes the increased production of ROS; this increase in ROS may interact with the mitochondrial outer membrane, ultimately leading to decreased levels of PGC1α in mutant HD neurons (Cui et al. 2006; St-Pierre et al. 2006; Weydt et al. 2006). These PGC1α studies also suggest that molecules that activate PGC1α may be a therapeutic strategy not only for HD but also for other neurodegenerative diseases including AD, PD, and ALS.
Mutant Htt Association with Mitochondria: Recently
Orr et al. (2008) demonstrated that specific N-terminal mutant Htt fragments associate with mitochondria in Hdh(CAG)150 knock-in mouse brain and that this association increases with age. The interaction between soluble N-terminal mutant Htt and mitochondria interferes with the in vitro association of microtubule-based transport proteins with mitochondria. Mutant Htt reduces the distribution and transport rate of mitochondria in the processes of cultured neuronal cells. Reduced ATP level was also found in the synaptosomal fraction isolated from Hdh(CAG)150 knock-in mouse brain. These findings suggest that specific N-terminal mutant Htt fragments, before the formation of aggregates, can impair mitochondrial function directly and that this interaction may be a novel target for therapeutic strategies in HD (Orr et al. 2008).
Amyotrophic Lateral Sclerosis
ALS is a progressive, invariably fatal neurological disease that attacks the neurons responsible for controlling voluntary muscles. In ALS, the upper and lower motor neurons degenerate or die, thus silencing the messages they send to muscles. Unable to function, the muscles gradually weaken, waste away, and twitch. Eventually the brain loses its ability to control voluntary movement (Martin 2006; Hervias et al. 2006). Individuals with ALS lose their strength and the ability to move their arms, legs, and body. ALS has an incidence of 1–2 in a population of 100,000, with fairly uniform distribution worldwide and equal representation among racial groups (Boillee et al. 2006). The incidence of ALS is greatest in persons aged 50–70 years. The male:female ratio is approximately 1.5:1. Genetic studies have identified several missense mutations in the gene encoding Cu/Zn superoxide dismutase (SOD1) on the long arm of chromosome 21, in approximately one-fifth of autosomal-dominant cases of ALS. The genetic causes of ALS are known for only about 10% of total ALS cases, and mutations in gene encoding SOD1 are known to cause only a small protion of familial ALS cases (Hervias et al. 2006; Kwong et al. 2006). Causal factors are still unknown for the vast majority of cases. Cytoplasmic SOD1 protein aggregates have been observed in both sporadic and familial ALS cases, as well as in mutant SOD1 transgenic mice (Bruijn et al. 1998; Gurney et al. 1994; Watanabe et al. 2001; Wong et al. 1995). SOD1 protein has been used to define accumulations of detergent-insoluble forms of SOD1 that were detected by immunoblotting of filter-trappable material (Deng et al. 2006, Furukawa et al. 2006). The detergent-insoluble forms were detectable only in the affected tissues of mutant SOD1 mice and were most prominent at symptomatic stages of disease progression (Furukawa et al. 2006; and Jonsson et al. 2006). The most misfolded, unstable SOD1 mutants were the most prone to aggregation.
ALS and Mitochondrial Dysfunction
A common feature of many neurodegenerative diseases is damage to mitochondria that contributes to the degenerative phenotype. Mitochondria have been implicated as a target of toxicity in ALS by histopathological observations of vacuolated and dilated mitochondria with disorganized cristae and membranes in the motor neurons of ALS patients (Afifi et al. 1966; Hirano et al. 1984). Mitochondrial defects have been reported in spinal cords and muscle biopsies of ALS patients, with such defects ranging from impaired mitochondrial respiration to increased levels of uncoupling proteins (Chung and Suh 2002; Dupuis et al. 2003).
Mitochondrial abnormalities have been observed in the motor neurons of mice that developed ALS from an accumulation of dismutase active mutants (Wong et al. 1995) and in hSOD1G93A mice (Higgins et al. 2003) prior to any detectable motor neuron loss or other observable damage (Higgins et al. 2003; Wong et al. 1995). A proportion of the predominantly cytosolic SOD1 localizes to mitochondria in certain contexts, in brain samples from ALS rodent models and samples from ALS patients. In these samples, mutant SOD1 was present in fractions enriched with mitochondria that were derived from affected tissues, but not from unaffected tissues (Bergemalm et al. 2006; Deng et al. 2006; Liu et al. 2004; Vijayvergiya et al. 2005). Mitochondrial localization of SOD1 has been confirmed by electron microscopy in both isolated mitochondria (Liu et al. 2004) and motor neurons in situ (Sasaki et al. 2004). SOD1 mutants that cause disease at the lowest accumulated levels (hSOD1G85R and hSOD1G127X) have the highest relative proportions that are mitochondrially associated (Liu et al. 2004). Mutant SOD1 has been reported in the intermembrane space of mitochondria and in the matrix, as well as in the inner and outer membranes of mitochondria in the spinal cord and brain (Fig. 4) (Bergemalm et al. 2006; Deng et al. 2006; Liu et al. 2004; Pasinelli et al. 2004; Vijayvergiya et al. 2005).
Friedreich Ataxia
FRDA is an autosomal, recessively inherited degenerative disorder, characterized by progressive gait and limb ataxia, axonal sensory neuropathy, loss of deep tendon reflexes, spasticity, and extensor plantar responses. FRDA is the most common inherited ataxia and is prevalent in 1 of 50,000 individuals. Pathologically, FRDA involves the spinocerebellar tracts, dorsal columns, and pyramidal tracts, and, to a lesser extent, the cerebellum and medulla. Over 90% of FRDA patients are homozygous for an expansion of a GAA repeat in the first intron of the FRDA gene (Campuzano et al. 1996). Researchers have also identified a small number of FRDA patients heterozygous for the GAA repeat expansion and point mutations within the gene. Frataxin, a gene product of FRDA, is a highly conserved, mitochondrial protein involved in heme synthesis, the formation of iron–sulfur clusters, and the detoxification of iron (Fig. 4). Molecular and cell biology studies in yeast and mice have shown that frataxin defects lead to the accumulation of iron in mitochondria and a decrease in iron-sulfur containing enzymes, such as aconitase and complex I–III of the ETC (Fig. 4) (Wilson 2006). This abnormal accumulation of iron in the mitochondria may induce free radical production, oxidative damage, and subsequent cell death. The role of oxidative damage in FRDA is supported by studies of FRDA patients in which the antioxidant idebenone was found to reduce myocardial hypertrophy and to decrease markers of oxidative stress (Hausse et al. 2002; Schultz et al. 2000).
Why We Need Mitochondrial Medicine for Aging and Neurodegenerative Diseases
As discussed above, mitochondrial dysfunction has been implicated as a common event in aging and such neurodegenerative diseases as PD, AD, HD, ALS, and FRDA. If mitochondrial dysfunction proves to be significant in aging and these age-related neurodegenerative diseases, it may be possible to treat aging and age-related neurodegenerative diseases by developing molecules that treat mitochondria. These strategies would necessarily be based on targeting ROS in mitochondria (Fig. 5).
Fig. 5.
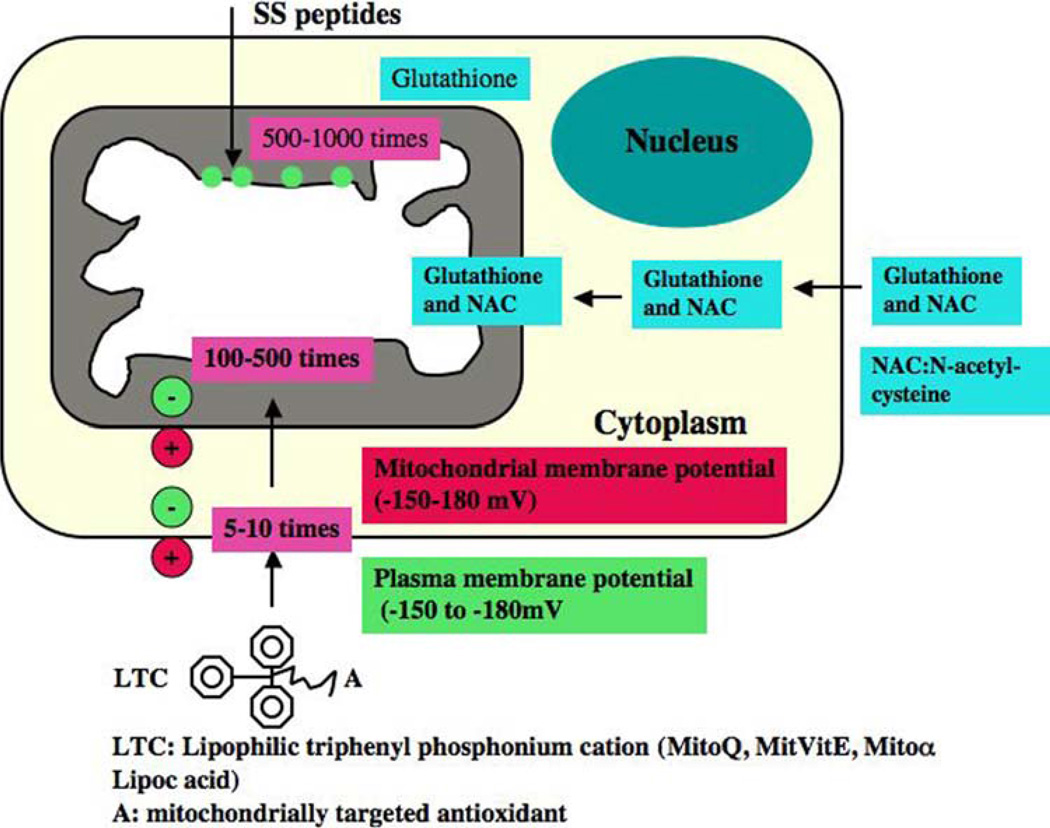
Strategies of neuronal protection in aging and neurodegenerative diseases. 1. A generic mitochondrially targeted antioxidant is shown constructed by the covalent attachment of an antioxidant molecule to the lipophilic triphenylphosphonium cation. Antioxidant molecules accumulate 5–10 fold in the cytoplasm, which is driven by plasma membrane potential, and then further accumulates 100–500 fold in the mitochondria. Mitochondrial antioxidants rapidly neutralize free radicals and reduce mitochondrial toxicity. 2. Glutathione is synthesized in the cytoplasm and transported to mitochondria. N-acetyl-L-cysteine provides s cysteine for glutathione synthase. Choline esters of glutathione and N-acetyl-L-cysteine have been used to increase mitochondrial glutathione and N-acetyl-L-cysteine. Increased glutathione can rapidly neutralize free radicals and protect mitochondria from oxidative insults. 3. SS peptides are cell-permeable, mitochondrially targeted antioxidants that are reported to protect mitochondria from oxidative damage. These SS peptides have a sequence motif that allows them to target mitochondria several hundred fold more than natural antioxidants. Once SS peptides reach mitochondria, the SS peptides rapidly neutralize free radicals and decrease mitochondrial toxicity
Drug Targets of ROS Production
The use of antioxidants to prevent and treat mitochondria in patients with neurodegenerative diseases has received much attention. Some epidemiologic studies suggest that an increased intake of standard antioxidant vitamins (e.g., vitamin E, vitamin C, and beta carotene) might reduce the risk of developing AD or PD, while other studies do not (Reddy 2007). Antioxidant approaches of treating age-related neurodegenerative diseases are promising and seems to have no adverse effects. However, currently available antioxidant approaches may not be effective for treating neurodegenerative diseases because: (1) naturally occurring antioxidants, such as vitamins E and C, may not likely cross the blood-brain barrier and so could not reach the relevant sites of free radical generation, especially if mitochondria are the primary source of ROS (Szeto 2008), (2) researchers have been unable to increase sufficient levels of antioxidants in mitochondria in patients with neurodegenerative diseases such as AD and PD, and (3) antioxidants may not have been administered to patients at early stages of disease development. To overcome these problems and to better assess whether antioxidant approaches may be valuable therapeutic treatments for neurodegenerative diseases, better delivery of antioxidants to the brain mitochondria of patients with neurodegenerative diseases is needed.
In the last decade, considerable progress has been made in developing mitochondrially targeted antioxidants. To increase the delivery of antioxidants into mitochondria, 3 types of mitochondrially targeted antioxidants have been developed: 1. triphenylphosphonium-based antioxidants––MitoQ, MitoVitE, MitoPBN, 2. the cell-permeable, small peptide-based antioxidants SS-02, SS-31, SS-19, and SS-20, and 3. choline esters of glutathione and N-acetyl-l-cysteine (Fig. 5) (Szeto 2006a; Reddy 2006b; Sheu et al. 2006; Murphy and Smith 2007). However, application of these mitochondrially targeted drugs to animal models of neurodegenerative diseases is still at primitive stages.
Triphenylphosphonium Cation Based Antioxidants
Recently, Murphy et al. developed a series of triphenylphosphonium cation based antioxidants (Murphy and Smith 2007). The liphophilic triphenylphosphonium cation is attached to antioxidants such as vitamin E, coenzyme Q, and these triphenylphosphonium cation attached antioxidants were preferentially taken up by mitochondria due to charge difference between mitochondria (with high negative charge) and liphophilic triphenylphosphonium cation based antioxidants (with high positive charge) (Fig. 5). These antioxidants initially accumulate in the cytoplasm of cells due to a negative plasma membrane potential and subsequently enter mitochondria and accumulate several hundred folds within the mitochondrial matrix.
MitoQ
MitoQ is a strong therapeutic antioxidant that has been successfully targeted to mitochondria. MitoQ consists of 2 redox forms of mitochondrially targeted ubiquinone derivatives: reduced mitoquinol and oxidized mitoquinone (Murphy and Smith 2007). MitoQ is a respiratory chain component buried within the lipid core of the inner membrane of mitochondria where it accepts 2 electrons from complexes I and II, to form the reduction-product ubiquinol, which donates electrons to complex III (Reddy 2006a). The ubiquinone pool exists in vivo largely in a reduced ubiquinol form, acting as an antioxidant and a mobile electron transfer. Ubiquinol has been reported to function as an antioxidant by donating a hydrogen atom from one of its hydroxyl groups to a lipid peroxyl radical, thereby decreasing lipid peroxidation within the mitochondrial inner membrane. The semi-ubiquinone radical formed during this process may then disproportionate into ubiquinone and ubiquinol. The respiratory chain may subsequently recycle ubiquinone back to ubiquinol, restoring the antioxidant function. MitoQ may excessively accumulate in the mitochondria and convert H2O2 to H2O and O2, and reduce toxic insults from free radicals in the mitochondria. This reduction may ultimately lead to the protection of neurons from age-related and AD-related mitochondrial insults. However, higher concentrations (above 0.3 µM) of MitoQ are toxic to neuronal cells (Reddy, unpublished observations). Optimization of MitoQ dosage is critical for treatment of cell and animal models of human mitochondrial diseases (Cocheme, et al. 2007).
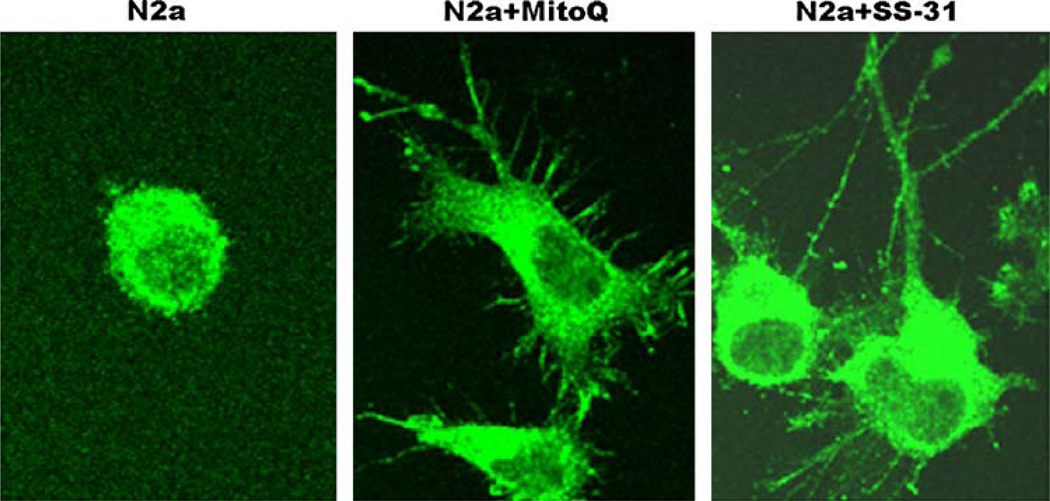
In a preliminary investigation of N2a cells treated with MitoQ (at 0.3 µM concentration), we found increased neurite outgrowth in N2a cells grown in serum-free media, suggesting that MitoQ may have a role in neuroprotection and synaptic connectivity (Figs. 6, 7). MitoQ is in the early stages of being administered in animal models of mitochondrial diseases (Murphy and Smith 2007). Using MitoQ, phase II clinical trial in PD patients has been completed and data yet to be reported.
Fig. 6.
Neuroprotective effects of MitoQ and SS-31. N2a cells were grown in a serum-free medium for 7 days and treated with MitoQ (0.3 µM) and SS-31 (0.1nM). After 48 h of treatment, neurite outgrowth was examined after immunostaining with an anti-dynamin-related protein 1 or Drp1 (enriched in neurons, particularly at synapses). As shown, increased neurite outgrowth has been observed in neurons treated with MitoQ and SS-31, in contrast to untreated N2a cells. This observation indicates that mitochondrially targeted antioxidants at very low concentrations are cytoprotective, and may have a role in neurite outgrowth and synaptic connectivity
Fig. 7.
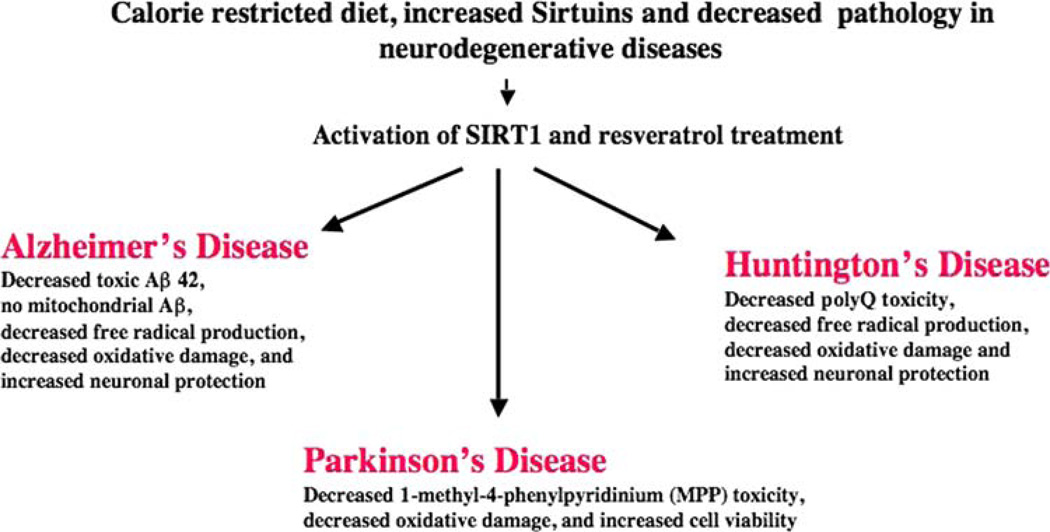
Neuronal protection of calorie-restricted diet and Sirtuins in aging and neurodegenerative diseases
MitoVitE
MitoVitE is an antioxidant that consists of [2–3,4dihydro-6-hydroxy-2,5,7,8tetra-methyl-2H-1-benzopyran-2-yl] and triphenylphosphonium bromide (TPPB) (Reddy 2007). MitoVitE, a derivative of vitamin E, was developed to study the mitochondrial toxicity and cell protection. Similar to MitoQ, MitoVitE is rapidly taken up by mitochondria. Accumulation ratios of 5000–6000 units have been achieved after incubating mitochondria with 1–20 µM MitoVitE (Smith et al. 1999).
Several lines of evidence suggest that MitoVitE in cytoprotective (Jauslin et al. 2003; Dhanasekaran et al. 2004; Siler-Marsiglio et al. 2005). MitoVitE was found to reduce H2O2-induced caspase activity and to prevent oxidative stress-induced cell death in cultured fibroblasts from FDRA patients (Jauslin et al. 2003). Jauslin et al. (2003) also found that MitoVit E was 350-fold more potent than the water soluble analog, Trolox, and prevents cell death that arises in response to endogenous oxidative damage in cultured fibroblasts from FRDA patients (Jauslin et al. 2003). Dhanasekaran et al. (2004) found that at a 1 µM concentration, MitoVitE was found to inhibit cytochrome c release and caspase-3 activation, to inactivate complex 1, and to restore mitochondrial membrane potential and proteosomal activity in bovine aortic epithelial cells (Dhanasekaran et al. 2004).
In a recent study, Siler-Marsiglio et al. (2005) found that at 1 nM concentration, MitoVit E renders significant neuroprotection against ethanol concentrations as high as 1600 mg/dL in cerebellar granule cells. MitoVit E mitigates ethanol-induced accumulation of intracellular oxidants and maintains the glutathione peroxidase/glutathione reductase functions, protein expression of gamma-glutamylcysteine synthetase, and total cellular glutathione levels. Overall, MitoVit E ameliorates ethanol toxicity through non-oxidant mechanisms-modulations of endogenous cellular proteins-and antioxidant means (Siler-Marsiglio et al. 2005). Further research is required to explore MitoVitE applications to elderly humans and humans with neurodegenerative diseases.
MitoPBN
MitoPBN is an antioxidant consisting of [4-[4 (1,1-dimethylethyl) oxidoimino]––methyl]phenoxy]butyl] and triphenylphosphonium bromide. The PBN is reported to protect against mitochondrial toxicity in animal models of ischemia, endotoxin shock, and experimental models of neurodegenerative diseases (Kotake 1999). The spin trap of PBN was chosen because of its high reactivity with carbon-centered radicals, whereas its reactivity with superoxide and peroxyl radicals is low, and it does not prevent lipid peroxidation by a chain-breaking antioxidant mechanism (Murphy et al. 2003). MitoPBN is accumulated within mitochondria and there reacts preferentially with carbon-centered radicals but not with superoxide. A carbon-centered radical generator stimulates uncoupling proteins, and this activation is also blocked by MitoPBN. These data suggest that superoxide and lipid peroxidation products are the components of a single oxidative damage pathway that activates uncoupling proteins (Murphy et al. 2003).
A mitochondrially targeted analog of MitoPBN was prepared to determine the effect of ROS and mitochondrial function in mitochondria, based on a selective α-phenyl-N-tert-butyl nitrone reaction with carbon-centered radicals. Similar to MitoQ and MitoVitE, MitoPBN was rapidly taken up by mitochondria, with a resulting concentration ranging from 2.2 to 4.0 mM. It has been reported that MitoPBN blocks the O-induced activation of uncoupled proteins (Reddy 2007).
Cell Permeable, Small Peptide Antioxidants (SS Peptides)
Recently, Szeto and Schiller developed a series of 4 small, cell-permeable, mitochondrially targeted antioxidant peptides (Szeto-Schiller or SS peptides) that are known to protect mitochondria from oxidative damage: (1) SS-01 H-Tyr-D-Arg-Phe-Lys-NH2, (2) SS-02 H-Dmt-D-Arg- Phe-Lys-NH2, (3) SS-31 H-D-Arg-Dmt-Lys-Phe-NH2, and (4) SS-20 H-Phe-D-Arg-Phe-Lys-NH2 (Szeto et al. 2001; Szeto 2006a, b; Szeto 2008). The structural motif of these SS peptides centers on alternating aromatic residues and basic amino acids (aromatic-cationic peptides). These SS peptides have a sequence motif that allows them to target mitochondria. They scavenge H2O2 and ONOO-, and inhibit lipid peroxidation. Their antioxidant action can be attributed to the tyrosine, or dimethyltyrosine (Dmt), residue. Tyrosine scavenges oxyradicals and forms relatively unreactive tyrosyl radicals; the unreactive tyrosyl radicals are then followed by radical-radical coupling, giving rise to dityrosine which reacts with a superoxide to form tyrosine hydroperoxide. Dmt is more effective than tyrosine in scavenging mitochondria for ROS. The specific location of the tyrosine or Dmt residue does not appear to be significant, as SS-31 was found to be as effective as SS-02 in scavenging H2O2 and in inhibiting LDL oxidation.
SS-02 contains an amino acid sequence that allows SS-02 to freely penetrate cells even when they carry a 3 + net charge at physiologic pH (Zhao et al. 2003). These aromatic-cationic peptides are taken up into cells in an energy-independent, non-saturable manner. Uptake studies with [3H]SS-02 showed rapid uptake with steady-state achieved in less than 30 min (Zhao et al. 2004). This finding suggests that these peptides freely pass through the plasma membrane in both directions. Unlike the larger cationic peptides, such as the Tat peptide (Drin et al. 2003; Derossi et al. 1996), there is no evidence of vesicular localization that would result from endocytosis. Incubation of isolated mitochondria with [3H]SS-02 confirmed that it is taken up and concentrated more than 1000-fold in mitochondria (Zhao et al. 2004). The ability of SS-02 to penetrate the blood-brain barrier in mice is also supported by the observation that SS-02, which also possesses high affinity for the μ opioid receptor, is a very potent analgesic after subcutaneous administration (Zhao et al. 2002). The duration of analgesia achieved with a single subcutaneous dose of SS-02 was 4 times longer than the duration of analgesia with an equipotent dose of morphine. However, the efficacies of SS-02 and SS-31 peptides have not been tested so far in AD, HD and FRDA mice.
Recently, the efficacy of the SS-31 peptide was studied in rodent models of ischemic brain injury (Cho et al. 2007a), with a diabetic condition (Thomas et al. 2007), undergoing myocardial infarction (Cho et al. 2007b) and in ALS (Petri et al. 2006). Researchers found that SS-31 protects cells from mitochondrial toxicity in all these disease states. Petri et al. (2006) investigated SS-31 peptide for its therapeutic effects both in vitro and in vivo in the G93A mouse model of ALS. SS-31 protected against cell death induced by H2O2 in vitro, in neuronal cells stably transfected with either wild-type or mutant Cu/Zn superoxide dismutase (SOD1). Daily intraperitoneal injections of SS-31 (5 mg/kg), starting at 30 days of age, led to a significant improvement in mouse survival and motor performance. In comparison with vehicle-treated G93A mice, SS-31-treated mice showed a decrease in the loss of cells and a decrease in oxidative stress in the lumbar spinal cord. These findings suggest that pharmacological modification of oxidative stress with SS-31 may hold therapeutic potential for the treatment of ALS (Petri et al. 2006).
In a preliminary investigation of N2a cells treated with SS-31 (1nM final concentration) and grown in serum-free media, our laboratory found an increase in the growth of the neurites (Fig. 6), suggesting that SS-31 may have a role in neuroprotection and synaptic connectivity (Reddy, unpublished observations).
Choline esters of Glutathione and N-acetyl-l-cysteine
Glutathione is an antioxidant that has been found to play a key role in reducing oxidants in the mitochondria. Glutathione choline ester and N-acetyl-l-cysteine choline ester are hydrophilic antioxidants and have been targeted to mitochondria (Fig. 5). Dicarboxylate and 2-oxoglutarate carry glutathione into mitochondria. Glutathione neutralizes free radicals in the mitochondria very effectively and protects mitochondria from oxidative damage. These molecules have been designed to develop the highly negative, internal potential of mitochondria.
Several recent studies using glutathione or glutathione inducers (such as γ-glutamylcysteine ethyl ester, glutathione- ethyl-ester, N-acetyl-l-cysteine choline) and AD cell lines or primary neurons expressing mutant APP demonstrated that increased glutathione levels protect neurons against protein oxidation, loss of mitochondrial function, and DNA fragmentation induced by Aβ (Pocernich et al. 2001; Boyd-Kimball et al. 2005a, b; Abe et al. 2004). These mitochondrially targeted antioxidants preferentially enter the mitochondria where they neutralize free radicals and decrease oxidative damage, and may protect neurons. Further research is needed to determine whether these mitochondrially targeted molecules can be used in mouse models of aging and neurodegenerative diseases.
These ROS drug targets may not increase ATP production in the mitochondria. Further, in the case of AD, these ROS targeted drugs may not have any effect on nuclear encoded mitochondrial proteins transport to mitochondria. This issue can be addressed by less or no production of Aβ in the cytosol.
Calorie-restricted Diets, Activation of SIRTUINS, and Increased Longevity of Neurons
Another way to decrease the production of ROS in mitochondria and protect mitochondria is through a calorie-restricted (CR) diet. CR diet is reported to activate longevity genes called SIRTUINS in lower organisms including yeast, worms, and flies (Guarente 2000; Tissenbaum and Guarente 2001; Blander and Guarente 2004; Lammings et al. 2004; Wood et al. 2004; Parker et al. 2005; Anekonda and Reddy 2006). Recent studies of a CR diet in rodents and non-human primates suggest that SIR-TUINS are activated and increase the lifespan of the healthy rodents and non-human primates studied (Fig. 6) (Wang et al. 2005a, b; Patel et al. 2005; Qin et al. 2006a, b). In other studies of aging and neurodegenerative diseases using animal models, a CR diet was found to reduce pathology and also to increase the lifespan of non-human primates, rodents, fruit flies, and worms (Guarente 2000; Tissenbaum and Guarente 2001; Blander and Guarente 2004; Lammings et al. 2004; Wood et al. 2004; Parker et al. 2005; Anekonda and Reddy 2006). These studies suggest that if humans eat a CR diet, their lifespan may increase.
Calorie Restricted Diet and AD
Several recent studies revealed that a CR diet reduces defects in the ETC, decreases mitochondrial ROS, and decreases mitochondrial damage in neurons from AD mice (Anekonda and Reddy 2006). Wang et al. (2005a, b) demonstrated that a CR regimen for mice diminished Aβ generation and Aβ plaque deposition, compared to control, well-fed mice. They observed a larger-than-two-fold increase in the concentration of brain sAPPα in CR animals compared to control mice, suggesting that CR diet may reduce Aβ production via the activation of SIRT1 and α-secretase activity. Using APP (swe/ind) and APP + PS1 adult mice, Patel et al. (2005) found that a CR diet correlated substantially with a decrease in the accumulation of Aβ-plaques by 40% in the APP mice (CR, 6 weeks) and by 55% in APP + PS1 mice (CR, 14 weeks), suggesting that dietary intervention in adult life might slow disease progression. In another CR APP mouse study, Qin et al. (2006a) reported that the predicted attenuation of Aβ in the brain during CR diet was reproduced in mouse neurons in vitro by manipulating the expression and activity of cellular mammalian SIRT1 (yeast Sir2) through mechanisms involving the regulation of the serine/threonine Rho kinase ROCK1, known in part for its role in inhibiting the non-amyloidogenic α-secretase processing of APP. In addition, Qin et al. (2006b) tested the benefits of a CR diet in squirrel monkeys with Aβ neuropathology. The monkeys were maintained on normal and CR diets throughout their lifespan, until they died of natural causes. They found reduced levels of Aβ1-40 and Aβ1-42 peptides in the temporal cortex of 30% of the CR monkeys, compared to the control monkeys. The decreased contents of the cortical Aβ peptide in the monkeys is inversely correlated with SIRT1 protein concentrations in the same brain region. These results suggest that CR may decrease Aβ pathology by activating SIRT1 and non-amyloidogenic α-secretase activity.
Further, several studies have reported that resveratrol, by activating SIRT1, reduces defects in electron transfer in ETC, decreases mitochondrial ROS, increases oxygen consumption, and maintains ATP production in brain neurons (Anekonda and Reddy 2006; Anekonda 2006).
Neuroprotective Role of PGC-1α in HD
Peroxisome proliferator-activated receptor- coactivator (PGC)-1α is a member of a family of transcription coactivators that play a central role in regulating the metabolism of cells. PGC-1α is a transcription coactivator that interacts with a broad range of transcription factors involved in a wide variety of biological responses, including adaptive thermogenesis and mitochondrial biogenesis of several tissues such as brain tissues (Liang and Ward 2006). Recently, using tissues from HD mice and patients, and cells from HD patients, 3 research groups independently studied PGC-1α and mitochondrial bioenergetics of HD in order to determine the role of PGC-1α in HD pathogenesis (Cui et al. 2006; St-Pierre et al. 2006; Weydt et al. 2006). Cui et al. (2006) studied striatal neurons from postmortem HD patient brains, from an HD knock-in mouse model that over-expresses mutant Htt, and from a STHdhQ111-cultured HD striatal cell line. They found a decrease in mRNA expression of PGC-1α in all 3 sources of striatal neurons, suggesting that mutant Htt interferes with the formation of the CREB/TAF4 complex that regulates transcription of the gene encoding PGC-1α. Using HD mice lines, Weydt et al. (2006) studied the connection between PGC-1α and adaptive thermogenesis in HD (Weydt et al. 2006). They found marked hypothermia at baseline temperatures, following cold exposure in both the N171-82Q and R6/2 HD mouse models. After cold exposure, UCP-1 expression was decreased in brown adipose tissue from N171-82Q HD mice relative to wild-type mice, implicating impaired PGC-1α function in these mice. St-Pierre et al. (2006) demonstrated that PGC-1α is capable of scavenging ROS and increasing the production of antioxidant enzymes. They also found an increased expression of genes encoding ROS defense enzymes, including copper/zinc superoxide dismutase (SOD1), manganese SOD (SOD2), catalase, and glutathione peroxidase. In a study of PGC-1α-deficient mice, these same researchers found that the basal expression of SOD1, SOD2, and catalase was considerably lower in the heart and brain, regions known to be sensitive to oxidative stress, suggesting that the activation of PGC-1α protects HD neurons from mitochondrial toxicity caused by mutant Htt. Overall, these studies suggest that PGC-1α may play a significant role in protecting neurons against mitochondrial toxicity and oxidative damage in HD pathogenesis by increasing its transcription and interaction with several transcription factors in HD neurons. Therapeutic molecules that target mitochondria or SIRTUIN activators may be useful in treating HD and other neurodegenerative diseases (Reddy 2007). CR studies are yet to be carried out in mouse models of HD, PD, FRDA, and other mitochondrial diseases, to determine neuroprotective efficacies of CR diet. Currently, researchers are actively testing CR diets and Sirtuin activators in mouse models of PD, ALS, FRDA, and other neurodegenerative diseases.
Conclusions
Several lines of evidence suggest that mitochondria play a significant role in aging and age-related neurological diseases, including AD, PD, HD, ALS, and FRDA. Mitochondria are the major source of energy for the normal function of brain cells. Mitochondrial dysfunction has been found to lead to the increased generation of ROS, abnormal protein-protein interactions, and decreased mitochondrial ATP production. Increased production of ROS with compromised mitochondrial function has been found to damage neurons in early-onset, inherited, and late-onset, non-inherited neurodegenerative diseases. Recent studies of neurons from postmortem brain specimens and from transgenic mouse models revealed that mutant proteins enter mitochondria, interact with mitochondrial proteins, and disrupt the ETC. This sequence of events ultimately generates ROS and inhibits the production of cellular ATP, which prevents neurons from functioning normally Further, the accumulation of mtDNA changes may contribute to mitochondrial dysfunction in an age-dependent manner, suggesting that age-related mitochondrial dysfunction plays a large role in neurodegenerative diseases.
Currently, several strategies are being developed to treat mitochondrial diseases, including: (1) targeting mitochondria with antioxidants, (2) developing molecules that activate human longevity genes “SIRTUINS” that suppress oxidative damage in cells, and (3) mitochondrial DNA therapy. These strategies are at early research stages, with results promising thus far. However, our current knowledge of mitochondrial involvement in aging and neurodegenerative diseases is still quite limited, with many issues still needing to be addressed, such as: (1) If mitochondrial dysfunction is critical to the progression of disease in neurodegenerative diseases, what is the mechanism for mitochondrial involvement in each of these diseases? Is there a selective accumulation of mitochondrial DNA defects in affected brain regions in each of these neurodegenerative diseases? (2) In AD, PD, HD, ALS, and FRDA, specific populations of neurons are affected, although both normal and mutant proteins express in all cells of the body. Are mitochondria preferentially dysfunctional, particularly in a specific population of neurons, in each of these diseases? (3) How do mutant proteins (Aβ in AD, mutant SOD1 in ALS, mutant Htt in HD, mutant α-synuclein in PD, and mutant frataxin in FRDA) selectively interact with mitochondrial proteins? (4) What are the pathomechanisms involved in inherited and non-inherited neurodegenerative diseases? Learning answers to these important questions may allow us to develop therapeutic strategies and/or develop molecules to treat devastating neurodegenerative diseases.
Acknowledgments
I sincerely thank Drs. Maria Manczak and Wei Zhao (postdoctoral scientists in the lab) for their technical assistance of MitoQ and SS-31 treatments of N2a cells. The research presented in this article was supported by grants from the American Federation for Aging Research, National Institutes of Health (AG028072 and AG026051), and KaloBios Pharmaceuticals, Inc.
Abbreviations
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- APP
Amyloid precursor protein
- ATP
Adenosine triphosphate
- CR
Caloric restricted
- ETC
Electron transport chain
- FRDA
Freidriech ataxia
- H2O2
Hydrogen peroxide
- HD
Huntington’s disease
- mtDNA
Mitochondrial DNA
- PGC-1α
Peroxisome proliferator-activated receptor-coactivator
Superoxide radical
- OXPHOS
Oxidative phosphorylation
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- SS peptide
Szeto-Schiller peptide
References
- Abe Y, Hashimoto Y, Tomita Y, Terashita K, Aiso S, Tajima H, et al. Cytotoxic mechanisms by M239 V presenilin 2, a little-analyzed Alzheimer’s disease-causative mutant. Journal of Neuroscience Research. 2004;77:583–595. doi: 10.1002/jnr.20163. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Beal MF. Parkinsonism genes: Culprits and clues. Journal of Neurochemistry. 2006;99:1062–1072. doi: 10.1111/j.1471-4159.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- Afifi AK, Aleu FP, Goodgold J, MacKay B. Ultrastructure of atrophic muscle in amyotrophic lateral sclerosis. Neurology. 1966;16:475–481. doi: 10.1212/wnl.16.5.475. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. Journal of Cell Biology. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JK. Iron dysregulation and Parkinson’s disease. Journal of Alzheimer’s Disease. 2004;6:S47–S52. doi: 10.3233/jad-2004-6s602. [DOI] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anekonda T. Resveratrol—a boon for treating Alzheimer’s disease? Brain Research Reviews. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer’s disease. Journal of Neurochemistry. 2006;96:305–313. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Hultenby K. Presenilin-1 is located in rat mitochondria. Biochemical and Biophysical Research Communications. 2002;295:766–770. doi: 10.1016/s0006-291x(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO Journal. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GP. History of genetic disease: The molecular genetics of Huntington disease—a history. Nature Reviews. Genetics. 2005;6:766–773. doi: 10.1038/nrg1686. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Annals of Neurology. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Behbahani H, Shabalina IG, Wiehager B, et al. Differential role of Presenilin-1 and -2 on mitochondrial membrane potential and oxygen consumption in mouse embryonic fibroblasts. Journal of Neuroscience Research. 2006;84:891–902. doi: 10.1002/jnr.20990. [DOI] [PubMed] [Google Scholar]
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, et al. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, et al. Mitochondrial bioenergetics and structural network organization. Journal of Cell Science. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- Bergemalm D, Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Rehnmark A, et al. Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models. Journal of Neuroscience. 2006;26:4147–4154. doi: 10.1523/JNEUROSCI.5461-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annual Review of Biochemistry. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Current Opinion in Cell Biology. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Abdul HM, Butterfield DA. Gamma-glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Abeta(1–42)-mediated oxidative stress and neurotoxicity: Implications for Alzheimer’s disease. Journal of Neuroscience Research. 2005a;79:700–706. doi: 10.1002/jnr.20394. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Mohmmad-Abdul H, Lynn BC, Klein JB, et al. Gamma-glutamylcysteine ethyl ester protection of proteins from Abeta(1–42)-mediated oxidative stress in neuronal cell culture: A proteomics approach. Journal of Neuroscience Research. 2005b;79:707–713. doi: 10.1002/jnr.20393. [DOI] [PubMed] [Google Scholar]
- Bozner P, Grishko V, LeDoux SP, Wilson GL, Chyan YC, Pappolla MA. The amyloid beta protein induces oxidative damage of mitochondrial DNA. Journal of Neuropathology and Experimental Neurology. 1997;56:1356–1362. doi: 10.1097/00005072-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Browne SE, Beal MF. The energetics of Huntington’s disease. Neurochemical Research. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- Browne SE, Beal MF. Oxidative damage in Huntington’s disease pathogenesis. Antioxidants Redox Signaling. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, et al. Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia. Annals of Neurology. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- Campuzano V, Montermini L, Molto MD, et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Canet-Avilés RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, et al. Mitochondrial Abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB Journal. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annual Review of Cell and Developmental Biology. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chan SL, Furukawa K, Mattson MP. Presenilins and APP in neuritic and synaptic plasticity: Implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Medicine. 2002;2:167–196. doi: 10.1385/NMM:2:2:167. [DOI] [PubMed] [Google Scholar]
- Chang S, Ran MAT, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18694–18689. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Progress in Neurobiology. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiology of Disease. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Chen X, Liang H, Van Remmen H, Vijg J, Richradson A. Catalase transgenic mice: Characterization and sensitivity to oxidative stress. Archives of Biochemistry and Biophysics. 2004;422:197–210. doi: 10.1016/j.abb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. Journal of Biological Chemistry. 2007a;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, et al. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coronary Artery Disease. 2007b;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- Chung MJ, Suh YL. Ultrastructural changes of mitochondria in the skeletal muscle of patients with amyotrophic lateral sclerosis. Ultrastructural Pathology. 2002;26:3–7. doi: 10.1080/01913120252934260. [DOI] [PubMed] [Google Scholar]
- Cocheme HM, Kelso GF, James AM, Ross MF, Trnka J, Mahendrian T, Asin-Cayuela J, Blaike FH, Manas AR, Porteous CM, Adlam VJ, Smith RA, Murphy MP. Mitochondrial targeting of quinones: Therapeutic implications. Mitochondrion. 2007 Suppl:S94–S102. doi: 10.1016/j.mito.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: Effect of ageing. Journal of the Neurological Sciences. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- Copeland WC. Inherited mitochondrial diseases of DNA replication. Annual Review of Medicine. 2008;59:131–146. doi: 10.1146/annurev.med.59.053006.104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, et al. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23:471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- Crouch PJ, Blake R, Duce JA, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. Journal of Neuroscience. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Deng HX, Shi Y, Furukawa Y, Zhai H, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. Journal of Biological Chemistry. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. Journal of Neuroscience. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. Journal of Biological Chemistry. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Jpseph J, Kalyanaraman B. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. Jounal of Biological Chemistry. 2004;279:37575–37587. doi: 10.1074/jbc.M404003200. [DOI] [PubMed] [Google Scholar]
- Ding Q, Cecarini V, Keller JN. Interplay between protein synthesis and degradation in the CNS: Physiological and pathological implications. Trends in Neurosciences. 2007;30:31–36. doi: 10.1016/j.tins.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. Journal of Biological Chemistry. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- Dupuis L, di Scala F, Rene F, de Tapia M, Oudart H, Pradat PF, et al. Up-regulation of mitochondrial uncoupling protein 3 reveals an early muscular metabolic defect in amyotrophic lateral sclerosis. FASEB Journal. 2003;17:2091–2093. doi: 10.1096/fj.02-1182fje. [DOI] [PubMed] [Google Scholar]
- Farrer M, Stone J, Mata IF, Lincoln S, Kachergus J, Hulihan M, et al. LRRK2 mutations in Parkinson disease. Neurology. 2005;65:738–740. doi: 10.1212/01.wnl.0000169023.51764.b0. [DOI] [PubMed] [Google Scholar]
- Fromenty B, Grimbert S, Mansouri A, Beaugrand M, Erlinger S, Rotug A, et al. Hepatic mitochondrial DNA deletion in alcoholics: Association with microvesicular steatosis. Gastroenterology. 1995;108:193–200. doi: 10.1016/0016-5085(95)90024-1. [DOI] [PubMed] [Google Scholar]
- Folstein SE. Huntington’s Disease. Baltimore: Johns Hopkins University Press; 1990. [Google Scholar]
- Fukui H, Moraes CT. Extended polyglutamine repeats trigger a feedback loop involving the mitochondrial complex III, the proteasome and huntingtin aggregates. Human Molecular Genetics. 2007;16:783–797. doi: 10.1093/hmg/ddm023. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Fu R, Deng HX, Siddique T, O’Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Larochette N, Zamzami N, Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene. 2006;25:4812–4830. doi: 10.1038/sj.onc.1209598. [DOI] [PubMed] [Google Scholar]
- Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Annals of Neurology. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood NW. Molecular pathogenesis of Parkinson’s disease. Human Molecular Genetics. 2005;14:2749–2755. doi: 10.1093/hmg/ddi308. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. Journal of Neural Transmission. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, et al. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes and Development. 2000;14:1021–1026. [PubMed] [Google Scholar]
- Guo Q, Sebastian L, Sopher BL, Miller MW, Ware CB, Martin GM, et al. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: Central roles of superoxide production and caspase activation. Journal of Neurochemistry. 1999;72:1019–1029. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, et al. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. Journal of Neuroscience. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hansson CA, Frykman S, Farmery MR, et al. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. Journal of Biological Chemistry. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hausse AO, Aggoun Y, Bonnet D, Sidi D, Munnich A, Rotig A, et al. Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart. 2002;87:346–349. doi: 10.1136/heart.87.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervias I, Beal MF, Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle and Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neuroscience. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer’s disease. Journal of Neuroscience. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology. 1984;43:461–470. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, et al. A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Horton TM, Graham BH, Corral-Debranski M, Shoffner JM, Kaufman AE, Beal MF, et al. Marked increase in mitochondrial DNA deletion levels in the cerebral cortex of Huntington’s disease patients. Neurology. 1995;45:1879–1883. doi: 10.1212/wnl.45.10.1879. [DOI] [PubMed] [Google Scholar]
- Howell N, Elson JL, Chinnery PF, Turnbull DM. mtDNA mutations and common neurodegenerative disorders. Trends in Genetics. 2005;21:583–586. doi: 10.1016/j.tig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Huang CC, Faber PW, Persichetti F, Mittal V, Vonsattel JP, MacDonald ME, et al. Amyloid formation by mutant huntingtin: Threshold, progressivity and recruitment of normal polyglutamine proteins. Somatic Cell and Molecular Genetics. 1998;24:217–233. doi: 10.1023/b:scam.0000007124.19463.e5. [DOI] [PubMed] [Google Scholar]
- Ikebe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, et al. Increase of deleted mitochondrial DNA in the striatum in Parkinson’s disease and senescence. Biochemical and Biophysical Research Communications. 1990;170:1044–1048. doi: 10.1016/0006-291x(90)90497-b. [DOI] [PubMed] [Google Scholar]
- Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB Journal. 2003;17(13):1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- Jin J, Meredeith GE, Chen L, Zhou Y, Xu J, Shie FS, Lockhart P, Zhang J. Quantitative proteomic analysis of mitochondrial proteins: Relevance to Lewy body formation and Parkinson’s disease. Brain Research. Molecular Brain Research. 2005;134:119–138. doi: 10.1016/j.molbrainres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Lindberg M, Oliveberg M, et al. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- Kao S, Chao HT, Wei YH. Mitochondrial deoxyribonucleic acid 4977-bp deletion is associated with diminished fertility and motility of human sperm. Biology of Reproduction. 1995;52:729–736. doi: 10.1095/biolreprod52.4.729. [DOI] [PubMed] [Google Scholar]
- Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. Journal of Neuroscience. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Timmer PA, et al. Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Annals of Neurology. 2000;48:148–155. [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO Journal. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxidants Redox Signaling. 1999;1:481–499. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Mitochondria in cancer. Oncogene. 2006;25:4630–4632. doi: 10.1038/sj.onc.1209589. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–448. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations and apoptosis in mammalian aging. Cancer Research. 2006;66:7386–7389. doi: 10.1158/0008-5472.CAN-05-4670. [DOI] [PubMed] [Google Scholar]
- Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. Journal of Neurochemistry. 2006;97:1659–1675. doi: 10.1111/j.1471-4159.2006.03990.x. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Gree KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nature Reviews. Neuroscience. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: Evidence for xenohormesis. Molecular Microbiology. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Leroy E, Boyer R, Auburger G, et al. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. Journal of Neurochemistry. 2004;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Li S, Li XJ. Multiple pathways contribute to the pathogenesis of Huntington disease. Molecular Neurodegeneration. 2006;1:19. doi: 10.1186/1750-1326-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF. PGC-1alpha: A key regulator of energy metabolism. Advances in Physiology Education. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu J, Lillo C, Jonsson PA, et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: Implications for free radical generation and oxidative damage in disease progression. Human Molecular Genetics. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: Implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Medicine. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. Journal of Cell Biology. 2004;167:99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology. 2006;65:1103–1110. doi: 10.1097/01.jnen.0000248541.05552.c4. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiology of Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Mohmmad Abdul H, Sultana R, Keller JN, St Clair DK, Markesbery WR, Butterfield DA. Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid beta-peptide (1–42), HO and kainic acid: Implications for Alzheimer’s disease. Journal of Neurochemistry. 2006;96:1322–1335. doi: 10.1111/j.1471-4159.2005.03647.x. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. Journal of the Neurological Sciences. 2007;257:206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Echtay KS, Blaikie FH, et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: Studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. Journal of Biological Chemistry. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annual Review of Pharmacology and Toxicology. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- Nichols WC, Pankratz N, Hernandez D, Paisan-Ruiz C, et al. Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- Orr AL, Li S, Wang CE, Wang J, Rong J, Xu X, et al. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. Journal of Neuroscience. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T, Tanaka M, Ikebe S, Ohno K, Kondo T, Mizuno Y. Quantitative determination of deleted mitochondrial DNA relative to normal DNA in parkinsonian striatum by a kinetic PCR analysis. Biochemical and Biophysical Research Communications. 1990;172:483–489. doi: 10.1016/0006-291x(90)90698-m. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, et al. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nature Neuroscience. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Panov AV, Lund S, Greenamyre JT. Ca2 + -induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Molecular and Cellular Biochemistry. 2005;269:143–152. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature Genetics. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of Neurology. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelmann RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiology of Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Perez MK, Paulson HL, Pendse SJ, Saionz SJ, Bonini N, Pittman RN. Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. Journal of Cell Biology. 1998;143:1457–1470. doi: 10.1083/jcb.143.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Roder H, Nunomura A, et al. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. NeuroReport. 1999;10:2411–4215. doi: 10.1097/00001756-199908020-00035. [DOI] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. Journal of Neurochemistry. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: Relevance to brain lipid peroxidation in neurodegenerative disease. Neurochemistry International. 2001;39:141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Qin W, Chachich M, Lane M, et al. Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) Journal of Alzheimer’s Disease. 2006a;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. Journal of Biological Chemistry. 2006b;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Rabol R, Boushel R, Dela F. Mitochondrial oxidative function and type 2 diabetes. Applied Physiology, Nutrition, and Metabolism. 2006;31:675–683. doi: 10.1139/h06-071. [DOI] [PubMed] [Google Scholar]
- Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ, et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. Journal of Biological Chemistry. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer’s disease. Journal of Neurochemistry. 2006a;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: Implications for mitochondrially targeted antioxidant therapeutics. Journal of Biomedicine & Biotechnology. 2006b;31372:13. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: Strategies to protect neurons. Antioxidants Redox Signaling. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Research. Brain Research Reviews. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction, and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends in Molecular Medicine. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S. Mapping cellular transcriptosomes in autopsied Alzheimer’s disease subjects and relevant animal models. Neurobiology of Aging. 2006;27:1060–1077. doi: 10.1016/j.neurobiolaging.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: Up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Human Molecular Genetics. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Tagle DA. Biology of trinucleotide repeat disorders. In: Mattson PM, editor. Genetic aberrancies and neurodegenerative disorders. Advances in cell aging and gerontology. Vol. 3. Stanford, Connecticut: Jai press Inc.; 1999. [Google Scholar]
- Reddy PH, Williams M, Charles V, Garrett L, Pike-Bucanan L, Whetsell WO, Jr, Miller G, Tagle DA. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nature Genetics. 1998;20:198–202. doi: 10.1038/2510. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Williams M, Tagle DA. Recent advances in understanding the pathogenesis of Huntington’s disease. Trends in Neurosciences. 1999;22:248–255. doi: 10.1016/s0166-2236(99)01415-0. [DOI] [PubMed] [Google Scholar]
- Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nature Genetics. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicology and Applied Pharmacology. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop HP, Perricak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Warita H, Murakami T, Abe K, Iwata M. Ultrastructural study of mitochondria in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathologica. 2004;107:461–474. doi: 10.1007/s00401-004-0837-z. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Manczak M, Akileswaran L, Reddy PH, Coghlan VM. Interaction of the nuclear matrix protein NAKAP with HypA and huntingtin: Implications for nuclear toxicity in Huntington’s disease pathogenesis. Neuromolecular Medicine. 2005;7:297–310. doi: 10.1385/NMM:7:4:297. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Journal of Neurochemistry. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Human Molecular Genetics. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, Schachner M. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Molecular Psychiatry. 2007 doi: 10.1038/sj.mp.4002077. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, et al. Extension of murine lifespan by overexpression of catalase targeted to mitochondria. Science. 308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Schultz D, Harrison DG. Quest for fire: Seeking the source of pathogenic oxygen radicals in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1412–1423. doi: 10.1161/01.atv.20.6.1412. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiological Reviews. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Seong IS, Ivanova E, Lee JM, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Human Molecular Genetics. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochimica et Biophysica Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Shimohata T, Nakajima T, Yamada M, et al. Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nature Genetics. 2000;26:29–36. doi: 10.1038/79139. [DOI] [PubMed] [Google Scholar]
- Shirane M, Nakayama KI. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nature Cell Biology. 2003;5:28–37. doi: 10.1038/ncb894. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, et al. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Annals of Neurology. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Kurana NC, Heato MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Research. 2005;1052:202–211. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, John DR. Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology. 1999;53:1787–1793. doi: 10.1212/wnl.53.8.1787. [DOI] [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of diabetes: The role of oxidative stress. Free Radical Biology and Medicine. 2006;40:917–922. doi: 10.1016/j.freeradbiomed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. Journal of Neurochemistry. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, et al. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. European Journal of Biochemistry. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons & enhances oxidative stress. Journal of Cell Biology. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Human Molecular Genetics. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: Insights into mechanism of neurodegeneration from redox proteomics. Antioxidants Redox Signaling. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. Journal of Neuroscience Research. 2007a;85:3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Treating neurodegeneration by modifying mitochondria: Potential solutions to a complex problem. Antioxidants Redox Signaling. 2007b;9:1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Medical Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, et al. Cybrids in Alzheimer’s disease: A cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted peptide antioxidants: Novel neuroprotective agents. AAPS Journal. 2006a;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS Journal. 2006b;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-Targeted Cytoprotective Peptides for Ischemia-Reperfusion Injury. Antioxidants Redox Signaling. 2008;10:601–620. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Lovelace JL, Fridland G, Soong Y, Fasolo J, Wu D, et al. In vivo pharmacokinetics of selective muopioid peptide agonists. Journal of Pharmacology and Experimental Therapeutics. 2001;298:57–61. [PubMed] [Google Scholar]
- Szeto HH, Schiller PW, Zhao K, Luo G. Fluorescent dyes alter intracellular targeting and function of cell-penetrating tetrapeptides. FASEB Journal. 2005;19:118–120. doi: 10.1096/fj.04-1982fje. [DOI] [PubMed] [Google Scholar]
- Tabrizin SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Annals of Neurology. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. 45:1≤25::AID-ART6≥3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ–1 has a role in antioxidative stress to prevent cell death. EMBO Reports. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, et al. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB Journal. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Gugglielmotto M, Argno M, Borghi R, Autelli R, Giliberto L, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. Journal of Neurochemistry. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. Journal of Neuroscience. 2007;27:2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Beal MF. Parkinson’s disease. Human molecular genetics. 2007;16(Spec No. 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Stauffer C, Zhao K, Yang H, Sharma VK, Szeto HH, et al. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. Journal of the American Society of Nephrology. 2007;18:213–222. doi: 10.1681/ASN.2006080825. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Swerdlow RH, Park JK, Keeney P, Bennet JP, Miller SW, et al. Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Experimental Neurology. 2004;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- Trushina E, Dyer RB, Badger JD., 2nd Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Molecular and Cellular Biology. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reproductive Biomedicine Online. 2008;16:553–569. doi: 10.1016/s1472-6483(10)60463-4. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Warby SC, Hayden MR. Selective degeneration in YAC mouse models of Huntington disease. Brain Research Bulletin. 2007;72:124–131. doi: 10.1016/j.brainresbull.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Qi W, Sabia M, Freeman G, Estlack L, Yang H, et al. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radical Biology and Medicine. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. Journal of Neuroscience. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov AD, Grivennikova VG. Generation of superoxide-radical by the NADH:ubiquinone oxidoreductase of heart mitochondria. Biochemistry (Mosc) 2005;70:120–127. doi: 10.1007/s10541-005-0090-7. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. Journal of Neuropathology and Experimental Neurology. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1448. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic & degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annual Review of Genetics. 2005a;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. The mitochondrial genome in human adaptive radiation and disease: On the road to therapeutics and performance enhancement. Gene. 2005b;354:169–180. doi: 10.1016/j.gene.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB Journal. 2005a;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Nakaya Y, Du Z, et al. Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2. Human Molecular Genetics. 2005b;14:1889–1902. doi: 10.1093/hmg/ddi195. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiology of Disease. 2001;8:933–941. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- West AB, Morre DJ, Biskup S, Bugayenko A, Smith W·W, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16842–1687. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metabolism. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Wilson RB. Iron dysregulation in Friedreich ataxia. Seminars in Pediatric Neurology. 2006;3:166–175. doi: 10.1016/j.spen.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin Ctivators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Yoon DS, Lim IK, et al. Formation of elongated giant mitochondria in DFO-induced cellular senescence: Involvement of enhanced fusion process through modulation of Fis1. Journal of Cellular Physiology. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawso TM, Dawson VL. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: Implications for pathogenesis. Human Molecular Genetics. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zhao K, Luo G, Zhao GM, Schiller PW, Szeto HH. Transcellular transport of a highly polar 3 + net charge opioid tetrapeptide. Journal of Pharmacology and Experimental Therapeutics. 2003;304:425–432. doi: 10.1124/jpet.102.040147. [DOI] [PubMed] [Google Scholar]
- Zhao GM, Wu D, Soong Y, Shimoyama M, Berezowska I, Schiller PW, et al. Profound spinal tolerance after repeated exposure to a highly selective mu-opioid peptide agonist: Role of delta-opioid receptors. Journal of Pharmacology and Experimental Therapeutics. 2002;302:188–196. doi: 10.1124/jpet.302.1.188. [DOI] [PubMed] [Google Scholar]
- Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. Journal of Biological Chemistry. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]