Abstract
Narcolepsy with cataplexy is characterized by daytime sleepiness, cataplexy (sudden loss of bilateral muscle tone triggered by emotions), sleep paralysis, hypnagogic hallucinations and disturbed nocturnal sleep. Narcolepsy with cataplexy is most often associated with human leucocyte antigen-DQB1*0602 and is caused by the loss of hypocretin-producing neurons in the hypothalamus of likely autoimmune aetiology. Noting that children with narcolepsy often display complex abnormal motor behaviours close to disease onset that do not meet the classical definition of cataplexy, we systematically analysed motor features in 39 children with narcolepsy with cataplexy in comparison with 25 age- and sex-matched healthy controls. We found that patients with narcolepsy with cataplexy displayed a complex array of ‘negative’ (hypotonia) and ‘active’ (ranging from perioral movements to dyskinetic–dystonic movements or stereotypies) motor disturbances. ‘Active’ and ‘negative’ motor scores correlated positively with the presence of hypotonic features at neurological examination and negatively with disease duration, whereas ‘negative’ motor scores also correlated negatively with age at disease onset. These observations suggest that paediatric narcolepsy with cataplexy often co-occurs with a complex movement disorder at disease onset, a phenomenon that may vanish later in the course of the disease. Further studies are warranted to assess clinical course and whether the associated movement disorder is also caused by hypocretin deficiency or by additional neurochemical abnormalities.
Keywords: hypotonia, movement disorder, narcolepsy with cataplexy, streptococcal infection, chorea
Introduction
Narcolepsy with cataplexy was first described as a condition with repetitive attacks of sleep and/or loss of muscle tone (Westphal, 1877). In 1880, Gélineau (Gélineau, 1880) coined the term ‘narcolepsy’. The disease is characterized by excessive daytime sleepiness with repetitive and refreshing sleep attacks, cataplexy, sleep paralysis and hypnagogic hallucinations (Yoss and Daly, 1957; American Academy of Sleep Medicine, 2005; Dauvilliers et al., 2007a). Cataplexy, a symptom characterized by sudden and brief (5–30 s) losses of muscle tone (partial or total) triggered by emotions such as laughter and surprise, is considered pathognomonic of narcolepsy with cataplexy (American Academy of Sleep Medicine, 2005; Vetrugno et al., 2010). Additionally, nocturnal sleep in narcolepsy with cataplexy is not only fragmented by frequent awakenings, but also disturbed by symptoms reflecting abnormal motor control during sleep, such as periodic and non-periodic limb movements, restless legs syndrome and rapid eye movement (REM) sleep behaviour disorder (Ferri et al., 2006, 2008; Dauvilliers et al., 2007b; Mattarozzi et al., 2008; Knudsen et al., 2010; Plazzi et al., 2008a, 2010).
A loss of hypocretin-producing neurons (De Lecea et al., 1998; Sakurai et al., 1998) in the postero-lateral hypothalamus (Peyron et al., 2000; Thannickal et al., 2000) is characteristic of narcolepsy with cataplexy. Hypocretin-producing neurons have widespread projections within the CNS and play a key role in the regulation of sleep and wakefulness (Saper et al., 2005), thus explaining the major symptoms of narcolepsy with cataplexy. Animals with genetic modifications in hypocretin or the hypocretin receptor gene have narcolepsy and cataplexy, indicating causality for major narcolepsy symptoms (Chemelli et al., 1999; Lin et al., 1999).
Recent works strongly suggest that the loss of hypocretin neurons is of autoimmune origin. Indeed, as for many other autoimmune diseases, the disorder is strongly associated with human leucocyte antigen (HLA)-DQB1*0602 (Mignot et al., 1994), with modulating influences of other HLA subtypes (Mignot et al., 2001; Hong et al., 2006b; Hor et al., 2010). In addition, immune-related polymorphisms in the T-cell receptor α (Hallmayer et al., 2009; Hor et al., 2010) and the P2YR11 receptor loci (Kornum et al., 2011), a gene modulating immune cell function, are also associated with the condition. Finally, high levels of anti-tribbles 2 autoantibodies and anti-streptolysin O antibodies are found around disease onset in many patients (Aran et al., 2009; Cvetkovic-Lopes et al., 2010; Kawashima et al., 2010; Toyoda et al., 2010). These findings suggest that it may be possible to stop the progression of hypocretin cell loss in some patients using immune suppressive therapy in cases close to symptom onset when hypocretin cell damage may not yet be irreversible (Fontana et al., 2010). To date, however, immunoglobulin treatment in such cases has met with variable results (Lecendreux et al., 2003; Dauvilliers et al., 2004, 2009; Valko et al., 2008; Plazzi et al., 2008b).
Narcolepsy with cataplexy is a lifelong disorder arising mainly in young adults (Dauvilliers et al., 2001) or in children (Yoss and Daly, 1960; Aran et al., 2010); ∼5% of cases occur in the pre-pubertal stage (Nevsimalova, 2009). It is typically diagnosed 10–15 years after symptom onset (Morrish et al., 2004; Ohayon et al., 2005), although this is rapidly improving with increased awareness. As a consequence, most case series have characterized the disorder in adults after a long period of adaptation since onset, and thus the phenotype at presentation has been assessed only retrospectively. Cataplexy generally occurs within a year from sleepiness onset, but a delay between sleepiness and cataplexy of up to several decades, mostly in adult-onset cases with mild disease (Rye et al., 1998; Ohayon et al., 2005b), has been reported. In recent work in our centre, we have seen increasing numbers of children who were identified close to disease onset. In these cases, we found that childhood cataplexy often appears abruptly as both emotionally triggered episodes (e.g. watching cartoons, tickling), as in adults, as spontaneous falls to the ground (e.g. while walking, running, eating), or as generalized hypotonia with prominent facial involvement resulting in ‘cataplectic facies’ (Plazzi et al., 2006; Serra et al., 2008; Dhondt et al., 2009; Merino-Andreu and Martinez-Bermejo, 2009; Peraita-Adrados et al., 2011). Overall, 11 cases of cataplectic status with typical cataplectic facies have been reported in children (Hecht et al., 2003; Plazzi et al., 2006; Serra et al., 2008; Dhondt et al., 2009; Merino-Andreu and Martinez-Bermejo, 2009; Peraita-Adrados et al., 2011). This picture is generally associated with hypotonia, ptosis, tongue protrusion or unsteady gait at neurological examination (Plazzi et al., 2006; Serra et al., 2008; Dhondt et al., 2009), and rarely with ‘unconscious’ active motor behaviours (e.g. scratching, head shaking) that have been only briefly characterized (Serra et al., 2008). Precocious puberty has also been reported in 3 out of 11 children (Plazzi et al., 2006; Peraita-Adrados et al., 2011), whereas obesity occurs at least in 25% of paediatric patients (Nevsimalova, 2009).
In an effort to document cataplexy features using video recordings, we noticed abnormal motor behaviours occurring around episodes of cataplexy with or without emotional triggering. These abnormal behaviours included the atypical features of childhood cataplexy described above and the occurrence of additional active, but apparently unconscious, movements ranging from normal patterns to frankly pathological motor behaviours. Indeed, given the common association with increased anti-streptolysin O titres in narcolepsy with cataplexy, we also searched for motor abnormalities reminiscent of features often observed in Sydenham's Chorea and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS), two autoimmune movement disorders linked to streptococcal infection (Cardoso et al., 2006). In this study, we report these novel features of childhood narcolepsy with cataplexy in comparison with a gender- and age-matched control group using representative figures and videos, and discuss their relevance for understanding pathophysiology of narcolepsy with cataplexy and to aid early correct diagnosis.
Materials and methods
We performed a prospective study to describe the clinical features of childhood narcolepsy with cataplexy at disease onset. The study was conducted at the Sleep Disorders Centre, University of Bologna, and approved by the local institutional review board.
Participants
We prospectively collected patients under the age of 18 years old who were newly diagnosed with narcolepsy with cataplexy between the years 2001–11, who met the international criteria for narcolepsy with cataplexy (American Academy of Sleep Medicine, 2005). None of the patients had positive family history for narcolepsy with cataplexy. Consent was obtained from parents and assent from patients. Patients had a complete clinical and neurological examination, conventional brain MRI and underwent a 48 h continuous polysomnographic recording followed by a standard five-nap Multiple Sleep Latency Test (Littner et al., 2005). HLA-DQB1*0602 typing was conducted in all patients, and CSF hypocretin-1 evaluations were performed when possible. All patients were drug-naïve for stimulant and anti-cataplectic drugs at the time of the study.
The presence and age of onset of all core narcolepsy with cataplexy symptoms (sleepiness, cataplexy, sleep paralysis, hypnagogic hallucinations and disturbed nocturnal sleep) and the presence of any abnormalities at neurological examination were systematically documented. Additionally, the history of tonsillectomy occurring before narcolepsy with cataplexy symptom onset, and biochemical data pertaining to streptococcal infections (anti-streptolysin O titres) were also collected. Disease duration was defined as the elapsed time from the onset of the first symptom (i.e. sleepiness or cataplexy, including ‘cataplectic facies’) to clinical observation. We enrolled 39 patients (19 females) with a mean ± standard deviation (SD) age of 11.5 ± 3.5 years. Following the launch of a media awareness campaign led by the Italian association of narcoleptic patients (Associazione Italiana Narcolettici) in 2001, we were able to observe and diagnose an increasing number of new narcolepsy with cataplexy cases every year, and also children close to disease onset. In particular, in the years from 2001 to 2005 we diagnosed one or two children per year; in the 2-year period from 2006 to 2007, four per year; and from 2008 to 2010, up to nine per year. The mean ± SD age of symptom onset was 9.3 ± 3.0 years; yearly narcolepsy with cataplexy onset ranged from one to three during the period from 1998 to 2006, from six to nine in the period from 2007 to 2009 and a single patient in 2010. Diagnostic delay was 1.8 ± 2.1 years (range 3 weeks–9 years). Tentative diagnoses made prior to narcolepsy with cataplexy included myopathy (two cases), Sydenham's chorea (three cases), PANDAS (one case), attention deficit hyperactivity disorder (three cases), epilepsy (six cases), malingering (one case), encephalitis (three cases) and depression (two cases). All patients had excessive daytime sleepiness (onset: 9.3 ± 3.0 years) and cataplexy (onset: 10.0 ± 3.1 years). Sleep paralysis was present in 26% (onset: 9.8 ± 4.8 years), hypnagogic hallucinations in 59% (onset: 10.4 ± 3.5 years) and disturbed nocturnal sleep in 51% (onset: 9.7 ± 3.0 years) of cases. A mean ± SD sleep latency of 3.9 ± 3.3 min and 4.2 ± 0.9 sleep onset REM periods were found on the Multiple Sleep Latency Test. The mean ± SD Epworth sleepiness scale score was 14.9 ± 3.2. HLA-DQB1*0602 was positive in 36 out of 39 (92%) patients. CSF hypocretin-1 was <110 pg/ml in all the 27 tested cases (mean ± SD of 24.9 ± 29.7 pg/ml), including three DQB1*0602-negative patients. Eight patients (23%) had a clinical history of allergy (atopic dermatitis, allergic asthma and allergic rhinitis), while two (7%) had evidence of tonsillectomy occurring before the onset of narcolepsy with cataplexy symptoms. Mean ± SD anti-streptolysin O values were 333 ± 254, and 33% had levels >400. Conventional brain MRI findings were unremarkable.
Interictal neurological examination revealed frequently overlapping abnormal findings in 17 cases (44%): 16 patients (41%) had mildly reduced muscle tone overall and 10 subjects (26%) had an abnormal gait, characterized by wide-based swaying. Overall, nine children (23%) had a combination of hypotonia and abnormal gait, whereas seven (18%) and one (3%) presented only mild hypotonia or abnormal gait, respectively. The rest of the examination was unremarkable.
Twenty-five healthy controls were also enrolled. They were matched to patients for gender (52% males, P = 0.955), and age (mean ± SD age = 12.3 ± 3.2 years, P = 0.408). At a clinical interview all subjects denied symptoms of sleep disorders, and none of them had pathological signs on neurological examination. Their mean ± SD Epworth sleepiness scale score was 6 ± 2.
Video recordings
Video recordings were performed with the subject sitting and/or standing for 10–40 min, enough for the child to feel at ease but not long enough for sleepiness to set in. Patients were also asked to take a brief nap prior to video recordings to reduce sleepiness, and were asked again whether they felt sleepy at the end of the procedure; this was done to avoid the confounding effects of sleepiness on the observation of cataplexy. Videos of patients who reported sleepiness during the recordings were excluded from further analysis. Prior to each video recording, patients were interviewed (C.F., V.P.) to determine humour preference and asked to choose between various funny videos; these were selected to best stimulate each subject. Video recordings included a 5 min baseline recording and up to 30 min while subjects were watching videos.
Two independent neurologists, experts in the field of movement disorders and blind to patients’ or controls’ clinical features (M.J.E., P.C.), independently analysed video recordings, rating ‘negative’ and ‘active’ motor symptoms at baseline and during emotional stimulation. The scoring procedure of each video encompassed two steps allowing the separate evaluation of movements occurring at baseline and during emotional stimulation. Video recordings of patients and controls were shown to the two raters in a random order. ‘Negative’ motor phenomena were classified as: (i) paroxysmal head drops and falls; (ii) persistent eyelid narrowing and tongue protrusion; (iii) persistent facial hypotonia; and (iv) persistent generalized hypotonia. ‘Active’ motor phenomena were classified as: (i) raising of the eyebrows; (ii) perioral and tongue movements; (iii) facial grimaces; (iv) swaying of the head and/or trunk; (v) stereotyped motor behaviour; and (vi) dyskinetic or dystonic movements. Each item was scored on an ordinal scale ranging from 0 (absent) to 3 (continuously present).
Statistical analysis
Clinical, polygraphic and laboratory data are reported as frequencies or mean ± SD.
Inter-observer reliability estimates for each motor phenomenon score were assessed by Cohen's κ statistics. According to Fleiss (Fleiss, 1981), a κ > 0.75 indicates excellent agreement between the two scorers, whereas a κ between 0.40 and 0.75 suggests a fair to good agreement, and a κ < 0.40 a poor agreement.
A composite score of ‘negative’ (range 0–12) and ‘active’ (range 0–18) motor features at baseline and during emotional stimulation was then obtained by summing each sub-item score for each rater. A mean value between the two scorers for each ‘negative’ and ‘positive’ motor feature and for the composite scores was calculated, and this mean score was used for comparison between patients and controls using the Mann–Whitney U-test.
Mean scores at baseline and during emotional stimulation conditions were compared using the Wilcoxon signed-rank test for both patients and controls. Additionally, the difference between each score after emotional trigger and at baseline was calculated for each motor feature, and a comparison between patients and controls was then performed using the Mann–Whitney U-test.
Associations between motor phenomena scores in patients with and without pathological signs at neurological examination (hypotonia- and/or wide-based swaying gait) and with or without elevated anti-streptolysin O titres (i.e. >400) were tested using Mann–Whitney U-tests.
The relationships between motor phenomena scores, age at narcolepsy with cataplexy onset, disease duration, sleepiness severity at Multiple Sleep Latency Test (sleep latency, number of sleep-onset REM periods), CSF hypocretin-1 and plasma anti-streptolysin O values were assessed using Spearman's correlation analysis.
A P ≤ 0.05 was considered to be statistically significant.
Results
Motor phenomena assessment: inter-observer reliability estimates
Mean, median and SD of the scores of each ‘negative’ and ‘positive’ motor phenomenon assessed by each observer are reported in Supplementary Table 1, together with inter-observer reliability estimates according to κ statistics. Reliability was in the excellent range for most of the motor phenomena assessed, good for some motor scores (facial hypotonia after emotional trigger, perioral and tongue movements at baseline, facial grimacing after emotional trigger and stereotyped motor behaviour at baseline), and on the limit for poor agreement for a single item (facial grimacing at baseline).
Motor phenomena assessment in patients versus controls: the effect of emotional trigger
Mean values of ‘negative’ and ‘active’ motor phenomena scores at baseline and during emotional stimuli are reported in Table 1 for both patients and controls, together with statistical comparison using Mann–Whitney U-tests.
Table 1.
Motor phenomena scores in patients and controls
| Motor phenomena | Narcolepsy with cataplexy, mean ± SD | Controls, mean ± SD | P-value |
|---|---|---|---|
| Negative | |||
| Head drop and falls | |||
| Baseline | 0.31 ± 0.72 | 0.00 ± 0.00 | 0.017 |
| Trigger | 1.25 ± 0.84 | 0.04 ± 0.20 | <0.0005 |
| Ptosis and tongue protrusion | |||
| Baseline | 0.78 ± 0.83 | 0.00 ± 0.00 | <0.0005 |
| Trigger | 1.66 ± 0.86 | 0.00 ± 0.00 | <0.0005 |
| Facial hypotonia | |||
| Baseline | 0.73 ± 0.94 | 0.00 ± 0.00 | <0.0005 |
| Trigger | 1.42 ± 0.93 | 0.00 ± 0.00 | <0.0005 |
| Generalized hypotonia | |||
| Baseline | 0.55 ± 0.90 | 0.00 ± 0.00 | 0.001 |
| Trigger | 0.88 ± 1.02 | 0.00 ± 0.00 | <0.0005 |
| Positive | |||
| Eyebrow raising | |||
| Baseline | 0.29 ± 0.52 | 0.02 ± 0.10 | 0.013 |
| Trigger | 1.11 ± 0.81 | 0.44 ± 0.46 | 0.001 |
| Perioral and tongue movements | |||
| Baseline | 0.55 ± 0.64 | 0.26 ± 0.41 | 0.047 |
| Trigger | 1.59 ± 0.59 | 0.74 ± 0.50 | <0.0005 |
| Facial grimaces | |||
| Baseline | 0.22 ± 0.55 | 0.08 ± 0.19 | 0.404 |
| Trigger | 1.12 ± 0.68 | 0.50 ± 0.43 | <0.0005 |
| Head/trunk swaying | |||
| Baseline | 0.42 ± 0.53 | 0.00 ± 0.00 | <0.0005 |
| Trigger | 1.29 ± 0.72 | 0.14 ± 0.34 | <0.0005 |
| Stereotyped motor behaviour | |||
| Baseline | 0.22 ± 0.44 | 0.02 ± 0.10 | 0.024 |
| Trigger | 0.82 ± 0.80 | 0.30 ± 0.38 | 0.006 |
| Dyskinetic–dystonic movements | |||
| Baseline | 0.13 ± 0.32 | 0.00 ± 0.00 | 0.041 |
| Trigger | 0.74 ± 0.71 | 0.12 ± 0.30 | <0.0005 |
| Consistent patterns | |||
| Neck extension viewing | 0.24 ± 0.43 | 0.00 ± 0.00 | 0.009 |
| Puppet-like behaviour | 0.13 ± 0.34 | 0.00 ± 0.00 | 0.064 |
| Composite scores | |||
| Composite negative | |||
| Baseline | 2.37 ± 3.21 | 0.00 ± 0.00 | <0.0005 |
| Trigger | 5.21 ± 3.33 | 0.04 ± 0.20 | <0.0005 |
| Composite active | |||
| Baseline | 1.83 ± 2.35 | 0.38 ± 0.46 | 0.001 |
| Trigger | 5.55 ± 2.69 | 1.80 ± 1.05 | <0.0005 |
All ‘negative’ motor phenomena and the composite score evaluated at baseline and during emotional stimuli were significantly more common in the patients, with only a single control participant showing an isolated brief head drop during emotional stimuli (Fig. 1; Supplementary Videos 1–3]. Patients and controls obtained a mean score ≥1 in the following percentages at baseline and during emotional stimuli, respectively: head drops and falls, 18 and 82% versus 0 and 4%; ptosis and tongue protrusion, 51 and 82% versus 0 and 0%; facial hypotonia, 39 and 71% versus 0 and 0%; and generalized hypotonia, 31 and 47% versus 0 and 0%.
Figure 1.
‘Negative’ motor phenomena. (A) Head drop: sequence of head drop while watching cartoons in one patient. (B) Persistent eyelid narrowing and tongue protrusion in three different cases. (C) Persistent facial hypotonia in three subjects. (D) Persistent generalized hypotonia in three patients: wide-based gait (left), hypotonia leading to forced squatting position (centre) and unsteady gait (right).
All the ‘active’ motor phenomena except facial grimacing at baseline were significantly more common in patients compared with controls (Fig. 2; Supplementary Videos 4–8]. The ‘active’ composite score was also significantly higher in patients compared with controls. Patients and controls obtained a mean score ≥1 in the following percentages at baseline and during emotional stimuli, respectively: eyebrow raising, 23 and 76% versus 0 and 36%; perioral and tongue movements, 39 and 100% versus 20 and 68%; facial grimaces, 10 and 82% versus 0 and 36%; head/trunk swaying, 36 and 90% versus 0 and 12%; stereotyped motor behaviour, 13 and 61% versus 0 and 16%; and dyskinetic–dystonic movements, 10 and 55% versus 0 and 8%.
Figure 2.
‘Active’ motor phenomena. (A) Raising of the eyebrows in three different subjects. (B) Perioral and tongue movements: lip licking (left), lip biting (centre) and lip chewing (right). (C) Facial grimaces: asymmetric facial contraction (left), repetitive tongue protrusion (centre) and asymmetric mouth contraction (right). (D) Swaying of the head and/or trunk: swaying of the head (left), swaying of the head and trunk (centre) and neck extension viewing (right). (E) Stereotyped motor behaviour: repetitive/rhythmic bilateral finger movements with mouth stimulation (left), bilateral repetitive/rhythmic arms and hands movements (centre) and rhythmic purposeless finger tapping on the lips (right). (F) Dyskinetic or dystonic movements: sequence of choreic trunk and limb movements in a patient.
Two additional abnormal complex behaviours could not be simply classified as ‘active’ or ‘negative’ motor phenomena: (i) a posture characterized by neck extension, eyelid ptosis with eyebrow raising while watching videos (‘neck extension viewing’) observed exclusively in nine patients (24%); and (ii) a rapid and rhapsodic set of choreic movements of the whole body and limbs with hypotonia that closely recalled the motion of puppets observed exclusively in five patients (13%) (‘puppet-like’ movements; Supplementary Videos 9 and 10). These complex behaviours were scored as present/absent during video recordings, without further differentiating their frequency or the impact of the emotional trigger.
We tested the impact of emotional stimuli on motor phenomena in patients and controls comparing the scores at baseline and during emotional stimulation for each group using the Wilcoxon signed-rank test. Patients showed a significant increase in all the ‘negative’ and ‘active’ motor phenomena during the emotional stimuli, whereas controls displayed a significant increase of most of the ‘active’ motor features (Table 2, first two columns). To better dissect the effect of emotional stimulation in the two populations, we calculated the difference (delta) between baseline and emotional stimulation conditions for each motor phenomenon, and we then compared these values between patients and controls using Mann–Whitney U-tests. Patients showed significantly higher deltas for all the ‘negative’ and ‘active’ motor phenomena during emotional stimuli (Table 2) compared with control participants.
Table 2.
Impact of emotional stimulation on motor phenomena scores of patients and controls
| Motor phenomena | Narcolepsy with cataplexy, P-value | Controls, P-value | Narcolepsy with cataplexy, mean ± SD | Controls, mean ± SD | P-value |
|---|---|---|---|---|---|
| Head drop and falls | 0.000 | 0.317 | 1.01 ± 0.63 | 0.04 ± 0.20 | <0.0005 |
| Ptosis and tongue protrusion | 0.000 | 1.000 | 0.93 ± 0.48 | 0.00 ± 0.00 | <0.0005 |
| Facial hypotonia | 0.000 | 1.000 | 0.75 ± 0.42 | 0.00 ± 0.00 | <0.0005 |
| Generalized hypotonia | 0.000 | 1.000 | 0.39 ± 0.51 | 0.00 ± 0.00 | <0.0005 |
| Eyebrow raising | 0.000 | 0.001 | 0.80 ± 0.50 | 0.42 ± 0.49 | 0.004 |
| Perioral and tongue movements | 0.000 | 0.001 | 1.03 ± 0.35 | 0.48 ± 0.57 | <0.0005 |
| Facial grimaces | 0.000 | 0.001 | 0.89 ± 0.47 | 0.42 ± 0.43 | <0.0005 |
| Head/trunk swaying | 0.000 | 0.059 | 0.86 ± 0.45 | 0.14 ± 0.34 | <0.0005 |
| Stereotyped motor behaviour | 0.000 | 0.004 | 0.59 ± 0.59 | 0.28 ± 0.38 | 0.037 |
| Dyskinetic–dystonic movements | 0.000 | 0.063 | 0.63 ± 0.55 | 0.12 ± 0.30 | <0.0005 |
| Composite negative | 0.000 | 0.317 | 3.09 ± 1.58 | 0.04 ± 0.20 | <0.0005 |
| Composite active | 0.000 | 0.000 | 3.70 ± 1.49 | 1.42 ± 1.20 | <0.0005 |
Motor phenomena assessment in patients: relation to baseline neurological examination findings
Dichotomizing our narcolepsy with cataplexy population on the presence or absence of subtle alterations at neurological examination (mild hypotonia and/or wide based swaying gait), we found that the 17 patients with narcolepsy with cataplexy presenting with such abnormalities showed: (i) higher overall ‘negative’ and ‘active’ composite scores; (ii) higher scores for all the ‘negative’ motor phenomena; (iii) higher scores for most ‘active’ motor phenomena occurring in the facial area (raising of the eyebrows, facial grimacing), and involving postural control (swaying of the head or trunk); and (iv) more frequently had the patterns of ‘neck extension viewing’ and ‘puppet-like movements’ (Table 3).
Table 3.
Comparison of motor phenomena in patients with and without hypotonic features at neurological examination and with or without increased anti-streptolysin O titres at presentation
| Motor phenomena | Normal neurological examination (n = 22), mean ± SD | Hypotonic neurological examination (n = 17), mean ± SD | P-value | Anti-streptolysin O < 400 (n = 26), mean ± SD | Anti-streptolysin O > 400 (n = 13), mean ± SD | P-value |
|---|---|---|---|---|---|---|
| Negative | ||||||
| Head drop and falls | ||||||
| Baseline | 0.05 ± 0.21 | 0.65 ± 0.98 | 0.005 | 0.21 ± 0.64 | 0.5 ± 0.87 | 0.237 |
| Trigger | 0.86 ± 0.71 | 1.78 ± 0.73 | 0.001 | 1.14 ± 0.77 | 1.46 ± 0.97 | 0.336 |
| Ptosis and tongue protrusion | ||||||
| Baseline | 0.34 ± 0.45 | 1.35 ± 0.88 | <0.0005 | 0.65 ± 0.76 | 1.04 ± 0.95 | 0.216 |
| Trigger | 1.20 ± 0.57 | 2.28 ± 0.80 | <0.0005 | 1.48 ± 0.84 | 2 ± 0.82 | 0.105 |
| Facial hypotonia | ||||||
| Baseline | 0.16 ± 0.32 | 1.47 ± 0.98 | <0.0005 | 0.60 ± 0.82 | 1 ± 1.14 | 0.280 |
| Trigger | 0.89 ± 0.55 | 2.16 ± 0.85 | <0.0005 | 1.30 ± 0.92 | 1.65 ± 0.94 | 0.282 |
| Generalized hypotonia | ||||||
| Baseline | 0.07 ± 0.23 | 1.18 ± 1.06 | <0.0005 | 0.40 ± 0.75 | 0.85 ± 1.13 | 0.167 |
| Trigger | 0.27 ± 0.53 | 1.72 ± 0.95 | <0.0005 | 0.76 ± 0.96 | 1.12 ± 1.14 | 0.321 |
| Positive | ||||||
| Eyebrow raising | ||||||
| Baseline | 0.05 ± 0.21 | 0.62 ± 0.63 | <0.0005 | 0.17 ± 0.37 | 0.54 ± 0.69 | 0.059 |
| Trigger | 0.70 ± 0.63 | 1.66 ± 0.70 | <0.0005 | 0.94 ± 0.68 | 1.42 ± 0.95 | 0.111 |
| Perioral and tongue movements | ||||||
| Baseline | 0.39 ± 0.46 | 0.76 ± 0.77 | 0.094 | 0.35 ± 0.44 | 0.96 ± 0.78 | 0.006 |
| Trigger | 1.43 ± 0.50 | 1.81 ± 0.66 | 0.068 | 1.44 ± 0.49 | 1.88 ± 0.68 | 0.046 |
| Facial grimaces | ||||||
| Baseline | 0.14 ± 0.32 | 0.32 ± 0.75 | 0.419 | 0.08 ± 0.23 | 0.5 ± 0.84 | 0.014 |
| Trigger | 0.86 ± 0.52 | 1.47 ± 0.74 | 0.004 | 0.92 ± 0.43 | 1.5 ± 0.91 | 0.035 |
| Head/trunk swaying | ||||||
| Baseline | 0.25 ± 0.43 | 0.65 ± 0.58 | 0.021 | 0.35 ± 0.46 | 0.58 ± 0.64 | 0.281 |
| Trigger | 1.00 ± 0.53 | 1.69 ± 0.77 | 0.002 | 1.22 ± 0.71 | 1.42 ± 0.76 | 0.400 |
| Stereotyped motor behaviour | ||||||
| Baseline | 0.14 ± 0.32 | 0.32 ± 0.56 | 0.222 | 0.15 ± 0.31 | 0.35 ± 0.63 | 0.460 |
| Trigger | 0.59 ± 0.59 | 1.13 ± 0.96 | 0.058 | 0.70 ± 0.60 | 1.04 ± 1.09 | 0.479 |
| Dyskinetic–dystonic movements | ||||||
| Baseline | 0.05 ± 0.21 | 0.24 ± 0.40 | 0.040 | 0.12 ± 0.33 | 0.15 ± 0.32 | 0.433 |
| Trigger | 0.55 ± 0.51 | 1.00 ± 0.88 | 0.136 | 0.68 ± 0.68 | 0.85 ± 0.8 | 0.564 |
| Consistent patterns | ||||||
| Neck extension viewing | 0.05 ± 0.21 | 0.50 ± 0.52 | 0.001 | 0.24 ± 0.44 | 0.23 ± 0.44 | 0.950 |
| Puppet-like behaviour | 0.00 ± 0.00 | 0.29 ± 0.47 | 0.007 | 0.12 ± 0.33 | 0.15 ± 0.38 | 0.738 |
| Composite scores | ||||||
| Composite negative | ||||||
| Baseline | 0.61 ± 0.84 | 4.65 ± 3.70 | <0.0005 | 1.87 ± 2.79 | 3.38 ± 3.83 | 0.177 |
| Trigger | 3.23 ± 1.91 | 7.94 ± 2.92 | <0.0005 | 4.68 ± 3.16 | 6.23 ± 3.54 | 0.175 |
| Composite active | ||||||
| Baseline | 1.00 ± 1.19 | 2.91 ± 3.01 | 0.008 | 1.21 ± 1.27 | 3.08 ± 3.4 | 0.025 |
| Trigger | 4.43 ± 1.47 | 7.09 ± 3.23 | 0.004 | 4.96 ± 1.91 | 6.69 ± 3.58 | 0.160 |
Patients with and without abnormalities at neurological examination obtained a mean score ≥1 in the following percentages at baseline and during emotional stimuli, respectively: head drops and falls, 35 and 100% versus 4 and 68%; ptosis and tongue protrusion, 82 and 94% versus 27 and 91%; facial hypotonia, 77 and 94% versus 9 and 54%; generalized hypotonia, 65 and 81% versus 4 and 23%; eyebrow raising, 47 and 100% versus 4 and 59%; perioral and tongue movements, 47 and 100% versus 32 and 100%; facial grimaces, 12 and 100% versus 9 and 68%; head/trunk swaying, 53 and 94% versus 23 and 86%; stereotyped motor behaviour, 18 and 69% versus 9 and 54%; and dyskinetic–dystonic movements, 18 and 56% versus 4 and 54%; ‘neck extension viewing’, 50% versus 4%; and ‘puppet-like’ movements 29% versus 0%.
Correlations of motor phenomena scores with age at onset, disease duration, multiple sleep latency test data, and cerebrospinal fluid hypocretin-1 levels
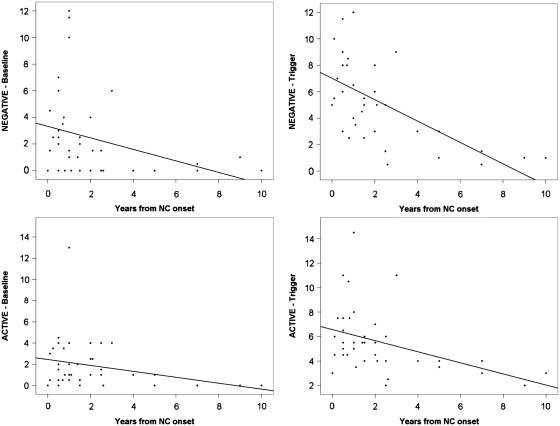
We tested whether the observed motor features correlated with severity or clinical presentation of narcolepsy with cataplexy. To do so, we performed a Spearman's correlation analysis between age at onset and time from narcolepsy with cataplexy onset, sleep latency and sleep-onset REM period numbers during the Multiple Sleep Latency Test, CSF hypocretin-1 levels and the composite scores of ‘negative’ and ‘active’ motor phenomena. We found that the age at narcolepsy with cataplexy onset was inversely related to ‘negative’ composite scores, and that disease duration was inversely related to both ‘negative’ and ‘active’ composite scores, the latter reaching statistical significance only during emotional stimulation (Fig. 3). No other significant correlations (sleep latency and sleep-onset REM periods at Multiple Sleep Latency Test, hypocretin-1 levels) to composite scores were found (Table 4).
Figure 3.
Scattered graphs of composite scores and disease duration. Scattered graphs showing the negative correlations between disease duration and ‘negative’ motor phenomena at baseline (upper left panel), under emotional trigger (upper right panel), and between disease duration and ‘active’ motor phenomena at baseline (lower left panel) and under emotional trigger (lower right panel). NC = narcolepsy with cataplexy.
Table 4.
Spearman's correlation coefficients between composite scores of negative and active motor features and clinical and investigational parameters of narcolepsy with cataplexy
| Clinical and investigational parameters | Negative |
Active |
||
|---|---|---|---|---|
| Baseline | Trigger | Baseline | Trigger | |
| Age at narcolepsy with cataplexy onset | ||||
| ρ value | −0.511 | −0.330 | −0.128 | −0.158 |
| P-value | 0.001 | 0.043 | 0.437 | 0.343 |
| Disease duration | ||||
| ρ value | −0.493 | −0.657 | −0.277 | −0.501 |
| P-value | 0.001 | <0.0005 | 0.088 | 0.001 |
| Multiple Sleep Latency Test | ||||
| Sleep latency | ||||
| ρ value | 0.284 | 0.153 | 0.216 | 0.163 |
| P-value | 0.079 | 0.358 | 0.187 | 0.329 |
| Sleep-onset REM periods | ||||
| ρ value | −0.042 | 0.026 | 0.062 | −0.051 |
| P-value | 0.801 | 0.875 | 0.709 | 0.762 |
| Hypocretin-1 | ||||
| ρ value | 0.119 | −0.059 | −0.067 | 0.120 |
| P-value | 0.555 | 0.774 | 0.738 | 0.559 |
| Anti-streptolysin O | ||||
| ρ value | 0.153 | 0.182 | 0.284 | 0.220 |
| P-value | 0.374 | 0.296 | 0.093 | 0.205 |
Motor phenomena in patients with and without biochemical evidence of past streptococcal infections
Motor phenomena scores were assessed with relation to biochemical evidence suggesting recent streptococcal infection. This comparison was performed between 13 patients with, and 26 patients without increased anti-streptolysin O titres (i.e. >400) at first assessment. Patients with anti-streptolysin O levels of >400 showed higher composite scores for some ‘active’ motor features at baseline and during emotional stimulation, and scored particularly high in subscales involving facial muscles (perioral and tongue movements, and facial grimacing), as well as in the ‘active’ composite score at baseline. In contrast, anti-streptolysin O-positive patients did not differ from negative patients on any of the ‘negative’ motor features score (Table 3). Spearman's analysis did not show a significant relationship between anti-streptolysin O titres and the composite scores (Table 4). These results did not change when the two patients that underwent tonsillectomy before the onset on narcolepsy with cataplexy were excluded (data not shown).
Discussion
In this study, we found that cataplexy in children close to narcolepsy with cataplexy onset commonly presents as a complex movement disorder that includes not only ‘negative’ (hypotonic) motor features but also ‘active’ movement abnormalities. This presentation likely evolves into cataplexy with usual emotional triggers such as laughing (American Academy of Sleep Medicine, 2005) as previously reported (Serra et al., 2008). ‘Negative’ features ranged from partial (with ptosis and tongue protrusion in 51%, and facial hypotonia in 39% of cases) to generalized hypotonia (31% of our population, also evident at the neurological examination in 41% of children), generally enhanced by emotional triggering, thus consistent with the classic definition of cataplexy. In addition, ‘active’ motor phenomena were also observed, including eyebrow raising (23%), perioral and tongue movements (39%), facial grimacing (10%), body swaying (36%), dyskinetic and/or dystonic movements of the arms and of the tongue (10%), and stereotyped motor behaviours (13%). ‘Active’ phenomena were also increased by emotional stimulation, suggesting a link with genuine cataplexy. Both ‘negative’ and ‘active’ motor phenomena correlated inversely with disease duration, suggesting increased severity around onset, whereas ‘negative’ motor phenomena inversely correlated with age at narcolepsy with cataplexy onset, suggesting a discrete phenotype of childhood narcolepsy with cataplexy.
Our study extends previous preliminary observations (Serra et al., 2008; Peraita-Adrados et al., 2011) documenting generalized hypotonia and prominent facial involvement (‘cataplectic facies’) in childhood cataplexy close to disease onset. Most notably, we found that facial hypotonia in these cases commonly presents as continuous muscular weakness affecting the jaw (‘cataplectic gape’, intermittent or continuous jaw opening/dropping with tongue protrusion) or the eyelids (ptosis), with all these features enhanced by emotions. This presentation was highly stereotyped and specific for rapidly evolving narcolepsy with cataplexy, suggesting it is a direct reflection of an acute failure of hypocretinergic neurotransmission. In this context, the prominent facial involvement may reflect the primary importance of the hypocretinergic facilitation of motoneuron activity at the level of the multiply-innervated muscle fibres of the facial muscles (Schreyer et al., 2009) and of the trigeminal motor nuclei (Mascaro et al., 2009), while the mild generalized hypotonia could correspond to an abrupt general lack of facilitation of spinal cord motoneurons (Yamuy et al., 2004).
For the first time, we have described and documented though videotaping a wide range of ‘active’ movements in childhood narcolepsy with cataplexy in patients close to disease onset. These were represented by both an increase in normal purposeful movements (e.g. lip licking) and the occurrence of purposeless movements (e.g. dyskinetic–dystonic arm movements, tongue movements, stereotypies). The pathophysiology of these abnormalities is unknown, but it could be hypothesized that they represent a countermeasure to sleepiness, as hyperactivity is a frequent manifestation of sleepiness in children, or to hypotonia, given the evidence of a semi-permanent condition in a high proportion of cases. Several points however argue against this functional ‘fighting of sleepiness’ interpretation. First, such pathological movements have never been reported in children with daytime sleepiness secondary to other causes (Chervin et al., 2002), and we took great precautions to exclude daytime sleepiness during testing (see ‘Materials and methods’ section). When asked, patients reported that they were not sleepy and never stopped watching funny videos despite falls and movements. Secondly, when polygraphic recordings were available, EEG patterns associated with sleep or sleepiness were not detected. Thirdly, the severity of the movements (intensity, frequency) did not correlate with objective sleepiness assessments. Finally, these ‘active’ movements were enhanced by emotions, suggesting they are related to cataplexy. The reported movements are not commonly described in adulthood cataplexy that is typically characterized by jaw dropping, facial flickering or head dropping (Anic-Labat et al. 1999), as well as by twitches of the face and of the limbs occurring during a cataplectic spell and generally assessed by means of questionnaires (Sturzenegger and Bassetti, 2004; Overeem et al., 2011). Moreover, we also documented clear-cut pathological movements (e.g. dyskinetic–dystonic patterns, grimacing, stereotipies) for the first time in the context of narcolepsy. These findings are therefore in favour of a complex movement disorder that overlaps with cataplexy and occurs close to its onset in children.
We wish to highlight that we documented a single fragment of hypotonia (i.e. head drop) during laughter in one of our controls, and an intensification of ‘active’ movements in the stimulated versus baseline condition in the control group as a whole. These findings are not surprising, and in particular the former is confirmatory of sayings in popular use, for example ‘being weak with laughter’, of the occurrence of cataplexy-like phenomena in young adults (Bassetti and Aldrich, 1996) or in non-narcoleptic subjects (Anic-Labat et al., 1999), and of the decreased H-reflex amplitude, a correlate of reduced muscle tone, during laughter in normal subjects (Overeem et al., 1999) as well as in narcoleptic patients (Lammers et al., 2000). These data suggest that laughter induces hypotonia in healthy individuals, which in patients with cataplexy precipitates a pattern of more severe inhibition of normal tone (Overeem et al., 1999).
Considering the key role of dopaminergic transmission in many movement disorders and in regulating both muscle tone and alertness, we speculate that ‘active’ motor phenomena observed at disease onset could represent the acute destabilization of dopaminergic–hypocretinergic interactions. It is possible that sudden loss of hypocretinergic activity engages dopaminergic transmission to compensate for hypersomnia and hypotonia, resulting in temporary instability, a model suggested by both anatomical and pharmacological experiments. Indeed, hypocretinergic neurons have direct dopaminergic efferents (e.g. via the ventro-tegmental area to the medial prefrontal cortex, or activating the mesolimbic dopamine pathway) (Fadel and Deutch, 2002; Vittoz and Berridge, 2006; Narita et al., 2006; Vittoz et al., 2008), and are also modulated by dopamine (Bubser et al., 2005; Alberto et al., 2006). Dopaminergic transmission is essential for the stimulant effect of modafinil and amphetamines (Nishino and Mignot, 1997; Kanbayashi et al., 2000; Wisor et al., 2001; Wisor and Eriksson, 2005; Madras et al., 2006; Murillo-Rodrıguez et al., 2007; Qu et al., 2008). Further, cataplexy is modulated by D2/D3 receptors and sleep attacks by D1 receptors in mice (Burgess et al., 2010). Blockade or activation of D2/D3 receptors improves or aggravates cataplexy, respectively, without modifying REM sleep in canine narcolepsy (Okura et al., 2000, 2004). Dopaminergic drugs acting on D2/D3 receptors in the ventral tegmental area and the globus pallidus/putamen, as well as dopaminergic drugs acting on D3 receptors in the substantia nigra increase cataplexy without affecting sleep in canine narcolepsy with cataplexy (Reid et al., 1996; Honda et al., 1999).
Conflicting studies have shown either the presence (Eisensehr et al., 2003) or the absence (MacFarlane et al., 1997; Rinne et al., 2004) of in vivo alterations of the human striatal dopaminergic system in adult narcolepsy with cataplexy, and a significant impact of stimulant treatment on dopaminergic transmission (Staedt et al., 1996; Volkow et al., 2009). The most consistent monoaminergic abnormality reported in canine narcolepsy has been increased dopamine content in the amygdala (Miller et al., 1990). Cerebral single-photon emission CT during cataplexy disclosed increased perfusion of different cortico-subcortical networks consistently encompassing the basal ganglia (Hong et al., 2006a; Chabas et al., 2007), and frequently involving the right amygdala (Hong et al., 2006a), a structure functionally activated during cataplexy (Gulyani et al., 2002). Functional MRI studies in adulthood narcolepsy with cataplexy have shown altered emotional processing with increased right amygdala activity (Schwartz et al., 2008; Reiss et al., 2008), and correlations between disease duration and activation of the ventral–medial prefrontal cortex (Ponz et al., 2010), a cerebral structure involved in conflict monitoring and motor inhibition (Brass et al., 2007), and found to be abnormal in narcolepsy with cataplexy (Kim et al., 2008). It is therefore possible that the complex movement disorder we have described in childhood narcolepsy with cataplexy close to disease onset results from increased dopaminergic transmission and the transient imbalance of basal ganglia and cortical networks. Altered emotional processing engaging cortico-subcortical networks may also be involved in the transition to clear-cut cataplexy (Schwartz et al., 2008; Ponz et al., 2010).
The association between ‘active’ motor phenomena and evidence of recent streptococcal infection was the second notable finding of this study. We wish to indicate that the cases reported in this study occurred prior to 2009–10, and thus are unlikely to involve H1N1 vaccination or the H1N1 pandemic previously involved in selected cases (Dauvilliers et al., 2010). Indeed, the H1N1 pandemic reached Italy only in May 2009 and none of our cases occurred after H1N1 vaccination (http://www.who.int/csr/don/2009_05_03a/en/index.html). Rather, increased diagnosis in our Sleep Disorders Center may reflect increased awareness of narcolepsy with cataplexy linked to a media campaign by the Italian association of narcoleptic patients (Plazzi et al., 2006, 2008b; Serra et al., 2008). Group A β-haemolytic streptococcus infections have been reported as possible environmental triggers for narcolepsy with cataplexy by several studies (Aran et al., 2009; Dauvilliers et al., 2010; Koepsell et al., 2010). In this context, childhood narcolepsy with cataplexy could be therefore considered related to brain autoimmune post-streptococcal diseases such as Sydenham's chorea, obsessive-compulsive disorder, tics and PANDAS (Dale, 2005). All these conditions share an episodic course, a childhood-onset with acute presentation, and coexistence of motor and behavioural symptoms. It is also notable that when narcolepsy with cataplexy is misdiagnosed, a choreiform movement disorder is often proposed as the cause: PANDAS and Sydenham's chorea were previous diagnoses in our case series and an initial diagnosis of Huntington's chorea was reported in the first paediatric case series (Yoss and Daly, 1960). Moreover, typical characteristics of narcolepsy with cataplexy are reported in Sydenham's chorea, such as being ‘clumsy’, ‘withdrawn’, ‘troubled’, ‘day-dreaming’, ‘transient intellectual impairment’ and ‘having nightmares’ (Gatti and Rosenheim, 1969; Cimaz et al., 2010). Strikingly, motor impersistence demonstrated by ptosis or tongue protrusion is a common sign in Sydenham's chorea, a condition also frequently associated with grimacing and hypotonia (Oosterveer et al., 2010), and recurs in ‘cataplectic facies’ (Serra et al., 2008; Dhondt et al., 2009; Merino-Andreu and Martinez-Bermejo, 2009). Finally, the motor signs of Sydenham's chorea tend to vanish within a few years from onset in most cases (Cardoso et al., 2006), mirroring our cross-sectional observation in narcolepsy with cataplexy. Whether or not the choreic features of recent-onset narcolepsy genuinely reflect a post-streptococcal disorder deserves additional investigation.
In conclusion, we report that childhood narcolepsy with cataplexy when close to disease onset frequently manifests with a movement disorder. Some of the clinical features of this complex movement disorder are reminiscent of chorea and some patients have high anti-streptolysin O titres. These movement abnormalities seem transient, and the clinical phenotype may rapidly evolve into a more characteristic picture that includes typical cataplexy. The rapid loss of hypocretin neurons is likely to be involved in the pathophysiology of the movement disorder, with the possible involvement of secondary dopaminergic abnormalities.
Funding
nEUroped (PHEA) grant 2007122 from EU (to G.Plazzi and F.Poli, in part).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank J. Faraco for reading and commenting on the manuscript. We are indebted to all the participants of the study, most notably the Italian association of narcoleptic patients (Associazione Italiana Narcolettici). Without their contributions, this study would not have been possible.
Glossary
Abbreviations
- HLA
human leucocyte antigen
- PANDAS
paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections
- REM
rapid eye movement
References
- Alberto CO, Trask RB, Quinlan ME, Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J Neurosci. 2006;26:10043–50. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- American Academy of Sleep Medicine. Diagnostic and coding manual. 2nd edn. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- Aran A, Lin L, Nevsimalova S, Plazzi G, Hong SC, Weiner K. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–83. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti C, Aldrich MS. Narcolepsy. Neurol Clin. 1996;14:545–71. doi: 10.1016/s0733-8619(05)70273-5. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. To do or not to do: the neural signature of self-control. J Neurosci. 2007;27:9141–5. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Burgess CR, Tse G, Gillis L, Peever JH. Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep. 2010;33:1295–304. doi: 10.1093/sleep/33.10.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, Seppi K, Mair KJ, Wenning GK, Poewe W. Seminar on choreas. Lancet Neurol. 2006;5:589–602. doi: 10.1016/S1474-4422(06)70494-X. [DOI] [PubMed] [Google Scholar]
- Chabas D, Habert MO, Maksud P, Tourbah A, Minz M, Willer JC, et al. Functional imaging of cataplexy during status cataplecticus. Sleep. 2007;30:153–6. doi: 10.1093/sleep/30.2.153. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Archbold KH, Dillon JE, Panahi P, Pituch KJ, Dahl RE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002;109:449–56. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- Cimaz R, Gana S, Braccesi G, Guerrini R. Sydenham's chorea in a girl with juvenile idiopathic arthritis treated with anti-TNFalpha therapy. Mov Disord. 2010;25:511–4. doi: 10.1002/mds.22923. [DOI] [PubMed] [Google Scholar]
- Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–9. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol. 2005;47:785–91. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Montplaisir J, Molinari N, Carlander B, Ondze B, Besset A, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology. 2001;57:2029–33. doi: 10.1212/wnl.57.11.2029. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Carlander B, Rivier F, Touchon J, Tafti M. Successful management of cataplexy with intravenous immunoglobulins at narcolepsy onset. Ann Neurol. 2004;56:905–8. doi: 10.1002/ana.20339. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007a;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Pennestri MH, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007b;16:333–9. doi: 10.1111/j.1365-2869.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Abril B, Mas E, Michel F, Tafti M. Normalization of hypocretin-1 in narcolepsy after intravenous immunoglobulin treatment. Neurology. 2009;73:1333–4. doi: 10.1212/WNL.0b013e3181bd1344. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Montplaisir J, Cochen V, Desautels A, Einen M, Lin L, et al. Post-H1N1 narcolepsy-cataplexy. Sleep. 2010;33:1428–30. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–27. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhondt K, Verhelst H, Pevernagie D, Slap F, Van Coster R. Childhood narcolepsy with partial facial cataplexy: a diagnostic dilemma. Sleep Med. 2009;10:797–8. doi: 10.1016/j.sleep.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Eisensehr I, Linke R, Tatsch K, von Lindeiner H, Kharraz B, Gildehaus FJ, et al. Alteration of the striatal dopaminergic system in human narcolepsy. Neurology. 2003;60:1817–9. doi: 10.1212/01.wnl.0000069608.84542.46. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Ferri R, Zucconi M, Manconi M, Bruni O, Ferini-Strambi L, Vandi S, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- Ferri R, Franceschini C, Zucconi M, Vandi S, Poli F, Bruni O, et al. Searching for a marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep. 2008;31:1409–17. [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Statistical methods for rates and proportions. New York: John Wiley and Sons; 1981. pp. 212–36. [Google Scholar]
- Fontana A, Gast H, Reith W, Recher M, Birchler T, Bassetti CL. Narcolepsy: autoimmunity, effector T cell activation due to infection, or T cell independent, major histocompatibility complex class II induced neuronal loss? Brain. 2010;133:1300–11. doi: 10.1093/brain/awq086. [DOI] [PubMed] [Google Scholar]
- Gatti FM, Rosenheim E. Sydenham's chorea associated with transient intellectual impairment. A case study and review of the literature. Am J Dis Child. 1969;118:915–8. doi: 10.1001/archpedi.1969.02100040917019. [DOI] [PubMed] [Google Scholar]
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux. 1880;53:626–8. [Google Scholar]
- Gulyani S, Wu MF, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112:355–65. doi: 10.1016/s0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M, Lin L, Kushida CA, Umetsu DT, Taheri S, Einen M, et al. Report of a case of immunosuppression with prednisone in an 8-year-old boy with an acute onset of hypocretin-deficiency narcolepsy. Sleep. 2003;26:809–10. doi: 10.1093/sleep/26.7.809. [DOI] [PubMed] [Google Scholar]
- Honda K, Riehl J, Mignot E, Nishino S. Dopamine D3 agonists into the substantia nigra aggravate cataplexy but do not modify sleep. Neuroreport. 1999;10:3717–24. doi: 10.1097/00001756-199911260-00046. [DOI] [PubMed] [Google Scholar]
- Hong SB, Tae WS, Joo EY. Cerebral perfusion changes during cataplexy in narcolepsy patients. Neurology. 2006a;66:1747–9. doi: 10.1212/01.wnl.0000218205.72668.ab. [DOI] [PubMed] [Google Scholar]
- Hong SC, Lin L, Jeong JH, Shin YK, Han JH, Lee JH, et al. A study of the diagnostic utility of HLA typing, CSF hypocretin-1 measurements, and MSLT testing for the diagnosis of narcolepsy in 163 Korean patients with unexplained excessive daytime sleepiness. Sleep. 2006b;29:1429–38. doi: 10.1093/sleep/29.11.1429. [DOI] [PubMed] [Google Scholar]
- Hor H, Kutalik Z, Dauvilliers Y, Valsesia A, Lammers GJ, Donjacour CE, et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat Genet. 2010;42:786–9. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- Kanbayashi T, Honda K, Kodama T, Mignot E, Nishino S. Implication of dopaminergic mechanisms in the wake-promoting effects of amphetamine: a study of D and L-derivatives in canine narcolepsy. Neuroscience. 2000;99:651–9. doi: 10.1016/s0306-4522(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Lin L, Tanaka S, Jennum P, Knudsen S, Nevsimalova S, et al. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33:869–74. doi: 10.1093/sleep/33.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133:568–79. doi: 10.1093/brain/awp320. [DOI] [PubMed] [Google Scholar]
- Kornum BR, Kawashima M, Faraco J, Lin L, Rico TJ, Hesselson S, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43:66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell TD, Longstreth WT, Ton TG. Medical exposures in youth and the frequency of narcolepsy with cataplexy: a population-based case-control study in genetically predisposed people. J Sleep Res. 2010;19:80–6. doi: 10.1111/j.1365-2869.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Lee YS, Sung YH, Kim HJ, Kim JH, et al. Increased GABA levels in medial prefrontal cortex of young adults with narcolepsy. Sleep. 2008;31:342–7. doi: 10.1093/sleep/31.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers GJ, Overeem S, Tijssen MA, van Dijk JG. Effects of startle and laughter in cataplectic subjects: a neurophysiological study between attacks. Clin Neurophysiol. 2000;111:1276–81. doi: 10.1016/s1388-2457(00)00306-0. [DOI] [PubMed] [Google Scholar]
- Lecendreux M, Maret S, Bassetti C, Mouren MC, Tafti M. Clinical efficacy of high-dose intravenous immunoglobulins near the onset of narcolepsy in a 10-year-old boy. J Sleep Res. 2003;12:347–8. doi: 10.1046/j.1365-2869.2003.00380.x. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- MacFarlane JG, List SJ, Moldofsky H, Firnau G, Chen JJ, Szechtman H, et al. Dopamine D2 receptors quantified in vivo in human narcolepsy. Biol Psychiatry. 1997;41:305–10. doi: 10.1016/s0006-3223(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Mascaro MB, Prosdócimi FC, Bittencourt JC, Elias CF. Forebrain projections to brainstem nuclei involved in the control of mandibular movements in rats. Eur J Oral Sci. 2009;117:676–84. doi: 10.1111/j.1600-0722.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- Mattarozzi K, Bellucci C, Campi C, Cipolli C, Ferri R, Franceschini C, et al. Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy. Sleep Med. 2008;9:425–33. doi: 10.1016/j.sleep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Merino-Andreu M, Martinez-Bermejo A. Narcolepsia con y sin cataplejia: una enfermedad rara, limitante e infradiagnosticada. An Pediatr. 2009;71:524–34. doi: 10.1016/j.anpedi.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin X, Arrigoni J, Macaubas C, Olive F, Hallmayer J, et al. DQB1*0602 and DQA1*0102 (DQ1) are better markers than DR2 for narcolepsy in Caucasian and black Americans. Sleep. 1994;17(Suppl. 8):S60–7. doi: 10.1093/sleep/17.suppl_8.s60. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin L, Rogers W, Honda Y, Qiu X, Lin X, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Faull KF, Bowersox SS, Dement WC. CNS monoamines and their metabolites in canine narcolepsy: a replication study. Brain Res. 1990;509:169–71. doi: 10.1016/0006-8993(90)90328-9. [DOI] [PubMed] [Google Scholar]
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodríguez E, Haro R, Palomero-Rivero M, Millán-Aldaco D, Drucker-Colín R. Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increases wakefulness in rats. Behav Brain Res. 2007;176:353–7. doi: 10.1016/j.bbr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevsimalova S. Narcolepsy in childhood. Sleep Med Rev. 2009;13:169–80. doi: 10.1016/j.smrv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. Frequency of narcolepsy symptoms and other sleep disorders in narcoleptic patients and their first-degree relatives. J Sleep Res. 2005;14:437–45. doi: 10.1111/j.1365-2869.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. How age influences the expression of narcolepsy. J Psychosom Res. 2005b;59:399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Okura M, Riehl J, Mignot E, Nishino S. Sulpiride, a D2/D3 blocker, reduces cataplexy but not REM sleep in canine narcolepsy. Neuropsychopharmacology. 2000;23:528–38. doi: 10.1016/S0893-133X(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Okura M, Fujiki N, Kita I, Honda K, Yoshida Y, Mignot E, et al. The roles of midbrain and diencephalic dopamine cell groups in the regulation of cataplexy in narcoleptic Dobermans. Neurobiol Dis. 2004;16:274–82. doi: 10.1016/j.nbd.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Oosterveer DM, Overweg-Plandsoen WC, Roos RA. Sydenham's chorea: a practical overview of the current literature. Pediatr Neurol. 2010;43:1–6. doi: 10.1016/j.pediatrneurol.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Overeem S, Lammers GJ, van Dijk JG. Weak with laughter. Lancet. 1999;354:838. doi: 10.1016/s0140-6736(99)80023-3. [DOI] [PubMed] [Google Scholar]
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12:12–8. doi: 10.1016/j.sleep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Peraita-Adrados R, García-Peñas JJ, Ruiz-Falcó L, Gutiérrez-Solana L, López-Esteban P, Vicario JL, et al. Clinical, polysomnographic and laboratory characteristics of narcolepsy-cataplexy in a sample of children and adolescents. Sleep Med. 2011;12:24–7. doi: 10.1016/j.sleep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Parmeggiani A, Mignot E, Lin L, Scano MC, Posar A, et al. Narcolepsy-cataplexy associated with precocious puberty. Neurology. 2006;66:1577–9. doi: 10.1212/01.wnl.0000216142.21375.71. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Serra L, Ferri R. Nocturnal aspects of narcolepsy with cataplexy. Sleep Med Rev. 2008a;12:109–28. doi: 10.1016/j.smrv.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Poli F, Franceschini C, Parmeggiani A, Pirazzoli P, Bernardi F, et al. Intravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexy. J Neurol. 2008b;255:1549–54. doi: 10.1007/s00415-008-0983-7. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Ferri R, Antelmi E, Bayard S, Franceschini C, Cosentino FI, et al. Restless legs syndrome is frequent in narcolepsy with cataplexy patients. Sleep. 2010;33:689–94. doi: 10.1093/sleep/33.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponz A, Khatami R, Poryazova R, Werth E, Boesiger P, Bassetti CL, et al. Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Ann Neurol. 2010;67:190–200. doi: 10.1002/ana.21825. [DOI] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Tafti M, Nishino S, Sampathkumaran R, Siegel JM, Mignot E. Local administration of dopaminergic drugs into the ventral tegmental area modulates cataplexy in the narcoleptic canine. Brain Res. 1996;733:83–100. doi: 10.1016/0006-8993(96)00541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Hoeft F, Tenforde AS, Chen W, Mobbs D, Mignot EJ. Anomalous hypothalamic responses to humor in cataplexy. PLoS One. 2008;3:e2225. doi: 10.1371/journal.pone.0002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Hublin C, Någren K, Helenius H, Partinen M. Unchanged striatal dopamine transporter availability in narcolepsy: a PET study with [11C]-CFT. Acta Neurol Scand. 2004;109:52–5. doi: 10.1034/j.1600-0404.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- Rye DB, Dihenia B, Weissman JD, Epstein CM, Bliwise DL. Presentation of narcolepsy after 40. Neurology. 1998;50:459–65. doi: 10.1212/wnl.50.2.459. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Schreyer S, Büttner-Ennever JA, Tang X, Mustari MJ, Horn AK. Orexin-A inputs onto visuomotor cell groups in the monkey brainstem. Neuroscience. 2009;164:629–40. doi: 10.1016/j.neuroscience.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Ponz A, Poryazova R, Werth E, Boesiger P, Khatami R, et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131:514–22. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]
- Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23:858–65. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- Staedt J, Stoppe G, Kögler A, Riemann H, Hajak G, Rodenbeck A, et al. [123I]IBZM SPET analysis of dopamine D2 receptor occupancy in narcoleptic patients in the course of treatment. Biol Psychiatry. 1996;39:107–11. doi: 10.1016/0006-3223(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Tanaka S, Miyagawa T, Honda Y, Tokunaga K, Honda M. Anti-Tribbles homolog 2 autoantibodies in Japanese patients with narcolepsy. Sleep. 2010;33:875–8. doi: 10.1093/sleep/33.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko PO, Khatami R, Baumann CR, Bassetti CL. No persistent effect of intravenous immunoglobulins in patients with narcolepsy with cataplexy. J Neurol. 2008;255:1900–3. doi: 10.1007/s00415-008-0996-2. [DOI] [PubMed] [Google Scholar]
- Vetrugno R, D’Angelo R, Moghadam KK, Vandi S, Franceschini C, Mignot E, et al. Behavioural and neurophysiological correlates of human cataplexy: a video-polygraphic study. Clin Neurophysiol. 2010;121:153–62. doi: 10.1016/j.clinph.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–95. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–40. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal C. Eigentümliche mit Einschlafen verbundene Anfälle. Archiv für Psychiatrie und Nervenkrankheiten. 1877;7:631–5. [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–34. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–45. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoss RE, Daly DD. Criteria for the diagnosis of the narcoleptic syndrome. Proc Staff Meet Mayo Clin. 1957;32:320–8. [PubMed] [Google Scholar]
- Yoss RE, Daly DD. Narcolepsy in children. Pediatrics. 1960;25:1025–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.