Abstract
Greater understanding of the risk factors and mechanisms of incident dementia in stroke survivors is needed for prevention and management. There is limited information on the long-term consequences and forms of incident dementia in older stroke survivors. We recruited 355 patients aged >75 years from hospital-based stroke registers into a longitudinal study 3 months after stroke. At baseline none of the patients had dementia. Patients were genotyped for apolipoprotein E and assessed annually for cognition and development of incident dementia over up to 8 years of follow-up. The effect of baseline vascular risk factors upon incidence of dementia and mortality were estimated by Cox proportional regression analyses adjusted for age and gender. Standard neuropathological examination was performed to diagnose the first 50 cases that came to autopsy. We found that the median survival from the date of the index stroke was 6.72 years (95% confidence intervals: 6.38–7.05). During the follow-up of a mean time of 3.79 years, 23.9% of subjects were known to have developed dementia and 76.1% remained alive without dementia or died without dementia. The incidence of delayed dementia was calculated to be 6.32 cases per 100 person years whereas that for death or dementia was 8.62. Univariate and multivariate regression analyses showed that the most robust predictors of dementia included low (1.5 standard deviations below age-matched control group) baseline Cambridge Cognitive Examination executive function and memory scores, Geriatric Depression Scale score and three or more cardiovascular risk factors. Autopsy findings suggested that remarkably ≥75% of the demented stroke survivors met the current criteria for vascular dementia. Demented subjects tended to exhibit marginally greater neurofibrillary pathology including tauopathy and Lewy bodies and microinfarcts than non-demented survivors. Despite initial improvements in cognition following stroke in older stroke survivors, risk of progression to delayed dementia after stroke is substantial, but is related to the presence of vascular risk factors. Careful monitoring and treatment of modifiable vascular risk factors may be of benefit in preventing post-stroke dementia in the general population.
Keywords: Alzheimer's disease, diagnosis, post-stroke dementia, stroke, vascular dementia
Introduction
Stroke and dementia individually are common, costly, and personally often devastating to patients and their carers. One in six older people suffer a stroke, and 30% of these individuals develop vascular dementia or vascular cognitive impairment (Savva and Stephan, 2010) and there is also a 9-fold increased risk of incident dementia in the first year after the first-ever cerebral infarct rising to a cumulative incidence of 23% within 10 years (Kokmen et al., 1996). Stroke patients may also experience cognitive dysfunction in the form of mild cognitive impairment. Recent meta-analysis suggested the pooled prevalence estimates of post-stroke dementia <1 year after the stroke ranged from 7.4% in population-based studies of first-ever stroke, in which pre-stroke dementia was excluded, to 41.3% (29.6–53.1) in hospital-based studies of recurrent stroke in which pre-stroke dementia was included. The cumulative incidence of dementia after the first year was slightly greater (3.0%, 1.3–4.7) per year in hospital-based studies than expected on the basis of recurrent stroke alone (Pendlebury and Rothwell, 2009a). Multiple lesions over time and the characteristics and complications of the stroke were found to be most strongly associated with post-stroke dementia. It was therefore suggested that stroke and recurrent strokes themselves, were more important than underlying vascular risk factors. However, this review included many studies that included post-stroke dementia diagnosed within 3 months after stroke. It is possible that the effect of background vascular risk factors will be more visible when studying their long-term effects on more delayed onset of post-stroke dementia, occurring after 3 months and much further beyond. Few studies (Melkas et al., 2009; Narasimhalu et al., 2009; Bejot et al., 2011) have followed participants over >2 years and therefore the long-term outcomes in terms of survival and underlying dementia processes still need elucidation. The lack of association of individual vascular risk factors does not take account of the potentially cumulative effect of multiple vascular risk factors in some patients, influencing the progression of both small vessel and large vessel disease. In addition, these risk factors place the brain at risk over a prolonged period of time after a stroke and therefore, long follow-up periods may be required to delineate their effect more fully. Knowledge of the effects of underlying vascular risk factors would be important for optimum care of post-stroke survivors and secondary prevention in reducing the burden of dementia (Pendlebury and Rothwell, 2009b).
We previously reported that although 41% of elderly survivors who were dementia-free at 3 months post-stroke, were stable and 50% improved in cognition at 15 months post-stroke (Ballard et al., 2003a), a substantial proportion of these subsequently progress to delayed dementia (Ballard et al., 2003a). Consistent with other studies utilizing the 3-month design before follow-up (Tatemichi et al., 1992; Pohjasvaara et al., 1998; Barba et al., 2000), delayed post-stroke dementia was estimated to occur in at least 25% of the subjects with various risk factors. The mechanisms accelerating cognitive decline and leading to delayed dementia are unclear. These need to be elucidated if effective treatment or management approaches to prevent cognitive decline after stroke are to be developed (Leys et al., 2005). More recently, we showed the association of medial temporal lobe atrophy but not white matter hyperintensities with subsequent cognitive decline in post-stroke survivors (Firbank et al., 2007). This suggests a greater role for Alzheimer-type pathology than vascular pathology in delayed cognitive impairment after stroke but, to our knowledge, there is no study to date reporting the neuropathological outcomes of older post-stroke survivors who develop dementia, and medial temporal lobe atrophy may be an important component, but not uniquely associated with Alzheimer-type pathology. Here, we hypothesized that although the characteristics of the stroke would be a strong predictor of post-stroke dementia, the burden of vascular risk factors will have an equally potent effect. We also report on the pathological diagnosis in the first 50 cases of this cohort that came to autopsy.
Materials and methods
Study design and participants
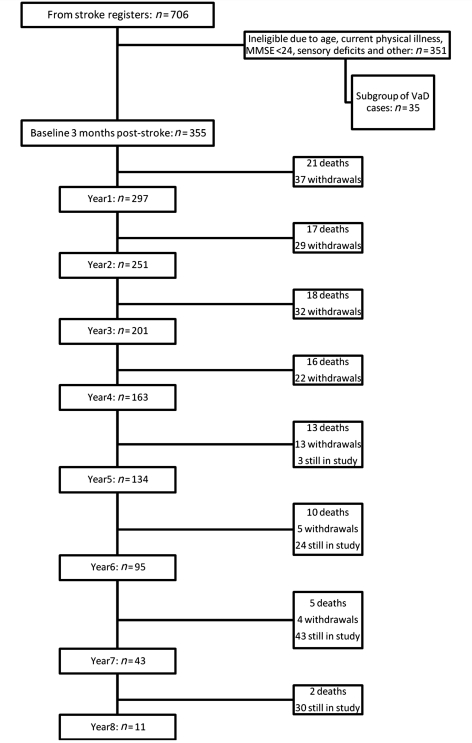
Older (≥75 years) stroke patients (n = 706) were screened consecutively from hospital-based stroke registers in Tyneside and Wearside in the North East of England (Fig. 1). Potential participants were evaluated at least 3 months after stroke [on the basis of the design of Desmond et al. (2000) and to enable resolution of acute post-stroke delirium] with a standardized battery comprised of medical history, Mini-Mental State Examination score, assessment of neurological deficits, a blood screen, and review of CT brain scan undertaken at the time of the stroke. Stroke was defined according to the World Health Organization definition and classified according to the Oxford Community Stroke project classification. We excluded those stroke subjects who (i) were younger than 75 years of age; (ii) were with significant physical illness and disabilities that precluded neuropsychological evaluation (e.g. visual impairment, aphasia, hemiparesis affecting the hand used for writing); (iii) who had a Mini-Mental State Examination score <24 at the 3-month assessment; (iv) had a diagnosis of dementia according to DSM IIIR (Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised) criteria; or (v) declined to take part. Following general practitioner approval and discussion of the study, 355 of the original 706 subjects were eligible for the post-stroke survivor study (Fig. 1). In addition to this full cohort, a subgroup of 35 of the stroke survivors who were already demented at baseline were recruited into a study of falls in dementia, ongoing within the same department (Allan et al., 2009). These patients received the same follow-up protocol as the main cohort (described below). The brains (n = 6) of this subgroup who came to autopsy were examined for comparative purposes of neuropathological changes in those who presented with immediate post-stroke dementia, as opposed to delayed dementia after stroke.
Figure 1.
Flow chart showing numbers screened, recruited and numbers of assessments at each year of the study, together with deaths and withdrawals. Where totals show ‘still in study’, this refers to individuals who have so far completed less than 5–8 years of follow-up but who have not yet died or withdrawn from the study. MMSE = Mini-Mental State Examination; VaD = vascular dementia.
Ethical approval was obtained from the Joint Ethics Committee of Newcastle and North Tyneside Health Authority, the University of Newcastle upon Tyne and the University of Northumbria at Newcastle and participants gave written informed consent in accordance with the Declaration of Helsinki.
Medical histories taken from the participants were supported by review of hospital charts for diagnoses of previous stroke (including whether there was any residual disability from previous stroke), hypertension (a documented history of blood pressure >140/90 mm Hg or treatment of hypertension), atrial fibrillation, ischaemic heart disease, peripheral vascular disease, hypercholesterolaemia, diabetes (documented or treated) and history of smoking prior to stroke. Table 1 provides a list of the cardiovascular risk factors including history of smoking. The characteristics of the full cohort have been described previously (Ballard et al., 2003a, b).
Table 1.
Baseline characteristics of post-stroke survivors
| All participants | |
|---|---|
| n | 355 |
| Age (years) mean (SD) | 80 (4.10) |
| Male gender, n (%) | 184 (51.8) |
| CAMCOG total score, mean (SD) | 85.1 (8.96) |
| Total CAMCOG score <80 (CIND), n (%) | 90 (25.4) |
| CAMCOG total memory score ≤17 (mild cognitive impairment), n (%) | 50 (14.1) |
| CAMCOG total executive function score ≤13, n (%) | 168 (47.3) |
| Geriatric depression score, median (IQR) | 3 (1–5) |
| Number of cardiovascular risk factors, median (IQR) | 2 (1–3) |
| Previous stroke, n (%) | 104 (29.3) |
| Previous disabling stroke, n (%) | 34 (9.6) |
| Ischaemic heart disease, n (%) | 113 (31.8) |
| Hypertension, n (%) | 193 (54.4) |
| Atrial fibrillation, n (%) | 55 (15.5) |
| Diabetes mellitus, n (%) | 29 (8.2) |
| Hypercholesterolaemia, n (%) | 45 (12.7) |
| Ever smoked, n (%) | 207 (58.3) |
| APOE є4 allele (one copy), n (%) | 64/273(23.4) |
Baseline and follow-up assessments
All participants completed a baseline neuropsychological assessment including Cambridge Cognitive Examination (CAMCOG) section B (mental disorders in the elderly), which is a standardized paper and pencil test (maximum score, 107) for global cognitive performance. The schedule includes subscores for memory (27 points), orientation (10 points), language comprehension (9 points), language expression (21 points), attention (7 points), praxis (12 points), calculation (2 points), abstract thinking (8 points) and perception (11 points). A separate executive function domain was scored out of 28 points including supplementary questions in the revised version. As in our previous report (Ballard et al., 2003a), mild cognitive impairment based on Petersen criteria were applied: those with cognitive scores >1.5 standard deviations (SD) below an age-matched control group were defined as having amnestic mild cognitive impairment (CAMCOG memory subscore ≤13) and/or non-amnestic mild cognitive impairment (CAMCOG executive subscore ≤17). In separate analyses, participants with a total CAMCOG score <80 were defined as having cognitive impairment with no dementia (CIND). Other components of the battery included the Cognitive Drug Research computerized battery, the Boston Naming Test, the FAS Verbal Fluency Test, activities of daily living assessed with the Bristol scale, and depression assessed with the DSM IV criteria for depression, the Geriatric depression scale and the Cornell scale.
Neuropsychological evaluations were repeated annually, as in the baseline assessment, during a follow-up period of up to 8 years. If the participant was too impaired to complete a Mini-Mental State Examination, or their score was ≤24, had fallen by ≥2 points or they had other evidence or suspicion of dementia, the assessor completed the Clinical Dementia Rating scale and IQCODE, with an informant. All assessments were reviewed by the assessor and a senior clinician (old age psychiatrist or geriatrician) to determine whether the participant met the DSM IV criteria for dementia. Vital status and dates of death were established immediately prior to analysis of the data by making use of all available sources (next of kin, care home records, hospital notes, primary care physician), even if the participant had withdrawn from the study prior to death.
The apolipoprotein genotype for each participant was determined as described previously (Ballard et al., 2004).
Neuropathological examination and dementia diagnoses
Brains were retrieved from a total of 56 stroke survivors who came to autopsy. Six of these were demented at baseline (Fig. 1) but were included in the assessment for comparative purposes. Table 4 provides demographic details and the highest and last CAMCOG scores. In the majority of cases bronchopneumonia was recorded as the cause of death.
Table 4.
Neuropathological diagnoses of dementia in post-stroke survivors
| Variable | Post-stroke no dementia | Incident dementia | Dementia at baseline | P-values* |
|---|---|---|---|---|
| No of cases | 27 | 23 | 6 | – |
| Age | 84 ± 4 | 88 ± 5 | 85 ± 8 | P = 0.009 PSND versus ID |
| Gender | 15M/12F | 12M/11F | 3M/3F | – |
| CAMCOG (highest) | 92 ± 6 | 82 ± 9 | 58 ± 11 | P = 0.000 PSND versus ID; versus DBaseline |
| CAMCOG (last) | 89 ± 6 | 59 ± 23 | 31 ± 33 | P = 0.000 PSND versus ID; versus DBaseline |
| CDR | 0.1 ± 0.4 | 1.4 ± 0.9 | 3.0 ± 0 | P = 0.000 PSND versus ID; versus DBaseline |
| Duration of survival (days from baseline stroke to death) | 1606 ± 857 | 1304 ± 811 | nd | P > 0.05 |
| Brain weight | 1267 ± 118 | 1229 ± 152 | 1219 ± 69 | P > 0.05 |
| Number of infarcts | 3.9 ± 2.0 | 4.5 ± 2.8 | 6.2 ± 3.9 | P > 0.05 |
| Microinfarction (>three lesions in four regions) | 41% | 50% | 50% | P = 0.05 PSND versus ID |
| CERAD score | 1.3 ± 0.7 | 0.9 ± 0.9 | 1.7 ± 1.5 | P > 0.05 |
| Braak staging | 2.5 ± 1.3 | 2.6 ± 1.1 | 3.7 ± 2.6 | P > 0.05 |
| Neurodegenerative pathology (at least one type) | 30% | 48% | 50% | P = 0.05 PSND versus ID |
| Diagnosis | PSND (n = 27) | Vascular dementia (n = 18); mixed: ATP with cerebrovascular disease (n = 2), LBP (n = 1), FTLD (n = 1), or PSP (n = 1) | Vascular dementia (n = 3); mixed Alzheimer's disease with cerebrovascular disease (n = 3) |
Values are mean ± SD, unless noted otherwise.
ATP = Alzheimer type pathology; CDR = clinical dementia rating; DBaseline = dementia at baseline; FTLD = frontotemporal lobar degeneration; ID = incident dementia; LBP = Lewy body pathology; n = number of autopsied subjects (total n = 56); nd = not defined; PSND = post-stroke no dementia; PSP = progressive supranuclear palsy.
Mixed = vascular and neurodegenerative pathology.
There were no differences in the distribution of apolipoprotein E genotypes with the demented versus non-demented groups.
*P-values for two independent samples, Mann–Whitney U test or Chi-square analysis.
Macroscopic and microscopic pathology was assessed with standardized protocols as described (Kalaria et al., 2004; Ihara et al., 2010). Briefly, macroscopic infarcts were detected by visual inspection while dissecting the brain, and their presence was subsequently confirmed by microscopy. Haematoxylin and eosin was used as standard stain for general neuropathological assessment of the structure of the brain, and for confirmation/detection of the infarcts. In this study, any infarct <5 mm in diameter was defined as a microinfarct. Gallyas and Bielschowsky's silver impregnation and tau immunohistochemistry were applied to assess neuritic plaques and neurofibrillary tangles for the ‘CERAD’ plaque score and ‘Braak and Braak’ neurofibrillary tangle staging. Additional staining included α-synuclein, ubiquitin and TDP-43 immunohistochemistry.
A clinical diagnosis of whether the dementia syndrome was present was made independently of neuropathological data prior to monthly clinicopathological consensus meetings where clinicians met with the pathologists to designate a final diagnosis for autopsied subjects. In all cases, additional pathologies such as Alzheimer's disease and dementia with Lewy bodies were noted. The pathological diagnosis of vascular dementia was then assigned if there was clinical evidence of dementia (DSM IV) and the presence of multiple or cystic infarcts involving cortical and subcortical structures, border-zone infarcts, lacunae, microinfarcts and small vessel disease in the general absence of a high burden of neurofibrillary pathology i.e. Braak staging <IV (Kalaria et al., 2004) or obvious other primary neurodegenerative disease, and consistent with the clinical diagnostic criteria proposed by others (Chui et al., 1992, 2000; Roman et al., 1993; Gold et al., 2002). After breakdown by diagnostic subgroups, the number of autopsies did not permit generation of sensitivity and specificity rates. Subjects were designated as mixed when there was pathological evidence of cerebrovascular disease with Alzheimer's disease-type pathology, Lewy bodies or tauopathy (frontotemporal lobar degeneration or progressive supranuclear palsy). The cases with mixed cerebrovascular disease and Alzheimer's disease had a Braak stage of V or VI and moderate to severe CERAD scores.
Neuroimaging
Fourteen participants who were MRI scanned in life at baseline also come to autopsy. In these, the extent of medial temporal lobe atrophy (right and left) was rated with a standardized scale (Scheltens et al., 1992) from hard copies of the baseline T1-weighted coronal images. This scale rates atrophy as 0, absent; 1, minimal; 2, mild; 3, moderate; and 4, severe, based on the width of the surrounding CSF spaces (temporal horn and choroid fissure) and the height of the hippocampal formation (which includes the hippocampus proper, subiculum, and parahippocampal and dentate gyri). Left and right scores were summed to give an overall combined medial temporal lobe atrophy score (maximum score 8). All scans were rated by consensus between two trained and experienced raters (M.J.F. and Dr Emma Burton, Newcastle University).
Statistical analysis
In order to examine the associations between exposure to putative risk factors for death or dementia, Cox proportional regression analyses were used to obtain univariate proportional hazard ratios for each risk factor, adjusted for age and sex, with time (days) from index stroke to death, dementia and death or dementia as the dependent variables. The date of onset of dementia was assumed to be at the midpoint between the two assessments where dementia status changed. Hazard ratios were given according to presence or absence of the risk factor, or per point on quantitative scales as appropriate.
Following identification in univariate models, significant predictors of death, dementia and death or dementia were entered into a multivariate forward stepwise Cox regression model, P<0.05 for entry, P>0.1 for removal. Age and sex were included even if not significant. Where similar clinical features were described by more than one significant predictor, the predictor with the higher level of significance in univariate analyses was entered into multivariate analyses, in order to avoid co-aggregation of predictors; e.g. baseline CIND rather than the baseline CAMCOG score as both these tests assess cognition. Comparisons or distributions between categories and numeric variables, e.g. age, measures of pathological lesions, were assessed by the Mann–Whitney U test or Chi square analysis. Associations between numeric variables were determined by the Pearson or Spearman's rank correlation analysis (coefficient estimate r).
Results
Participants
The 355 recruited subjects had a mean age of 80 (±4.1) years with 52% males. Hospital notes revealed that 19 (5.4%) patients had sustained a total anterior circulation stroke, 145 (41%) a partial anterior circulation stroke, 116 (33%) a lacunar stroke, 49 (14%) a posterior circulation stroke and 1 (0.3%) an unclassifiable stroke. A summary of baseline characteristics of the participants is shown in Table 1.
Incidence of death, dementia and death or dementia
The total duration of follow-up to establish vital status alone was 2039 person years (mean 5.74 years). During this time 176/355 (49.6%) participants were known to have died, 175 (49.3%) remained alive and it was not possible to establish vital status in four participants. Incidence of death was therefore 8.63 per 100 person years. The median survival from the date of the index stroke was 6.72 years [95% confidence intervals (CI): 6.38–7.05].
The total duration of follow-up to establish the incidence of dementia was 1346 person years (mean 3.79 years). During this time 85/355 (23.9%) participants were known to have developed dementia (either before death or currently alive with dementia), and 270 (76.1%) remained alive without dementia, or were without dementia at death or withdrew from the study. Incidence of dementia was calculated as 6.32 cases per 100 person years. Table 2 shows the number and per cent of dementia cases at each year of follow-up. The proportion of cases remaining in the study with dementia continued to rise throughout the follow-up period, such that although only 9.4% had dementia after 1 year as in our previous report, after 3 years 21.4% cases had dementia and by Year 7, 39.5% had dementia. One hundred and forty-two participants withdrew from neuropsychological follow-up during the study for reasons other than death; 11 had dementia at the time of withdrawal. Participants who withdrew from the study did not differ significantly from other participants in age, gender, baseline CAMCOG or the presence of baseline CIND.
Table 2.
The number of incident cases of dementia each year
| n | No dementia (%) | Dementia |
Cumulative number of incident cases of dementia | |||
|---|---|---|---|---|---|---|
| Established cases (%) | Incident cases (%) | Total cases (%) | ||||
| Baseline | 355 | 355 (100) | 0 (0) | 0 (0) | 0 (0) | 0 |
| Year 1 | 297 | 269 (90.6) | 0 (0) | 28 (9.4) | 28 (9.4) | 28 |
| Year 2 | 251 | 212 (84.5) | 24 (9.6) | 16 (6.4) | 40 (15.9) | 44 |
| Year 3 | 201 | 158 (78.6) | 25 (12.4) | 18 (9.0) | 43 (21.4) | 62 |
| Year 4 | 163 | 127 (77.9) | 32 (19.6) | 4 (2.5) | 36 (22.1) | 66 |
| Year 5 | 134 | 99 (73.9) | 27 (20.1) | 8 (6.0) | 35 (26.1) | 74 |
| Year 6 | 95 | 69 ((72.6) | 21 (22.1) | 5 (5.3) | 26 (27.3) | 79 |
| Year 7 | 43 | 26 (60.5) | 12 (27.9) | 5 (11.6) | 17 (39.5) | 84 |
| Year 8 | 12 | 3 (25.0) | 8 (66.7) | 1 (8.3) | 9 (75.0) | 85 |
n= number of participants completing assessments.
The total duration of follow-up to establish death or dementia was 1346 person years (mean 3.79 years). During this time, 116 participants died or developed dementia before their last examination of dementia status. Incidence of death or dementia was therefore 8.63 per 100 person years.
Univariate predictors of death, dementia and death or dementia
Significant univariate predictors of death, dementia and death or dementia are shown in Table 3. Significant predictors of death in descending order included previous disabling stroke, number of cardiovascular risk factors, CAMCOG executive function score, the presence of CIND at baseline, history of smoking, baseline Geriatric Depression Scale score and age.
Table 3.
Univariate and multivariate predictors of death, dementia and death or dementia
| Baseline characteristic | Death | Dementia | Death or dementia |
|---|---|---|---|
| Relative hazard ratio (95% CI) | Relative hazard ratio (95% CI) | Relative hazard ratio (95% CI) | |
| Univariate predictors | |||
| Age (years) | 1.06 (1.02–1.10) | 1.04 (0.99–1.10) | 1.06 (1.01–1.11) |
| Male gender | 1.13 (0.835–1.52) | 0.68 (0.44–1.04) | 0.825 (0.572–1.19) |
| CAMCOG score <80 (CIND) | 1.64 (1.19–2.26) | 4.02 (2.57–6.28) | 2.96 (2.01–4.35) |
| CAMCOG total memory score <17.6 (mild cognitive impairment) | 1.35 (0.902–2.03) | 3.04 (1.81–5.10) | 2.19 (1.36–3.52) |
| CAMCOG total executive function score <13.6 | 1.78 (1.31–2.40) | 2.84 (1.80–4.48) | 2.26 (1.55–3.29) |
| Geriatric Depression score | 1.08 (1.03–1.13) | 1.14 (1.07–1.22) | 1.13 (1.07–1.19) |
| No. of cardiovascular risk factors: | |||
| 1–2 versus none | 2.23 (1.07–4.63) | 2.13 (0.847–5.37) | 2.17 (0.994–4.74) |
| ≥3 versus none | 2.75 (1.28–5.89) | 3.61 (1.38–9.44) | 3.37 (1.49–7.63) |
| Previous stroke (prior to the index episode) | 1.70 (1.24–2.33) | 1.74 (1.10–2.75) | 1.65 (1.11–2.44) |
| Previous disabling stroke (prior to the index episode) | 2.89 (1.92–4.33) | 3.62 (2.01–5.651) | 2.69 (1.57–4.60) |
| Ever smoked | 1.58 (1.10–2.28) | 1.11 (0.672–1.84) | 1.45 (0.937–2.26) |
| Ischaemic heart disease | 1.11 (0.810–1.53) | 1.56 (1.00–2.43) | 1.31 (0.890–1.92) |
| Hypertension | 1.03 (0.753–1.40) | 1.28 (0.815–2.02) | 1.22 (0.829–1.79) |
| Atrial fibrillation | 0.935 (0.616–1.42) | 0.988 (0.553–1.76) | 1.06 (0.649–1.72) |
| Diabetes mellitus | 1.13 (0.649–1.95) | 1.54 (0.738–3.23) | 1.22 (0.611–2.42) |
| Hypercholesterolaemia | 0.772 (0.458–1.30) | 0.672 (0.307–1.47) | 0.635 (0.320–1.26) |
| APOE є4 allele | 1.04 (0.680–1.58) | 1.47 (0.866–2.49) | 1.37 (0.847–2.20) |
| Multivariate predictors | |||
| Age (years) | 1.06 (1.02–1.11) | ||
| Previous disabling stroke | 2.41 (1.58–3.72) | ||
| Ever smoked | 1.47 (1.02–2.11) | ||
| Geriatric depression score | 1.06 (1.01–1.12) | 1.09 (1.02–1.15) | |
| Number of cardiovascular risk factors | |||
| 1–2 versus none | 2.68 (0.83–8.70) | 3.09 (1.12–8.51) | |
| ≥3 versus none | 4.32 (1.28–14.6) | 4.60 (1.61–13.1) | |
| CAMCOG total executive function score ≤13 | 2.58 (1.54–4.28) | 2.32 (1.53–3.52) | |
| CAMCOG total memory score ≤17 (mild cognitive impairment) | 2.27 (1.29–3.98) | ||
Significant results are shown in bold (P<0.05).
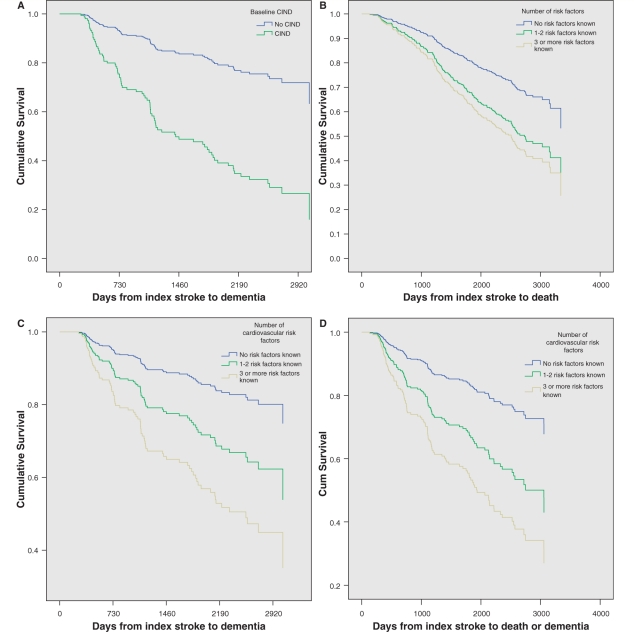
Significant predictors of dementia in descending order included the presence of CIND at baseline, previous disabling stroke, number of cardiovascular risk factors, baseline CAMCOG memory and executive function scores, ischaemic heart disease and Geriatric Depression Scale score. Figure 2A shows the survival curve to dementia in the presence and absence of CIND at baseline.
Figure 2.
Survival curves to show time (A) to dementia by presence of CIND at baseline, (B) to death by number of cardiovascular risk factors, (C) to dementia by number of cardiovascular risk factors and (D) to death or dementia by number of cardiovascular risk factors. The mean number of days from stroke to dementia or death in the non-demented and DSM IV demented groups was 1483 ± 922 and 1059 ± 676 (P = 0.001).
Significant predictors of death or dementia in descending order included three or more cardiovascular risk factors, the presence of CIND at baseline, previous disabling stroke, baseline CAMCOG executive and memory function scores, Geriatric Depression Scale score and age. Among the strongest vascular risk factors were ischaemic heart disease and hypertension (Table 3).
Multivariate predictors of death, dementia and death or dementia
For the multivariate analyses, three different models were adopted (Table 3). In the first model predicting time to death, predictors retained were age, smoking history, a history of previous disabling stroke and baseline Geriatric depression score. In the second model, predicting time to dementia, predictors retained were number of cardiovascular risk factors, CAMCOG total executive function score ≤13 and CAMCOG total memory score ≤17 (mild cognitive impairment).
In the third model, predicting time to death or dementia, predictors retained were number of cardiovascular risk factors, CAMCOG total executive function score <13 and baseline Geriatric depression score (Table 3). Figure 2B–D illustrates the differences in median survival times to death, dementia and death or dementia by the number of cardiovascular risk factors. Thus, we noted that the time to dementia was particularly affected by the number of cardiovascular risk factors (Table 3).
Neuropathological diagnoses of post-stroke survivors
Age, gender and baseline CAMCOG scores did not differ significantly between those who did and did not donate their brains. Of 176 deaths at the time of analysis of the survival data, 46 participants (26.1% of deaths) had donated their brains. A further 10 deaths resulted in brain donation following the analysis of the survival data and these are also included in the neuropathological analyses. Table 4 shows the age, gender distribution and neuropathological diagnoses in demented stroke survivors among the 56 subjects who came to autopsy. Similar to data for the whole cohort, the mean age of the demented subjects was greater but the mean CAMCOG scores were lower (P < 0.05) than non-demented subjects. The mean survival times from baseline stroke were not different between the demented and non-demented subjects (P > 0.05). We also found that given the presence of clinical dementia, the majority of the post-stroke cases (not demented at baseline) fulfilled currently used criteria for vascular dementia. There was no particular distribution of the vascular territory of the stroke or frequencies of the apolipoprotein ε4 allele that differentiated demented and non-demented subjects (data not shown).
Further routine analysis of the pathological data indicated that there was a greater number of individuals with Braak stage III or higher among the demented cases, who also tended to exhibit a higher proportion of microinfarctions (Table 4). The six cases that were demented at baseline had a diagnosis of vascular dementia (n = 3) and mixed dementia (n = 3) with both Alzheimer's disease and vascular dementia features (Table 4). Among the 56 cases with post-mortem examination, 14 subjects (n = 6 with dementia) had a MRI scan at baseline. The averaged left and right medial temporal lobe atrophy score was significantly greater (t = 2.7; P = 0.02) in those with (medial temporal lobe atrophy = 3.8, SD ± 1.0) versus without (medial temporal lobe atrophy 2.3, SD ± 1.2) dementia at death. Baseline medial temporal lobe atrophy in this group also significantly correlated with baseline CAMCOG score (Pearson r = −0.63; P 0.011). There were significant differences in the last CAMCOG scores between those with and without dementia at death (mean and SD, 88 ± 7 and 68 ± 11, P = 0.011). However, there were no differences in the mean brain weights, Braak staging or burden of infarcts between these demented and non-demented MRI scanned groups (P > 0.05).
Discussion
Our unique large study in older (>75 years age) stroke survivors in the North East of England reveals incidence and predictors of delayed post-stroke dementia during up to 8 years of follow-up in a hospital-based cohort of survivors of first or recurrent stroke. Having excluded those with pre-stroke dementia or a Mini-Mental State Examination score <24 at 3 months post-stroke, we found that almost 10% of these older participants developed post-stroke dementia between 3 and 15 months after the stroke. In the recent meta-analysis studies of post-stroke dementia in 22 separate cohorts (Pendlebury and Rothwell, 2009a), prevalence of post-stroke dementia up to 1 year was 20.3% in hospital-based studies, which included first or recurrent stroke, substantially higher than in our cohort. However, the hospital-based studies included people with early post-stroke dementia (i.e. at 3 months), in whom we believe dementia may have begun before the stroke. In support of this view are the ICD 10 criteria, which require a symptom duration of 6 months before making the diagnosis of dementia. We excluded these individuals and only assessed delayed post-stroke dementia occurring in the year after the first 3 months. Thus, although our cohort included patients with recurrent stroke, it was more similar to the two population-based studies of first-ever stroke, in which one also began measurement after 3 months (Kokmen et al., 1996), where prevalence of post-stroke dementia was 7.0%, and another (Kase et al., 1998) probably excluded more patients with pre-stroke dementia on the basis of the pre-stroke Mini-Mental State Examination (prevalence 12.2%), and so our cohort concords with rates of post-stroke dementia found where pre-stroke, or early post-stroke dementia were effectively excluded.
During the mean follow-up period of 3.79 years, we found that 24% of participants succumbed to delayed post-stroke dementia. Thus, the cumulative incident dementia rate, calculated to be 6.32 cases per 100 person years, was higher than those of recent studies showing 6.6 and 25.5% cases for mean follow-up of 3.4 and 5.1 years, respectively (Melkas et al., 2009; Narasimhalu et al., 2009). An obvious explanation for the differences relates to age. In these previous studies, stroke survivors were younger than in our study, with a mean age of 60 (Narasimhalu et al., 2009) and 72 years (Melkas et al., 2009) while our study was 80 ± 4 years. Our analysis showed that although the effect of age on dementia alone was not significant, older age was associated with higher mortality and the combined outcome of death or dementia. The older baseline age of our subjects may have attenuated our ability to show an effect of age on dementia. We did note that patients with CIND were older than those without CIND (data not shown), and CIND did predict dementia. This is in agreement with several studies to date (Altieri et al., 2004; Bejot et al., 2011) and supports the view that age is the strongest risk for all types of dementia, as well as stroke-associated cerebral impairment.
Our findings revealed that CIND at 3 months post-stroke (defined as <80 total score) is a significant predictor of long-term incidence of dementia. This corroborates with previous studies showing that moderate CIND or vascular mild cognitive impairment post-stroke consistently predict progression to post-stroke dementia (Ingles et al., 2002; Srikanth et al., 2006; Melkas et al., 2009; Narasimhalu et al., 2009; Sachdev et al., 2009) independently of pre-stroke cognitive impairment (Reitz et al., 2008). These observations suggest that poor cognitive function after the stroke is indicative of ongoing cognitive decline or that once a particular threshold of cognitive change is breached, recovery from it is impossible. Furthermore, in accord with recent studies (Narasimhalu et al., 2009; Oksala et al., 2009; Sachdev et al., 2009), we also noted that the presence of either executive dysfunction or global memory deficits at baseline more than doubled the risk of dementia after stroke. Although we did not specify all the individual domains including attention, speed processing or visuospatial and constructional abilities, we expected that in this age group incident dementia would encompass several executive function features that might adversely influence long-term survival after stroke, even after adjustment of physical health risk factors (Barba et al., 2002). Although we found that baseline CIND and poor executive function predicted poorer survival, in multivariate analyses, these factors were not retained, in contrast to Melkas et al. (2009) who found that post-stroke dementia predicted poor survival. We are uncertain of the cause of our finding, but perhaps physical health factors outweigh dementia in determining survival in an older cohort.
The effects of depression were slightly different from those of cognitive impairment. Partly keeping with recent studies suggesting depressed mood or depression after stroke is associated with disability (Willey et al., 2010), cardiovascular events and incident dementia (Peters et al., 2010), we noted that baseline depression was a predictor of death, dementia and death or dementia in older post-stroke survivors. In multivariate analyses, it was retained in the models predicting death or the combined endpoint of death and dementia, but not dementia alone. The mechanism by which depression leads to poor survival, and its interaction with dementia, urgently require further investigation.
Multivariate analyses revealed that presence of three or more cardiovascular risk factors, increased risk of dementia or death by 4-fold in our elderly stroke patient population. Our observations contrast with a recent systematic review (Savva and Stephan, 2010) where authors concluded that the effect of stroke on dementia incidence in the population is explained by recurrent stroke rather than cardiovascular risk factors. A limitation of our study is that it was not possible within the resources of the study to establish, with great accuracy, whether further strokes had occurred at each follow-up and so this information is not available to us. It is possible that the effect of multiple risk factors may have been mediated via an increased rate of recurrent stroke. It is likely that our subjects did go on to have further strokes, indeed the post-mortem findings of substantial vascular pathology support the argument that subsequent dementia is due to ongoing vascular pathology, including both macro- and microvascular disease. However, one aim was to examine whether modifiable risk factors known at the time of the index stroke would be useful in predicting dementia outcomes. Our results indicate to clinicians at the point of stroke assessment that the number of vascular risk factors is important for future outcome, matters that can be acted upon at that time, and plausibly influential in outcome. In contrast, where reports indicate that outcome depends on further stroke, the clinician will go back to the question, ‘what causes further stroke that I can do something about?’ This does beg the question of whether multiple vascular risk factors should be addressed to prevent post-stroke dementia, and we believe that trials of multiple interventions for vascular risk factors should be undertaken as soon as possible. In addition, our multivariate analyses suggest that it is the accumulation of vascular risk factors that is particularly detrimental for cognitive status in old age. A number of studies have found significant effects of individual vascular risk factors in both early and delayed post-stroke dementia, but have not examined their cumulative effect, e.g. a population-based study (Bejot et al., 2011) showed patients with post-stroke dementia had a higher prevalence of several vascular risk factors including hypertension, diabetes, atrial fibrillation, previous myocardial infarction and history of transient ischaemic attacks. However, such associations have not been widely found in other studies (Censori et al., 1996; Inzitari et al., 1998; Pohjasvaara et al., 1998; Barba et al., 2000; Reitz et al., 2008). The 24-year study (Bejot et al., 2011) also indicated that prevalence of post-stroke dementia associated with lacunar stroke was seven times higher than other types of stroke, including intracerebral haemorrhage. Even though we had noted the arterial territories of the initial strokes we did not have enough subjects to assess which types or arterial territories were more prevalent in post-stroke dementia cases.
The meta-analysis (Pendlebury and Rothwell, 2009a) reported that neither hypertension nor previous ischaemic heart disease was associated with post-stroke dementia, although recurrent strokes were. In this context, the Rotterdam study found that an association between incident stroke and risk of subsequent dementia was independent from all other assessed risk factors for cognitive decline including vascular disease factors such as hypertension, diabetes, body mass index and apolipoprotein E ε4 (Reitz et al., 2008). While different cohort ages and sizes may explain the conflicting results regarding the individual risk factors, or whether their cumulative effect is examined, these findings collectively emphasize that implementation of secondary prevention measures and sustained treatment strategies to control the risk of further strokes or vascular disease in stroke survivors are imperative (Mackowiak-Cordoliani et al., 2005). In the absence of other effective neurotransmission-based therapies, enhancement of cholinergic systems may also prove useful (Roman and Kalaria, 2006).
In this age group, we also showed that the risk of dementia remains increased even after initial improvements (Ballard et al., 2003a; del Ser et al., 2005) or stability (Tham et al., 2002). This could be related to factors other than those assessed in this study. It is plausible that factors such as initial intellectual abilities, level of education or anatomical variables such as brain size, structural integrity of the temporal lobe or the white matter at inception, contribute to the greater decline in some stroke patients.
One of the main strengths of our study is that unlike previous studies (Pendlebury, 2009), we provide neuropathological diagnosis on a sizeable number of stroke survivors. We were surprised to discover that there was no difference in the mean survival period between the demented and non-demented stroke survivors who came to autopsy. This was not expected given that clinical studies (Henon et al., 2003; Oksala et al., 2009; Brodaty et al., 2010; Narasimhalu et al., 2011) have indicated post-stroke cognitive deficits increase mortality rates and so demented subjects should exhibit shorter survival. We deem this is a relatively small sample (n = 25 each group) and may not hold true in a larger sample.
Given that accurate clinical diagnosis of vascular dementia and Alzheimer's disease has proved difficult in the light of autopsy results (Bowler et al., 1998; Nolan et al., 1998; Kalaria, 2002), we initially concentrated on diagnosing any type of dementia after stroke. However, it is important to consider the mechanisms causing dementia after stroke injury. Our previous imaging studies (in a subset of this post-stroke cohort) had suggested the presence of an Alzheimer's disease component in those who exhibit delayed dementia after stroke (Firbank et al., 2007). Although the subjects were found to have frontal lobe white matter abnormalities (Firbank et al., 2011) consistent with the Alzheimer's disease scenario (Burton et al., 2009), they exhibited medial temporal lobe atrophy as a prominent feature associated with cognitive decline after stroke (Bombois et al., 2008; Pendlebury and Rothwell, 2009a). Our findings from prospectively assessed individuals scanned in life and then examined at autopsy supports this notion that medial temporal lobe atrophy is associated with vascular dementia or stroke-related dementia although it may not be as apparent as in Alzheimer's disease (Jagust et al., 2008; Burton et al., 2009).
In our study most of the stroke survivors who were clinically demented at autopsy had high a burden of vascular pathology in the general absence of significant Alzheimer-type pathology (Kalaria et al., 2004). While stroke survivors may not be completely free of Alzheimer pathology or amyloid-β accumulation (Lewis et al., 2006; Mok et al., 2010), these data indicated that elderly stroke survivors, at least in the >75 age group, are likely to develop vascular dementia with minimal Alzheimer's disease features (Altieri et al., 2004). It is possible that stroke may trigger or unmask neurodegenerative processes characterized by primary pathologies (Bowler et al., 1998; Hsiung et al., 2006). On the other hand, our pathological findings are perhaps not surprising when considering the clinical inclusion criteria for each patient (Roman et al., 1993; Chui et al., 2000). They should have been cognitively intact despite being at least 75 years of age and had experienced at least one recent clinically detectable stroke. With few exceptions, the cerebrovascular changes in the brain appeared to explain the dementia better than the meagre presence of primary neurodegenerative disease pathology. Nonetheless, the clinical findings in the autopsied subpopulation indicate that the initial cognitive abilities after the stroke and age at death remain strong predictors of dementia even when the standard neuropathological lesions are taken into account.
Conclusion
Our report indicates that the prognosis for survival and cognitive function in older stroke survivors after 5 years from index stroke remains very poor. An increased number of cardiovascular risk factors worsens cognitive status after stroke whereby those who become demented largely appear to succumb to vascular dementia. Our results have strong implications for secondary prevention to abate vascular disease. They also suggest that the treatment or reduction of vascular risk factors in old age requires clinical trials with more than a single agent approach, especially if the burden of vascular dementia or vascular cognitive impairment is to be reduced (Hachinski et al., 2006).
Funding
Our work is supported by grants from the UK Medical Research Council (MRC, G0500247); Newcastle Centre for Brain Ageing and Vitality (BBSRC, EPSRC, ESRC and MRC, LLHW); and the Alzheimer's Research Trust (UK). Tissue for this study was collected by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK MRC (G0400074), by the Newcastle NIHR Biomedical Research Centre in Ageing and Age Related Diseases award to the Newcastle upon Tyne Hospitals NHS Foundation Trust, and by a grant from the Alzheimer's Society and ART as part of the Brains for Dementia Research Project.
Acknowledgements
We are very grateful to the patients, families and clinical house staff for their co-operation in the investigation of this study. We thank Michelle Widdrington, Carein Todd, Jean Scott, Deborah Lett and Anne Nicholson for assistance in managing and screening the cohort. We are indebted to Professors Clive Ballard and Rose Anne Kenny for guiding this longitudinal study in its early phases. L.A. contributed to the conception and design of the study, collected, analysed and interpreted the data and wrote the first draft of the article. R.K. and J.O. contributed to the conception and design of the study, interpretation of data and revisions of the article for important intellectual content. E.R. contributed to the collection and interpretation of the data and revision of the article for important intellectual content. A.T. and J.O. lead the dementia diagnosis consensus. T.M.P. and R.K. performed and interpreted the neuropathological examinations. M.F. and S.P. contributed to the imaging and clinical interpretation of data and revision of the article for important intellectual content. All authors gave final approval of the version to be published. L.A. and R.K. are the guarantors.
Glossary
Abbreviations
- CIND
cognitive impairment no dementia
- CAMCOG
Cambridge Cognitive Examination
References
- Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One. 2009;4:e5521. doi: 10.1371/journal.pone.0005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri M, Di Piero V, Pasquini M, Gasparini M, Vanacore N, Vicenzini E, et al. Delayed poststroke dementia: a 4-year follow-up study. Neurology. 2004;62:2193–7. doi: 10.1212/01.wnl.0000130501.79012.1a. [DOI] [PubMed] [Google Scholar]
- Ballard C, Rowan E, Stephens S, Kalaria R, Kenny RA. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke. 2003a;34:2440–4. doi: 10.1161/01.STR.0000089923.29724.CE. [DOI] [PubMed] [Google Scholar]
- Ballard C, Stephens S, Kenny R, Kalaria R, Tovee M, O'Brien J. Profile of neuropsychological deficits in older stroke survivors without dementia. Dement Geriatr Cogn Disord. 2003b;16:52–6. doi: 10.1159/000069994. [DOI] [PubMed] [Google Scholar]
- Ballard CG, Morris CM, Rao H, O'Brien JT, Barber R, Stephens S, et al. APOE epsilon4 and cognitive decline in older stroke patients with early cognitive impairment. Neurology. 2004;63:1399–402. doi: 10.1212/01.wnl.0000141851.93193.17. [DOI] [PubMed] [Google Scholar]
- Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000;31:1494–501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- Barba R, Morin MD, Cemillan C, Delgado C, Domingo J, Del Ser T. Previous and incident dementia as risk factors for mortality in stroke patients. Stroke. 2002;33:1993–8. doi: 10.1161/01.str.0000017285.73172.91. [DOI] [PubMed] [Google Scholar]
- Bejot Y, Aboa-Eboule C, Durier J, Rouaud O, Jacquin A, Ponavoy E, et al. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke. 2011;42:607–12. doi: 10.1161/STROKEAHA.110.595553. [DOI] [PubMed] [Google Scholar]
- Bombois S, Debette S, Bruandet A, Delbeuck X, Delmaire C, Leys D, et al. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke. 2008;39:2046–51. doi: 10.1161/STROKEAHA.107.505206. [DOI] [PubMed] [Google Scholar]
- Bowler JV, Munoz DG, Merskey H, Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1998;64:18–24. doi: 10.1136/jnnp.64.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodaty H, Altendorf A, Withall A, Sachdev PS. Mortality and institutionalization in early survivors of stroke: the effects of cognition, vascular mild cognitive impairment, and vascular dementia. J Stroke Cerebrovasc Dis. 2010;19:485–93. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132:195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- Censori B, Manara O, Agostinis C, Camerlingo M, Casto L, Galavotti B, et al. Dementia after first stroke. Stroke. 1996;27:1205–10. doi: 10.1161/01.str.27.7.1205. [DOI] [PubMed] [Google Scholar]
- Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, Tinklenberg J, et al. Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Arch Neurol. 2000;57:191–6. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–80. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- del Ser T, Barba R, Morin MM, Domingo J, Cemillan C, Pondal M, et al. Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke. 2005;36:2670–5. doi: 10.1161/01.STR.0000189626.71033.35. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Paik MC, Sano M, Mohr JP, Aboumatar S, et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54:1124–31. doi: 10.1212/wnl.54.5.1124. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Burton EJ, Barber R, Stephens S, Kenny RA, Ballard C, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging. 2007;28:1664–9. doi: 10.1016/j.neurobiolaging.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, He J, Blamire AM, Singh B, Danson P, Kalaria RN, et al. Cerebral blood flow by arterial spin labeling in poststroke dementia. Neurology. 2011;76:1478–84. doi: 10.1212/WNL.0b013e318217e76a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002;159:82–7. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Henon H, Durieu I, Lebert F, Pasquier F, Leys D. Influence of prestroke dementia on early and delayed mortality in stroke patients. J Neurol. 2003;250:10–6. doi: 10.1007/s00415-003-0917-3. [DOI] [PubMed] [Google Scholar]
- Hsiung GY, Donald A, Grand J, Black SE, Bouchard RW, Gauthier SG, et al. Outcomes of cognitively impaired not demented at 2 years in the Canadian Cohort Study of Cognitive Impairment and Related Dementias. Dement Geriatr Cogn Disord. 2006;22:413–20. doi: 10.1159/000095751. [DOI] [PubMed] [Google Scholar]
- Ihara M, Polvikoski TM, Hall R, Slade JY, Perry RH, Oakley AE, et al. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer's disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–89. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles JL, Wentzel C, Fisk JD, Rockwood K. Neuropsychological predictors of incident dementia in patients with vascular cognitive impairment, without dementia. Stroke. 2002;33:1999–2002. doi: 10.1161/01.str.0000024433.36590.1b. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Di Carlo A, Pracucci G, Lamassa M, Vanni P, Romanelli M, et al. Incidence and determinants of poststroke dementia as defined by an informant interview method in a hospital-based stroke registry. Stroke. 1998;29:2087–93. doi: 10.1161/01.str.29.10.2087. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria R. Similarities between Alzheimer's disease and vascular dementia. J Neurol Sci. 2002;203–204:29–34. doi: 10.1016/s0022-510x(02)00256-3. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Kase CS, Wolf PA, Kelly-Hayes M, Kannel WB, Beiser A, D'Agostino RB. Intellectual decline after stroke: the Framingham Study. Stroke. 1998;29:805–12. doi: 10.1161/01.str.29.4.805. [DOI] [PubMed] [Google Scholar]
- Kokmen E, Whisnant JP, O'Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984) Neurology. 1996;46:154–9. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- Lewis H, Beher D, Cookson N, Oakley A, Piggott M, Morris CM, et al. Quantification of Alzheimer pathology in ageing and dementia: age-related accumulation of amyloid-beta(42) peptide in vascular dementia. Neuropathol Appl Neurobiol. 2006;32:103–18. doi: 10.1111/j.1365-2990.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–9. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- Mackowiak-Cordoliani MA, Bombois S, Memin A, Henon H, Pasquier F. Poststroke dementia in the elderly. Drugs Aging. 2005;22:483–93. doi: 10.2165/00002512-200522060-00003. [DOI] [PubMed] [Google Scholar]
- Melkas S, Oksala NK, Jokinen H, Pohjasvaara T, Vataja R, Oksala A, et al. Poststroke dementia predicts poor survival in long-term follow-up: influence of prestroke cognitive decline and previous stroke. J Neurol Neurosurg Psychiatry. 2009;80:865–70. doi: 10.1136/jnnp.2008.166603. [DOI] [PubMed] [Google Scholar]
- Mok V, Leung EY, Chu W, Chen S, Wong A, Xiong Y, et al. Pittsburgh compound B binding in poststroke dementia. J Neurol Sci. 2010;290:135–7. doi: 10.1016/j.jns.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Narasimhalu K, Ang S, De Silva DA, Wong MC, Chang HM, Chia KS, et al. Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology. 2009;73:1866–72. doi: 10.1212/WNL.0b013e3181c3fcb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhalu K, Ang S, De Silva DA, Wong MC, Chang HM, Chia KS, et al. The prognostic effects of poststroke cognitive impairment no dementia and domain-specific cognitive impairments in nondisabled ischemic stroke patients. Stroke. 2011;42:883–8. doi: 10.1161/STROKEAHA.110.594671. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Lino MM, Seligmann AW, Blass JP. Absence of vascular dementia in an autopsy series from a dementia clinic. Journal of the American Geriatrics Society. 1998;46:597–604. doi: 10.1111/j.1532-5415.1998.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Oksala NK, Jokinen H, Melkas S, Oksala A, Pohjasvaara T, Hietanen M, et al. Cognitive impairment predicts poststroke death in long-term follow-up. J Neurol Neurosurg Psychiatry. 2009;80:1230–5. doi: 10.1136/jnnp.2009.174573. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST. Stroke-related dementia: rates, risk factors and implications for future research. Maturitas. 2009;64:165–71. doi: 10.1016/j.maturitas.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009a;8:1006–18. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc Dis. 2009b;27(Suppl 3):1–11. doi: 10.1159/000209260. [DOI] [PubMed] [Google Scholar]
- Peters R, Pinto E, Beckett N, Swift C, Potter J, McCormack T, et al. Association of depression with subsequent mortality, cardiovascular morbidity and incident dementia in people aged 80 and over and suffering from hypertension. Data from the Hypertension in the Very Elderly Trial (HYVET) Age Ageing. 2010;39:439–45. doi: 10.1093/ageing/afq042. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998;29:75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- Reitz C, Bos MJ, Hofman A, Koudstaal PJ, Breteler MM. Prestroke cognitive performance, incident stroke, and risk of dementia: the Rotterdam Study. Stroke. 2008;39:36–41. doi: 10.1161/STROKEAHA.107.490334. [DOI] [PubMed] [Google Scholar]
- Roman GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging. 2006;27:1769–85. doi: 10.1016/j.neurobiolaging.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Chen X, Brodaty H, Thompson C, Altendorf A, Wen W. The determinants and longitudinal course of post-stroke mild cognitive impairment. J Int Neuropsychol Soc. 2009;15:915–23. doi: 10.1017/S1355617709990579. [DOI] [PubMed] [Google Scholar]
- Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–6. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth VK, Quinn SJ, Donnan GA, Saling MM, Thrift AG. Long-term cognitive transitions, rates of cognitive change, and predictors of incident dementia in a population-based first-ever stroke cohort. Stroke. 2006;37:2479–83. doi: 10.1161/01.STR.0000239666.46828.d7. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, et al. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology. 1992;42:1185–93. doi: 10.1212/wnl.42.6.1185. [DOI] [PubMed] [Google Scholar]
- Tham W, Auchus AP, Thong M, Goh ML, Chang HM, Wong MC, et al. Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci. 2002;203–204:49–52. doi: 10.1016/s0022-510x(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Willey JZ, Disla N, Moon YP, Paik MC, Sacco RL, Boden-Albala B, et al. Early depressed mood after stroke predicts long-term disability: the Northern Manhattan Stroke Study (NOMASS) Stroke. 2010;41:1896–900. doi: 10.1161/STROKEAHA.110.583997. [DOI] [PMC free article] [PubMed] [Google Scholar]