Abstract
Myotonic dystrophy types 1 and 2 are progressive multisystemic disorders with potential brain involvement. We compared 22 myotonic dystrophy type 1 and 22 myotonic dystrophy type 2 clinically and neuropsychologically well-characterized patients and a corresponding healthy control group using structural brain magnetic resonance imaging at 3 T (T1/T2/diffusion-weighted). Voxel-based morphometry and diffusion tensor imaging with tract-based spatial statistics were applied for voxel-wise analysis of cerebral grey and white matter affection (Pcorrected < 0.05). We further examined the association of structural brain changes with clinical and neuropsychological data. White matter lesions rated visually were more prevalent and severe in myotonic dystrophy type 1 compared with controls, with frontal white matter most prominently affected in both disorders, and temporal lesions restricted to myotonic dystrophy type 1. Voxel-based morphometry analyses demonstrated extensive white matter involvement in all cerebral lobes, brainstem and corpus callosum in myotonic dystrophy types 1 and 2, while grey matter decrease (cortical areas, thalamus, putamen) was restricted to myotonic dystrophy type 1. Accordingly, we found more prominent white matter affection in myotonic dystrophy type 1 than myotonic dystrophy type 2 by diffusion tensor imaging. Association fibres throughout the whole brain, limbic system fibre tracts, the callosal body and projection fibres (e.g. internal/external capsules) were affected in myotonic dystrophy types 1 and 2. Central motor pathways were exclusively impaired in myotonic dystrophy type 1. We found mild executive and attentional deficits in our patients when neuropsychological tests were corrected for manual motor dysfunctioning. Regression analyses revealed associations of white matter affection with several clinical parameters in both disease entities, but not with neuropsychological performance. We showed that depressed mood and fatigue were more prominent in patients with myotonic dystrophy type 1 with less white matter affection (early disease stages), contrary to patients with myotonic dystrophy type 2. Thus, depression in myotonic dystrophies might be a reactive adjustment disorder rather than a direct consequence of structural brain damage. Associations of white matter affection with age/disease duration as well as patterns of cerebral water diffusion parameters pointed towards an ongoing process of myelin destruction and/or axonal loss in our cross-sectional study design. Our data suggest that both myotonic dystrophy types 1 and 2 are serious white matter diseases with prominent callosal body and limbic system affection. White matter changes dominated the extent of grey matter changes, which might argue against Wallerian degeneration as the major cause of white matter affection in myotonic dystrophies.
Keywords: myotonic dystrophy, neuropsychology, MRI, DTI, VBM
Introduction
Myotonic dystrophy types 1 and 2 are autosomal dominantly inherited, progressive multisystemic disorders. Myotonic dystrophy type 1 is the most common form of muscular dystrophy in adults. Both types are caused by untranslated nucleotide repeat expansions in two distinct genes leading to RNA pathology and aberrant alternative splicing of several genes (Day and Ranum, 2005). While myotonic dystrophy type 1 results from a CTG repeat expansion in the dystrophia myotonica-protein kinase gene (DMPK), a CCTG repeat expansion in the zinc finger protein 9 gene (ZNF9) has been identified in myotonic dystrophy type 2 (Brook et al., 1992; Liquori et al., 2001). In myotonic dystrophy type 1, the classic disease range of CTG repeat numbers is 50–4000, in which repeat sizes of 50–80 may be associated with mild clinical phenotypes, and large repeat expansions up to 4000 are often found in severe, mostly congenital forms of the disorder.
There is ample evidence of cerebral involvement in myotonic dystrophy type 1 and 2, albeit less severe in type 2 (Machuca-Tzili et al., 2005; Meola and Sansone, 2007). Mental impairment, executive dysfunctioning and avoidant personality traits—eventually deteriorating with age—have been described in myotonic dystrophy types 1 and 2 (Meola et al., 2003; Gaul et al., 2006; Modoni et al., 2008; Weber et al., 2010). Sleepiness and fatigue constitute major complaints in myotonic dystrophy type 1 and type 2 (Giubilei et al., 1999; Laberge et al., 2009a, b; Tieleman et al., 2010). The pathogenesis of CNS symptoms is not entirely clear. Neurofibrillary degeneration with intraneuronal accumulation of abnormally modified microtubuli-associated tau protein has been demonstrated in the brains of patients with myotonic dystrophy types 1 and 2 (Sergeant et al., 2001; Maurage et al., 2005; Itoh et al., 2010). Aberrant tau expression by dysregulated alternative splicing has been proven in myotonic dystrophies allocating these disorders to a subset of neurodegenerative diseases termed tauopathies (Jiang et al., 2004; Day and Ranum, 2005; Maurage et al., 2005; Leroy et al., 2006; Dhaenens et al., 2008; Ghanem et al., 2009).
Brain involvement in myotonic dystrophy types 1 and 2 has been demonstrated in vivo using different neuroimaging techniques. MRI studies revealed white matter lesions and diffuse brain atrophy in myotonic dystrophy types 1 and 2. White matter lesions located within anterior temporal lobes represent a characteristic feature in myotonic dystrophy type 1 (Hund et al., 1997; Kassubek et al., 2003; Kornblum et al., 2004; Romeo et al., 2010). Cellular markers in magnetic resonance spectroscopy were reduced in occipital and temporoparietal cortical regions as well as frontal white matter of patients with myotonic dystrophy types 1 and 2 (Vielhaber et al., 2006). Single photon emission CT and PET studies demonstrated hypoperfusion and glucose hypometabolism of frontal and temporal lobes in myotonic dystrophy type 1, more so than in type 2 (Meola et al., 1999; Weber et al., 2010).
In myotonic dystrophy type 1, grey matter reductions have been described in various cortical regions and recently also in hippocampi and thalami using voxel-based morphometry (VBM) (Antonini et al., 2004; Weber et al., 2010). T2 relaxometry, diffusion MRI studies applying diffusion tensor imaging (DTI), and magnetization transfer imaging showed white matter changes in myotonic dystrophy type 1 using region of interest-based approaches (Di Costanzo et al., 2001; Naka et al., 2002; Fukuda et al., 2005). Atrophy or hypoplasia of the corpus callosum had been described mainly in the congenital disease form of myotonic dystrophy type 1, but a recent diffusion MRI study applying DTI showed widespread white matter abnormalities in congenital, as well as patients with juvenile-onset myotonic dystrophy type 1 (Hashimoto et al., 1995; Wozniak et al., 2011). Giubilei et al. (1999) reported corpus callosum atrophy in a small group of adult patients with myotonic dystrophy type 1. Analysing the regional structural changes of the corpus callosum by the diffusion tensor in DTI, a previous diffusion MRI study found associations with volume loss in corresponding cortical regions. Thus, Wallerian degeneration was postulated as a major cause of white matter affection in myotonic dystrophy type 1 (Ota et al., 2006).
In patients with myotonic dystrophy type 2, we recently demonstrated callosal body affection applying VBM, while grey matter reduction was present in hypothalamic, thalamic and brainstem regions (Minnerop et al., 2008). Weber et al. (2010) found reduced grey matter volumes in their myotonic dystrophy type 2 sample in several cortical regions, including hippocampi and thalami.
While some studies showed correlations of brain morphological changes with neuropsychological and clinical parameters including CTG repeat sizes in myotonic dystrophy type 1 (Ota et al., 2006; Kuo et al., 2008; Romeo et al., 2010; Weber et al., 2010; Wozniak et al., 2011), others failed to do so (Kassubek et al., 2003; Antonini et al., 2004; Fukuda et al., 2005; Di Costanzo et al., 2008).
Disconnection of cortical regions by changes of the interconnecting white matter is a potential mechanism for cognitive dysfunction in various neurological disorders (Dineen et al., 2009), and may also be responsible for CNS symptoms in myotonic dystrophies. Imaging techniques differ regarding their ability to investigate white matter alterations. VBM allows a voxel-wise analysis independent of predefined regions of interests, but it is based on signal differences of grey and white matter in T1-weighted MRIs with a methodological-based focus for grey matter. Contrary, DTI is a quantitative technique that measures tissue properties, i.e. diffusivity and the directional dependence of microscopic diffusion of water molecules in the brain especially in white matter (Catani, 2006). In white matter, diffusion is usually hindered by the high degree of structural organization, resulting in anisotropic movement of water molecules predominantly parallel to the orientation of fibre tracts. A lower fractional anisotropy signifies less anisotropic diffusion and thus lower microstructural integrity (Basser and Jones, 2002). While previous diffusion MRI studies allowed only a region of interest-based analysis of fractional anisotropy values, the new technique ‘tract-based spatial statistics’ enables a voxel-wise analysis of the microstructural integrity of white matter across subjects (Smith et al., 2006).
For a comprehensive analysis of brain structure alterations in myotonic dystrophies, we examined the brains of 22 myotonic dystrophy type 1 and 22 myotonic dystrophy type 2 clinically and neuropsychologically well-characterized patients and a corresponding healthy control group with structural MRI. White matter lesions were rated visually on T2-weighted images, and voxel-based analyses of T1-weighted and diffusion-weighted images (VBM and DTI/tract-based spatial statistics) were applied.
We hypothesized that white matter abnormalities and callosal body affection are present in adult patients with both myotonic dystrophy types 1 and 2 and are more frequent and pronounced as previously suggested. We further aimed to analyse if abnormal white matter integrity was associated with distinct clinical parameters and neuropsychological performance; and whether brain morphological changes play a direct causative role in the development of mood disturbances and increased daytime sleepiness or fatigue. Finally, we intended to evaluate the influence of age and disease duration on brain structural damage to further examine the hypothesis of premature ageing in myotonic dystrophies.
Materials and methods
Subjects
All analyses were performed in 22 patients with myotonic dystrophy type 1 (male/female: 9/13, age 43.1 ± 12.6 years, disease duration 13.2 ± 7.0 years) and 22 patients with myotonic dystrophy type 2 (male/female: 12/10, age 52.5 ± 10.1 years, disease duration 11.9 ± 9.9 years) as well as age- and sex-matched healthy controls (male/female: 11/11, age 50.0 ± 10.2 years). Congenital or infantile-onset forms of myotonic dystrophy type 1 were excluded from this study. Diagnoses were confirmed by genetic testing in all cases. CTG repeat expansion sizes were determined in all patients with myotonic dystrophy type 1 (mean ± SD 614 ± 306, range 80–1100 repeats). None of the patients and control subjects had a past medical history of other neuromuscular or CNS disorders. All subjects underwent clinical–neurological examinations and neuropsychological testing. Education was assessed as a combination score of graduation and professional qualification (sum score), in which score points were given for each school year (up to 13 in Germany), and 3–5 points for the professional qualification (professional education and/or study). In myotonic dystrophy type 1, the Muscular Impairment Rating Scale was used to assess disease severity (Mathieu et al., 2001). Evaluated scales for the assessment of disease severity or muscular symptoms are not yet available in myotonic dystrophy type 2. Characteristics of patients and controls are given in Table 1.
Table 1.
Clinical characteristics of patients with myotonic dystrophy type 1 and myotonic dystrophy type 2 and healthy controls (mean ± SD)
| Myotonic dystrophy type 1 patient group | Myotonic dystrophy type 2 patient group | Control group | |
|---|---|---|---|
| n | 22 | 22 | 22 |
| Age | 43.1 ± 12.6* | 52.5 ± 10.1 | 50.1 ± 9.0 |
| Sex (M/F) | 9/13 | 12/10 | 11/11 |
| CTG repeat length (range) | 614 ± 306 (80–1100) | – | – |
| Educational level | 10.3 ± 2.6* | 11.6 ± 2.1 | 11.7 ± 1.8 |
| Duration of disease (years) | 13.2 ± 7.0 | 11.9 ± 9.9 | – |
| Severity of disease (Muscular Impairment Rating Scale) | 3.6 ± 0.9 | – | – |
| Motor performance (Purdue Pegboard bimanual, cut-off <11 pairs in 30 s) | 9.5 ± 2.5* | 10.3 ± 1.9* | 11.6 ± 1.3 |
| Depressed mood (BDI score, cut off >10) | 9.3 ± 7.8* | 8.7 ± 7.2* | 2.1 ± 3.5 |
Educational levels were assessed as a combination score of graduation and professional qualification (sum score).
*P < 0.05, i.e. significant difference compared with control group.
The local ethics committee approved the study protocol. Informed written consent was obtained from all participants.
Neuropsychological testing
Cognitive functioning was assessed in patients and controls using a comprehensive neuropsychological test battery. We applied several questionnaires to assess increased daytime sleepiness and fatigue as well as depression. Since motor function affected the majority of neuropsychological test performances, we included the bimanual task of the ‘Pegboard Puzzle’ (Tiffin, 1987; lower values corresponding to a worse test result) as covariate to correct for motor impairment in test performance. MRI correlation analyses were restricted to those clinical and neuropsychological parameters with a significant difference compared with controls.
A detailed description of the test battery together with extensive information on statistical analyses of clinical and neuropsychological data is provided in the Supplementary material.
Magnetic resonance imaging
We acquired all MRI data at the Life & Brain Centre Bonn, Germany, using a 3 T scanner (Magnetom Trio, Siemens). An eight-channel head coil was used for signal reception. All subjects underwent the same imaging protocol consisting of whole brain T1-weighted, T2-weighted and diffusion-weighted imaging using an in-house DTI sequence. The total study time was ∼40 min per subject, and all images were obtained in one session. Further details of MRI data acquisition are given in the Supplementary material. Image quality was controlled by visual inspection, and images with artefacts were excluded from further data analysis.
Grading of white matter hyperintensities
White matter lesions (≥5 mm) were quantified on T2-images according to the age-related white matter change score (ARWMC; Wahlund et al., 2001), which was used in myotonic dystrophy types 1 and 2 recently (Romeo et al., 2010). The grading within five regions, separately for each hemisphere, ranged from 0 (no lesions) to 3 (diffuse involvement of the entire region).
Voxel-based morphometry
Image processing and statistical analyses were carried out according to the optimized VBM protocol (Ashburner and Friston, 2000; Good et al., 2001) using MATLAB 7.4.0 and statistical parametric mapping (SPM 5; http://www.fil.ion.ucl.ac.uk/spm/software/spm5). Further details of VBM analysis are described in the Supplementary material. Using the smoothed tissue segments, we performed two-sample t-tests comparing each patient group with controls, separately for grey and white matter. The results were explored at a false discovery rate-corrected threshold of P < 0.05 at voxel-level with an extended cluster threshold of 10 voxels.
Diffusion tensor imaging
Preprocessing and analysis of diffusion data were done with an in-house protocol using FMRIB software library (FSL) 4.1.3 tools (available at www.fmrib.ox.ac.uk/fsl). A diffusion tensor was reconstructed, and the following indices were generated using DTIfit (Smith et al., 2004): fractional anisotropy, mean diffusivity, eigenvalues (λ1, λ2, λ3) of the diffusion tensor with axial diffusivity (λ1) presumed to be the diffusivity parallel/along the axon, and radial diffusivity [(λ2 + λ3)/2] presumed to be the diffusivity perpendicular to the axon. For voxel-wise analysis of the resulting maps, we used the tract-based spatial statistics (version 1.1) tool also included in FSL (Smith et al., 2006). Statistical analysis included (i) group comparisons using two-sample t-tests between myotonic dystrophy type 1/myotonic dystrophy type 2 groups and healthy controls with respect to fractional anisotropy, mean diffusivity, axial and radial diffusivity (PFWE < 0.05) and (ii) correlation analyses (only for fractional anisotropy maps) in both patient groups, using simple regression models with clinical parameters as determining regressors (Pthreshold-free cluster enhancement < 0.05). Further details of DTI data analysis are given in the Supplementary material.
Results
Clinical characteristics of myotonic dystrophy types 1 and 2
Patients with myotonic dystrophy type 1 were younger than controls (P = 0.04), had poorer motor performance (lower values indicating worse performance in the bimanual pegboard task, P = 0.001), fewer years of education (P = 0.049) and higher Beck Depression Inventory (BDI) scores (Hautzinger et al., 1995) indicating more depressive symptoms (P = 0.001), although the group mean score value was still below the clinical cut-off (BDI score 9.3 ± 7.8). BDI scores above the clinical cut-off were found in 7/22 patients with myotonic dystrophy type 1 (32%).
Patients with myotonic dystrophy type 2 did not differ from controls with respect to age and education, but showed poorer motor performance (P = 0.011) and higher BDI scores (P = 0.001). Although the group mean score value was still below the clinical cut-off (BDI score 8.7 ± 7.2), clinically relevantly elevated BDI scores were found in 8/22 patients with myotonic dystrophy type 2 (38%).
Patients with myotonic dystrophy types 1 and 2 did not differ from controls regarding sex distribution. Further, in both patient groups age did not correlate with disease duration, and BDI scores were not associated with age, disease duration or motor performance. In patients with myotonic dystrophy type 1, CTG repeat lengths did not correlate with age, disease duration, muscular impairment rating scale, motor performance or BDI scores.
Comparing patient groups, patients with myotonic dystrophy type 1 were younger than patients with myotonic dystrophy type 2 (P = 0.010), but did not differ with regard to depressed mood, education, motor performance and disease duration (Table 1).
Neuropsychological performance
Neuropsychological performance – group comparison – myotonic dystrophy type 1 and controls
Patients with myotonic dystrophy type 1 had a higher susceptibility to interference compared with our control group. However, the test value was still within the normal range, when compared with the clinical cut-off value for relevant impairment (P = 0.380; Table 2). In contrast, patients with myotonic dystrophy type 1 performed better than controls in the choice reaction time and the recognition task of the verbal memory test. Our control group showed poorer performance in the choice reaction time task in comparison to normative data (P = 0.036). Thus, choice reaction time task results in patients with myotonic dystrophy type 1 did not differ from normative data (P = 0.807). Patients with myotonic dystrophy type 1 performed better in the verbal memory task not only when compared with our control group but also when compared with normative values (P = 0.033; Table 2).
Table 2.
Neuropsychological function, controlled for motor performance, in patients with myotonic dystrophy type 1 and myotonic dystrophy type 2 compared with controls (mean ± SD)
| Myotonic dystrophy type 1 (n = 22) | Myotonic dystrophy type 2 (n = 22) | Controls (n = 22) | Myotonic dystrophy type 1 versus controls | Myotonic dystrophy type 2 versus controls | Myotonic dystrophy type 1 versus myotonic dystrophy type 2 | |
|---|---|---|---|---|---|---|
| F-value (P-value) | F-value (P-value) | F-value (P-value) | ||||
| Focused attention (c.I.T.S) | 19.64 (5.06) | 20.55 (5.48) | 15.76 (2.77) | 3.135 (0.084)a | 9.918 (0.003)** | 0.876 (0.355) |
| Interference (c.I.T.I) | 27.50 (7.84) | 25.82 (5.65) | 19.38 (4.71) | 6.411 (0.015)a,* | 8.932 (0.005)a,** | 0.057 (0.812)a |
| Psychomotoric speed (TMT A) | 33.45 (11.70) | 39.00 (16.29) | 36.24 (14.29) | 2.136 (0.152) | 0.208 (0.651)a | 3.660 (0.063)a |
| Attention shift, mental flexibility (TMT B) | 96.52 (48.75) | 90.27 (35.24) | 83.52 (32.70) | 0.001 (0.970) | 0.662 (0.421)a | 0.002 (0.961)a |
| Naming (Boston Naming) | 54.36 (4.09) | 56.32 (7.47) | 56.67 (3.48) | 1.199 (0.280) | 2.719 (0.107) | 0.627 (0.433) |
| Phonematic fluency | 29.50 (7.74) | 29.45 (8.96) | 33.57 (9.54) | 0.011 (0.918)a | 0.261 (0.612)a | 0.235 (0.631)a |
| Semantic fluency | 23.77 (5.77) | 22.18 (6.34) | 24.48 (4.57) | 0.204 (0.654) | 0.717 (0.402) | 1.420 (0.240) |
| Visual–spatial / visual–constructive abilities (Blocktest) | 22.32 (10.54) | 29.95 (9.79) | 27.90 (8.01) | 0.499 (0.484)a | 2.432 (0.127)a | 4.321 (0.044)a,* |
| Reaction time (NeurocogFX) | 263.82 (55.56) | 291.64 (73.85) | 258.52 (47.80) | 0.001 (0.974) | 1.555 (0.220) | 3.919 (0.054)a |
| Choice reaction time (NeurocogFX) | 98.64 (58.56) | 141.86 (60.30) | 135.76 (57.85) | 9.491 (0.004)a,** | 0.229 (0.635) | 6.534 (0.014)* |
| Interference (NeurocogFX) | 388.91 (57.14) | 450.59 (84.34) | 412.57 (71.22) | 3.528 (0.068) | 1.642 (0.207) | 13.345 (0.001)** |
| Verbal memory-recognition (NeurocogFX) | 43.16 (4.91) | 36.50 (6.86) | 40.88 (2.98) | 6.912 (0.012)* | 0.051 (0.823) | 6.457 (0.015)a,* |
| Figural memory-recognition (NeurocogFX) | 5.59 (7.43) | 7.68 (8.16) | 6.57 (8.53) | 0.711 (0.404)a | 2.863 (0.056)a | 0.072 (0.790)a |
a Significant influence of motor performance.
Names of the applied test or test battery for each neuropsychological function are given in brackets. For details regarding the neuropsychological tests, see Supplementary material.
*P < 0.05, **P < 0.01.
c.I.T.S = subtest (symbol counting) of the Cerebraler Insuffizienztest; c.I.T.I = subtest (response inhibition) of the Cerebraler Insuffizienztest; NeurocogFX = omputerised neuropsychological screening test battery; TMT = Trail-Making Test.
Analysis of increased daytime sleepiness/fatigue showed that our myotonic dystrophy type 1 patient group reached higher scores than controls in all applied scales. However, mean scores above the clinical cut-off of pathological performing were found in 15/22 (68%) patients with myotonic dystrophy type 1 with Krupp's Fatigue Severity Scale (KFSS; Krupp et al., 1989), in 12/22 (55%) patients with myotonic dystrophy type 1 with Daytime Sleepiness Scale (Johnson et al., 1999) and in 9/22 (41%) patients with myotonic dystrophy type 1 with Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989; Table 3).
Table 3.
Sleep scales in patients with myotonic dystrophy types 1 and 2 compared with controls (mean ± SD)
| Cut-off Values | Myotonic dystrophy type 1 (n = 22) | Myotonic dystrophy type 2 (n = 21) | Controls (n = 19) | Myotonic dystrophy type 1 versus controls | Myotonic dystrophy type 2 versus controls | Myotonic dystrophy type 1 versus myotonic dystrophy type 2 | |
|---|---|---|---|---|---|---|---|
| F-value (P-value) | F-value (P-value) | F-value (P-value) | |||||
| PSQI total-score | >5 | 5.67 (3.43) | 5.85 (3.82) | 3.11 (2.23) | 7.555 (0.009)** | 7.287 (0.011)* | 0.026 (0.872) |
| KFSS | >3.7 | 4.38 (1.59) | 4.61 (1.73) | 2.26 (0.72) | 30.387 (0.000)*** | 29.742 (0.000)*** | 0.118 (0.733) |
| Daytime Sleepiness Scale | >10 | 11.27 (3.87) | 8.76 (5.48) | 6.47 (3.06) | 17.088 (0.000)*** | 2.465 (0.125) | 2.601 (0.115) |
| Epworth Sleepiness Scale | >10 | 9.50 (3.20) | 7.19 (4.01) | 5.67 (2.35) | 16.524 (0.000)*** | 1.661 (0.206) | 4.005 (0.052) |
| Ullanlinna | >13 | 9.68 (5.02) | 6.10 (3.16) | 5.42 (1.92) | 11.925 (0.001)** | 0.866 (0.358) | 7.195 (0.011)* |
*P < 0.05, **P < 0.01, ***P < 0.001.
Ullanlinna = Ullanlinna-Narcolepsy Scale.
We did not find any correlations between neuropsychological test results, sleepiness/fatigue scores and CTG repeat expansion sizes. Correlation analyses, however, showed that worse results in interference and choice reaction tasks were associated with higher age and poorer motor performance, whereas the verbal memory task correlated with age only. Higher KFSS, Daytime Sleepiness Scale and PSQI scores were associated with more depressed mood, but not with higher age or longer disease duration. Only higher KFSS scores indicating more fatigue were associated with poorer motor performance (Table 4).
Table 4.
Pearson product–moment correlation coefficient for correlations (P-values are given in brackets) between neuropsychological tests and fatigue/sleepiness scales with age, disease duration, motor performance and BDI score
| Function | Focused attention | Interference | Choice reaction time | Verbal memory-rec. | Depressed mood | Fatigue | Daytime sleepiness | Sleep quality |
|---|---|---|---|---|---|---|---|---|
| (Test) | (c.I.T.S) | (c.I.T.I) | (Neuro-Cog FX) | (Neuro-Cog FX) | (BDI) | (KFSS) | (DSS) | (PSQI) |
| Myotonic dystrophy type 1 | ||||||||
| Age | 0.14 (0.534) | 0.44 (0.040)* | 0.55 (0.007)** | −0.65 (0.001)** | −0.30 (0.169) | −0.04 (0.877) | −0.42 (0.053) | −0.27 (0.233) |
| Disease duration | 0.02 (0.938) | 0.23 (0.295) | 0.07 (0.745) | −0.29 (0.187) | −0.20 (0.366) | −0.28 (0.206) | −0.15 (0.505) | −0.36 (0.113) |
| Motor performance | −0.43 (0.046)* | −0.44 (0.039)* | −0.45 (0.034)* | 0.40 (0.066) | −0.23 (0.301) | −0.62 (0.002)** | 0.016 (0.943) | −0.23 (0.308) |
| Depressed mood | −0.25 (0.912) | 0.68 (0.763) | −0.14 (0.536) | 0.91 (0.687) | − | 0.46 (0.031)* | 0.61 (0.003)** | 0.89 (0.000)*** |
| Myotonic dystrophy type 2 | ||||||||
| Age | 0.07 (0.768) | 0.28 (0.205) | −0.03 (0.879) | −0.33 (0.134) | 0.04 (0.850) | 0.05 (0.836) | 0.1 (0.667) | −0.41 (0.077) |
| Disease duration | −0.29 (0.195) | −0.02 (0.932) | −0.10 (0.657) | −0.34 (0.125) | 0.09 (0.684) | −0.08 (0.728) | 0.04 (0.857) | −0.10 (0.664) |
| Motor performance | −0.09 (0.682) | −0.48 (0.023)* | 0.24 (0.290) | 0.31 (0.166) | −0.23 (0.319) | −0.44 (0.048)* | −0.26 (0.249) | −0.03 (0.891) |
| Depressed mood | 0.18 (0.444) | 0.15 (0.947) | 0.16 (0.488) | −0.43 (0.853) | – | 0.28 (0.216) | 0.36 (0.111) | 0.34 (0.139) |
| Controls | ||||||||
| Age | 0.33 (0.144) | 0.33 (0.145) | 0.31 (0.170) | 0.22 (0.331) | −0.14 (0.558) | 0.01 (0.959) | 0.07 (0.784) | −0.12 (0.634) |
| Motor performance | 0.72 (0.758) | −0.22 (0.350) | −0.19 (0.403) | −0.02 (0.935) | 0.29 (0.234) | −0.18 (0.470)* | 0.19 (0.427) | 0.16 (0.508) |
| Depressed mood | 0.04 (0.866) | 0.18 (0.461) | −0.39 (0.98) | −0.33 (0.167) | – | −0.4 (0.887) | 0.64 (0.003)** | 0.44 (0.061) |
Names of the applied test or test battery for each neuropsychological function are given in brackets. For details regarding the neuropsychological tests, see Supplementary material.
*P < 0.05, **P < 0.01, ***P < 0.001.
c.I.T.S = subtest (symbol counting) of the Cerebraler Insuffizienztest; c.I.T.I = subtest (response inhibition) of the Cerebraler Insuffizienztest; DSS = Daytime Sleepiness Scale; NeurocogFX =computerised neuropsychological screening test battery.
Neuropsychological performance – group comparison – myotonic dystrophy type 2 and controls
Patients with myotonic dystrophy type 2 showed poorer performance in focused attention and interference tasks compared with controls (Table 2). Myotonic dystrophy type 2 differed from controls in KFSS and PSQI scores with mean values scoring above the clinical cut-off: 14/21 (70%) and 9/21 (43%) patients with myotonic dystrophy type 2 reached pathological values, respectively. Daytime Sleepiness Scale and Epworth Sleepiness Scale scores (Johns, 1991) did not differ between patients with myotonic dystrophy type 2 and controls (Table 3).
In patients with myotonic dystrophy type 2, poorer performance in an interference task was associated with poorer motor performance, whereas no associations with age or disease duration were found. While Daytime Sleepiness Scale and PSQI scores showed no correlations at all, higher KFSS scores were associated with poorer motor performance, but not with higher age, longer disease duration or higher BDI scores (Table 4).
Neuropsychological performance – group comparison – myotonic dystrophy types 1 and 2
Patients with myotonic dystrophy type 2 performed better than patients with myotonic dystrophy type 1 in visual–spatial and visual–constructive abilities when corrected for motor performance. Patients with myotonic dystrophy type 1 performed better than patients with myotonic dystrophy type 2 in tests for choice reaction time, interference and verbal memory (Table 2). Myotonic dystrophy type 1 had higher Ullanlinna scores (Ullanlinna-Narcolepsy Scale; Hublin et al., 1994) indicating more signs of narcolepsy than myotonic dystrophy type 2; however, mean score values were below the clinical cut-off in both groups (Table 3). All other neuropsychological parameters did not differ between patients with myotonic dystrophy types 1 and 2.
Grading of white matter hyperintensities
Patients with myotonic dystrophy type 1 had more frequent and severe white matter lesions than controls in total white matter lesions scores assessing the whole brain (sum of bihemispheric scores/total score, P = 0.03). Frontal brain regions were the most frequently and severely affected areas in patients with myotonic dystrophy types 1 and 2 compared with controls (sum of bihemispheric subscores, myotonic dystrophy type 1 P < 0.001, myotonic dystrophy type 2 P = 0.02). Temporal white matter lesions were restricted to patients with myotonic dystrophy type 1. Mild infratentorial white matter lesions (subscore 1) were only found in one control, mild parieto-occipital and unilateral frontal lesions were present in three and two controls, respectively (subscores 1). The remaining controls did not show white matter lesions (Table 5). Both patient groups did not differ with regard to total white matter lesions scores or subscores.
Table 5.
White matter hyperintensities rated according the ARWMC scale in patients with myotonic dystrophy types 1 and 2 and control subjects (mean ± SD)
| Myotonic dystrophy type 1 patient group | Myotonic dystrophy type 2 patient group | Control group | |
|---|---|---|---|
| Total score | 1.82 ± 2.79 (0.03)* | 1.0 ± 1.75 (0.15) | 0.39 ± 0.66 |
| Frontal subscore | 1.18 ± 1.68 (0.00)* | 0.55 ± 0.86 (0.02)* | 0.09 ± 0.29 |
| Parieto-occipital subscore | 0.45 ± 1.14 (0.52) | 0.45 ± 1.10 (0.51) | 0.26 ± 0.62 |
| Temporal subscore | 0.18 ± 0.85 (0.32) | 0 | 0 |
| Infratentorial subscore | 0 (0.32) | 0 (0.32) | 0.04 ± 0.21 |
*Significant differences (P < 0.05) between patient and control groups. P-values are given in brackets.
ARWMC = age-related white matter change score.
Voxel-based morphometry
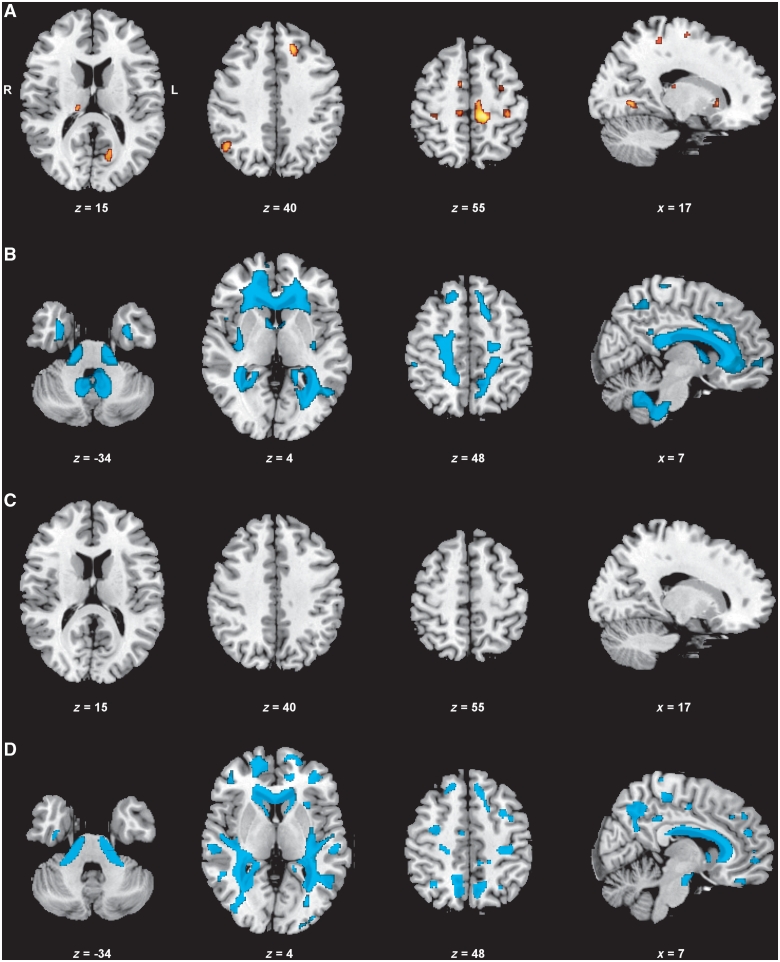
Compared with controls, we found grey matter alterations in patients with myotonic dystrophy type 1 in various cortical regions of both hemispheres, mainly located in frontal and parietal regions, whereas temporal lobes were spared. Most notably, pre- and postcentral gyrus and the supplementary motor area were bilaterally affected. Additional subcortical grey matter alteration was detected in the right posterior thalamus and in the most anterior part of the right putamen.
White matter decrease was more pronounced than grey matter decrease. It affected the entire corpus callosum, both fornices, cingulum bundle and white matter in every lobe. Further white matter decrease was located at brainstem level (pons), along middle cerebellar peduncles and in cerebellar white matter (Supplementary Table 1; Fig. 1).
Figure 1.
Neuroimaging results of the brain (VBM, group comparisons). Displayed results of VBM analyses are based on a threshold of Pfalse discovery rate < 0.05 at voxel-level with an extended cluster threshold of 10 voxels. The coordinates refer to the MNI reference space. (A) Grey matter decrease in patients with myotonic dystrophy type 1 compared with controls. (B) White matter decrease in patients with myotonic dystrophy type 1 compared with controls. (C) Grey matter decrease in patients with myotonic dystrophy type 2 compared with controls (no clusters detected). (D) White matter decrease in patients with myotonic dystrophy type 2 compared with controls.
In contrast to myotonic dystrophy type 1, no grey matter decrease was detected in patients with myotonic dystrophy type 2 compared with controls. White matter decrease, however, was pronounced and located along the entire corpus callosum and in every lobe, although the regions were less confluent than in myotonic dystrophy type 1. Further, white matter loss was present at brainstem level (pons), along middle cerebellar peduncles and in cerebellar white matter (Supplementary Table 2; Fig. 1).
Diffusion tensor imaging
Diffusion tensor imaging – group comparison – myotonic dystrophy type 1 and controls
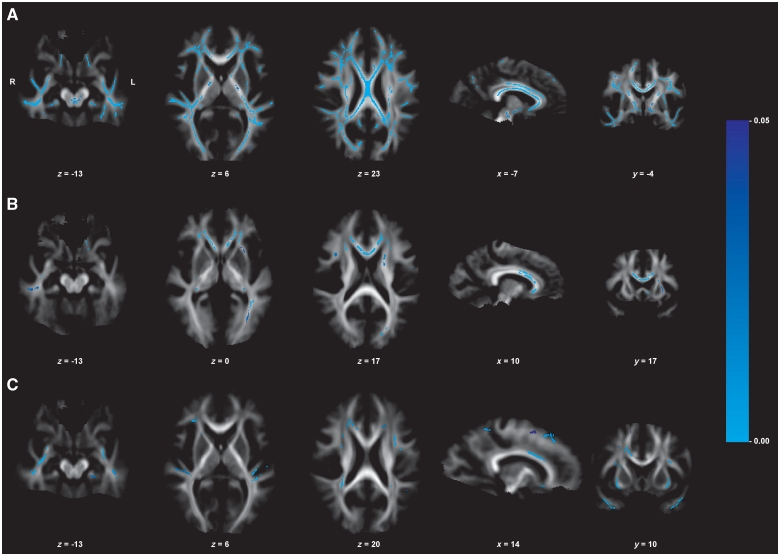
Compared with controls, in patients with myotonic dystrophy type 1 we detected ubiquitous fractional anisotropy reduction in association fibres: bilateral superior and inferior longitudinal fascicles, inferior fronto-occipital fascicles and uncinate fascicles, both fornices, cingulum bundles and hippocampal parts of the posterior cingulum bundle. The corpus callosum as main commissural tract was affected in all parts, sparing only parts of the splenium. In addition, projection fibres as internal capsules (anterior/posterior limbs, retrolenticular parts) and external capsules, both corticospinal tracts at the level of internal capsules and brainstem at pontine level were affected (Fig. 2, Supplementary Table 3).
Figure 2.
Neuroimaging results of the brain (DTI, group comparison of fractional anisotropy values). Displayed results of tract-based spatial statistics analyses of fractional anisotropy values (group comparison) are based on a corrected threshold of Pfamily wise error < 0.05. Mean tract-based spatial statistics tract skeleton is overlaid on the mean fractional anisotropy image (display threshold of 0.1). The coordinates refer to the MNI reference space. (A) Fractional anisotropy reduction in patients with myotonic dystrophy type 1 compared with healthy controls. (B) Fractional anisotropy reduction in patients with myotonic dystrophy type 2 compared with healthy controls. (C) Fractional anisotropy reduction in patients with myotonic dystrophy type 1 compared with patients with myotonic dystrophy type 2.
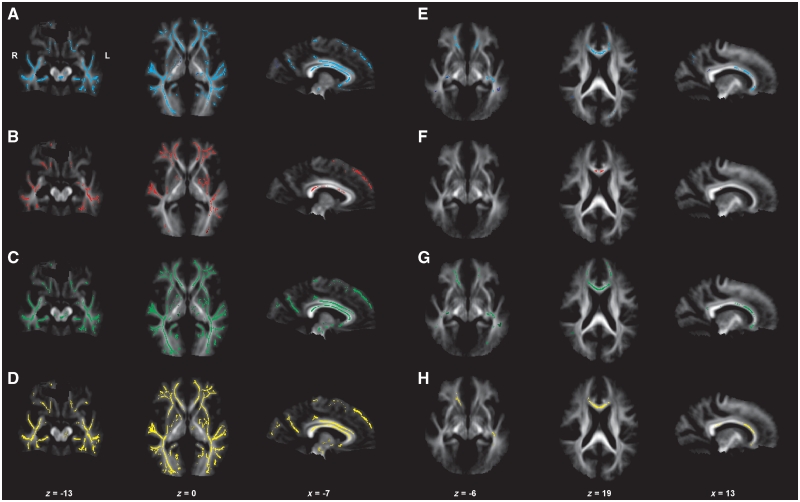
The areas of increased radial diffusivity and mean diffusivity closely mirrored the areas of fractional anisotropy reduction. Compared with fractional anisotropy reduction, some regions were even more continuously affected and reached more to the outlying border of affected fibre tracts. An increase of axial diffusivity was found in parts of regions with fractional anisotropy reduction (Fig. 3).
Figure 3.
Neuroimaging results of the brain (DTI, group comparison of fractional anisotropy values, axial, radial and mean diffusivity). Displayed results of tract-based spatial statistics analyses of different diffusivity indices (fractional anisotropy, axial diffusivity, radial diffusivity, mean diffusivity) are based on a corrected threshold of Pfamily wise error < 0.05. Mean tract-based spatial statistics tract skeleton is overlaid on the mean fractional anisotropy image (display threshold of 0.1). The coordinates refer to the MNI reference space. (A and E) Fractional anisotropy reduction in patients with myotonic dystrophy type 1 (A) and patients with myotonic dystrophy type 2 (E) compared with healthy controls. (B and F) Increase in axial diffusivity in patients with myotonic dystrophy type 1 (B) and patients with myotonic dystrophy type 2 (F) compared with healthy controls. (C and G) Increase in radial diffusivity in patients with myotonic dystrophy type 1 (C) and patients with myotonic dystrophy type 2 (G) compared with healthy controls. (D and H) Increase of mean diffusivity in patients with myotonic dystrophy type 1 (D) and patients with myotonic dystrophy type 2 (H) compared with healthy controls.
Diffusion tensor imaging – group comparison – myotonic dystrophy type 2 and controls
Compared with controls, in patients with myotonic dystrophy type 2 we detected prominent fractional anisotropy reduction in association fibres: bilateral superior longitudinal fascicles, inferior longitudinal fascicles, inferior fronto-occipital fascicles, uncinate fascicles, cingulum bundles and fornices. The corpus callosum was mainly affected in its anterior parts and more severely affected on the left side. Anterior limbs of both internal capsules (left > right) and both external capsules were affected as projection fibres (Fig. 2, Supplementary Table 3).
The areas of increased radial diffusivity mirrored those with fractional anisotropy reduction, but there were additional small significant areas with increased axial diffusivity in some fibre tracts. There was little overlap between fractional anisotropy reduction and increased axial diffusivity in areas of significance. An increase in mean diffusivity was again found in similar regions as fractional anisotropy reduction, although not all regions with fractional anisotropy reduction showed an increase of mean diffusivity (Fig. 3).
Diffusion tensor imaging – group comparison – myotonic dystrophy types 1 and 2
Direct comparison between both patient groups revealed lower fractional anisotropy values in patients with myotonic dystrophy type 1 in some parts of bilateral superior longitudinal fascicles and inferior longitudinal fascicles, right corticospinal tract—which was not affected in myotonic dystrophy type 2—and in parts of the corpus callosum. We did not find lower fractional anisotropy values in myotonic dystrophy type 2 when compared with myotonic dystrophy type 1 (Fig. 2).
Diffusion tensor imaging – correlation analyses with clinical and neuropsychological data – myotonic dystrophy type 1
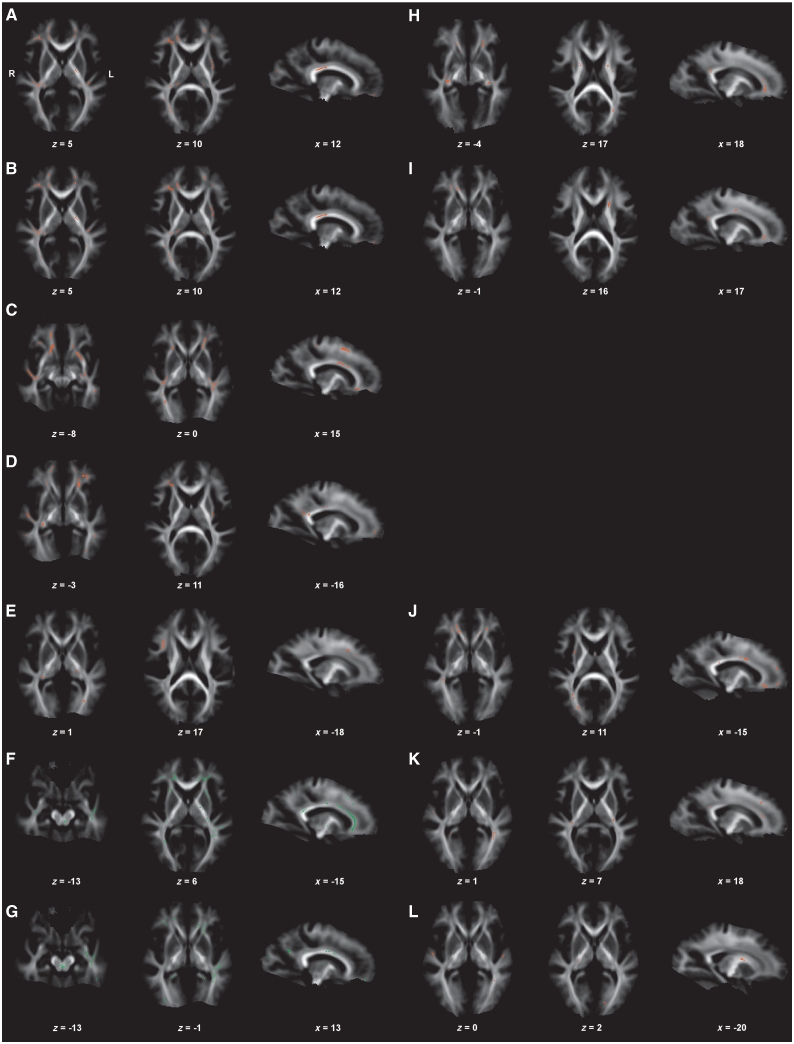
Lower fractional anisotropy values occurred with higher age, longer disease duration, larger CTG repeat expansion sizes, higher Muscular Impairment Rating Scale score and poorer manual motor performance in various brain regions, predominantly association fibres, fornix, cingulum bundles, corpus callosum and left external capsule. Most notably, regions associated with higher age were identical with those associated with longer disease duration. In addition, fractional anisotropy values of the posterior limb of the left internal capsule and left external capsule correlated not only with age and disease duration, but also with motor performance (and with depressed mood). Regions in which reduced fractional anisotropy values occurred with poorer motor performance overlapped only partially with regions in which reduced fractional anisotropy values correlated with the Muscular Impairment Rating Scale score (only right superior longitudinal fascicle and inferior longitudinal fascicle). Unexpectedly, fractional anisotropy values were lower in patients with less fatigue (lower KFSS scores) and less depressed mood (lower BDI scores). Whereas the corpus callosum was one of the most prominent regions affected in correlation analyses with the BDI score, we saw only minor callosal body involvement in correlation analyses with the KFSS score. Instead, there was a prominent involvement of the dorsal brainstem, not depicted in association with the BDI score. This region showed further correlations with age/disease duration (Fig. 4). Further details of fractional anisotropy correlation analyses with clinical parameters are given in Supplementary Table 4.
Figure 4.
Neuroimaging results of the brain (DTI, correlation analyses between white matter affection (fractional anisotropy values) and clinical parameters). Correlation analyses in myotonic dystrophy type 1 (A–G) and myotonic dystrophy type 2 (H–L). Displayed results of Tract-based spatial statistics analyses of fractional anisotropy values are based on a corrected threshold of Pthreshold-free cluster enhancement < 0.05. Mean tract-based spatial statistics tract skeleton is overlaid on the mean fractional anisotropy image (display threshold of 0.1). The coordinates refer to the MNI reference space. Correlations of fractional anisotropy values in patients with myotonic dystrophy type 1 with age and disease duration (A and B), CTG repeat length (C), Muscular Impairment Rating Scale score (D), motor performance (E). Positive correlation of fractional anisotropy values in patients with myotonic dystrophy type 1 with depressed mood (BDI score, F) and fatigue (KFSS score, G). Correlations of fractional anisotropy values in patients with myotonic dystrophy type 2 with age (H), disease duration (I) and motor performance (J). Negative correlations of fractional anisotropy values in patients with myotonic dystrophy type 2 with depressed mood (BDI score, K) and fatigue (KFSS score, L).
In contrast to correlation analyses of fractional anisotropy data with clinical parameters, correlation analyses with neuropsychological tests (interference, choice reaction time, verbal memory test) gave mixed results with marginal and inconsistent associations that involved only few voxels (data not shown).
Diffusion tensor imaging – correlation analyses with clinical and neuropsychological data – myotonic dystrophy type 2
Lower fractional anisotropy values occurred with higher age, longer disease duration and poorer motor performance in various brain regions, especially association fibres, cingulum bundles, fornices, anterior limb of internal capsules, corpus callosum and right external capsule. With respect to higher age and longer disease duration, we found more frontally accentuated reduced fractional anisotropy values compared with myotonic dystrophy type 1, affecting comparable but not identical fibre parts. Further, the changes were more pronounced with age than with disease duration. Correlation analyses of fractional anisotropy values with motor performance showed regions in the right external capsule, while in contrast to myotonic dystrophy type 1 no associations with posterior limbs of internal capsules were present. In contrast to myotonic dystrophy type 1, lower fractional anisotropy values were associated with a more depressed mood and increased fatigue in myotonic dystrophy type 2 (Fig. 4). Further details of fractional anisotropy correlation analyses with clinical parameters are given in Supplementary Table 4.
Correlation analyses with neuropsychological tests (interference, focused attention) did not show significant results (data not shown).
Discussion
We present a comprehensive study of brain structure and function in a comparably large series of patients with myotonic dystrophy types 1 and 2. Our neuroimaging findings confirmed the major hypotheses of our study: we found severe white matter effects in patients with myotonic dystrophy type 1, more than in patients with myotonic dystrophy type 2, which extensively exceeded grey matter involvement in both disorders. We applied DTI based on diffusion MRI, which is a suitable method for white matter evaluation. Even though VBM is not the method of choice for white matter evaluation, our DTI results were strongly supported by VBM analyses.
White matter effects were found throughout the whole brain in myotonic dystrophy types 1 and 2, affecting association fibres, commissural fibres (mainly corpus callosum), and projection fibres in the brainstem, internal and external capsules, the latter connecting prefrontal and temporal cortical areas with the striatum. Thalamocortical pathways (e.g. anterior thalamic radiation) and prefrontal connections are represented in anterior limbs of internal capsules, which were affected in myotonic dystrophy types 1 and 2. Interestingly, anterior limb lesions had previously been described in the context of behavioural problems and cognitive dysfunction (Mamah et al., 2010). In contrast, central motor pathways (e.g. corticospinal tracts located in posterior limbs of internal capsules) were exclusively affected in myotonic dystrophy type 1.
Our data on white matter in adult patients with myotonic dystrophy type 1 give evidence that callosal body affection is not restricted to congenital myotonic dystrophy type 1. Current results confirm callosal body affection in patients with myotonic dystrophy type 2 as previously demonstrated by our VBM and surface-based morphometry analyses (Minnerop et al., 2008), and demonstrate a predominant affection of anterior callosal fibres connecting frontal lobes in myotonic dystrophy type 2. We further found degradation of major pathways of the limbic system (e.g. fornix, cingulum bundle) in patients with myotonic dystrophy type 1, more than in patients with myotonic dystrophy type 2. This finding could be associated with behavioural abnormalities and emotional disturbances in myotonic dystrophies. This question should be addressed in future studies investigating personality traits in both disorders.
In the current patient series, cortical grey matter affection was only found in myotonic dystrophy type 1. Cortical grey matter loss was located in frontal and parietal regions, whereas subcortical grey matter loss was detected in thalamic and basal ganglia structures. This is in line with the literature, describing widespread cortical and subcortical grey matter alterations in myotonic dystrophy type 1 (Antonini et al., 2004; Weber et al., 2010). Previous analyses, including one of our own studies, also found grey matter changes in myotonic dystrophy type 2 (Minnerop et al., 2008; Weber et al., 2010). This discrepancy to our present data may be attributed to differences in sample size as earlier studies investigated smaller patient cohorts.
Though our patients with myotonic dystrophy type 2 were older than our patients with myotonic dystrophy type 1, the myotonic dystrophy type 2 cohort encompassed rather ‘young’ subjects regarding the common disease manifestation at higher ages. With respect to the clinical course of myotonic dystrophy type 2, an older cohort with longer disease durations might have been more appropriate. Less pronounced cerebral grey and white matter affection in patients with myotonic dystrophy type 2 compared with patients with myotonic dystrophy type 1 might be partially explained by this fact.
White matter lesions and brain atrophy are well known in myotonic dystrophy types 1 and 2, but current data stress that the underlying pathology seems to (i) affect white matter much more than grey matter and (ii) affect white matter far beyond circumscribed white matter lesions visible on T2-weighted MRIs. These findings link myotonic dystrophies to the growing group of brain disconnection disorders. The neuropathological background of white matter changes in myotonic dystrophies is still not fully understood. Neuropathological findings in myotonic dystrophy type 1 brains include abnormalities like intracytoplasmic inclusions in thalamus, striatum, cerebral cortex and brainstem (Rosman and Kakulas, 1966; Wiśniewski et al., 1975; Ono et al., 1987). Further studies in myotonic dystrophy type 1 and myotonic dystrophy type 2 brains found evidence of neurofibrillary degeneration with intracellular aggregation of microtubule-associated tau protein (Vermersch et al., 1996; Sergeant et al., 2001; Maurage et al., 2005; Itoh et al., 2010). Most changes are located in neurons; however, white matter alterations including disordered arrangement of myelin sheaths and axons have been described (Abe et al., 1994; Ogata et al., 1998; Itoh et al., 2010). It is widely accepted that the integrity of axonal membranes and myelin sheaths are the main biological causes of anisotropy in DTI (Beaulieu, 2002). Reduced fractional anisotropy values in the current study were primarily linked with an increase of radial diffusivity. Increased radial diffusivity can be either caused by reduced myelin sheaths/defect axonal membranes or as a consequence of neuronal cell loss with Wallerian degeneration. A likely explanation for our finding of an additional increase of axial diffusivity (theoretically leading to an increase of fractional anisotropy) in some areas with fractional anisotropy reduction is increased water content in the context of atrophy as described in the ‘healthy’ ageing brain (Moseley, 2002). This phenomenon leads to an increase of both radial more than axial diffusivity, finally resulting in a decrease of fractional anisotropy. The changes of diffusivity in white matter fibre tracts were more prominent in myotonic dystrophy type 1 than type 2. This is possibly a consequence of genetic differences with a disease-specific pathomechanism of tissue destruction, which however results in abnormal increases of water diffusion perpendicular to axons in both disorders. We have to consider that reduced fractional anisotropy values in genetically determined disorders might not reflect ongoing destructive processes, but instead a developmental defect with disturbed assembly of myelin and/or axonal membranes. The widespread changes in myotonic dystrophy type 1 may point towards a generalized white matter defect. However, the correlation of lower fractional anisotropy values with longer disease duration and higher age in myotonic dystrophy type 1 and myotonic dystrophy type 2 strongly argue for an ongoing destruction of myelin and/or axonal loss over time. The fact that white matter changes by far dominated the extent of grey matter changes in myotonic dystrophy types 1 and 2 might further argue against Wallerian degeneration as the major cause of white matter alterations as formerly postulated (Ota et al., 2006).
Relation of white matter integrity and motor function
Impaired motor function due to muscle affection is one of the most characteristic symptoms in myotonic dystrophy types 1 and 2. However, subclinical dysfunction of the central motor system had been described in myotonic dystrophy type 1 (Oliveri et al., 1997; Mitsuoka et al., 2003). We found reduced fractional anisotropy values in posterior limbs of internal capsules (corticospinal tract) in myotonic dystrophy type 1, not in myotonic dystrophy type 2, which correlated with motor performance in a simple motor task (bimanual pegboard). In the group comparison, we found reduced fractional anisotropy values along external capsules in patients with myotonic dystrophy types 1 and 2, correlating with the Muscular Impairment Rating Scale score (measuring muscular impairment) in myotonic dystrophy type 1 and motor performance in myotonic dystrophy type 1 and type 2. The external capsule contains corticostriatal projection fibres connecting (pre)-frontal and temporal areas with basal ganglia, known to play a major role in motion planning and execution. A recent functional MRI study in myotonic dystrophy type 1 showed activation patterns in central motor system areas that resembled those in healthy older subjects (Caramia et al., 2010). Taken together, these findings give evidence of impaired central motor functioning in myotonic dystrophies, potentially reflecting an accelerated or increased ageing process.
Neuropsychological performance
Neuropsychological deficits, especially of executive and visuospatial functions, have been frequently described in myotonic dystrophy types 1 and 2 (Meola et al., 1999, 2003; Gaul et al., 2006; Meola and Sansone, 2007; Romeo et al., 2010). However, most tests investigating frontal functions depend on some motor response, which may be impaired in myotonic dystrophies. While several neuropsychological studies did not address impaired motor performance as a possible confounding factor in their study design, other studies tried to solve this issue by excluding patients with a certain degree of muscular impairment (Meola et al., 2003; Gaul et al., 2006) or to select motor-independent tests (Modoni et al., 2004; Gaul et al., 2006). We tried to correct for motor performance by including a score for fine motor skills as covariate. We found only minor neuropsychological deficits in our patients. Patients with myotonic dystrophy type 1, usually thought to be more cognitively impaired than patients with myotonic dystrophy type 2, even showed a better performance in the verbal memory task compared with controls. This is not explained by age differences of patients with myotonic dystrophy type 1 and controls, since patients with myotonic dystrophy type 1 still performed above average in comparison to age-matched normative data. Increased liability for interference was the only significant neuropsychological deficit in our myotonic dystrophy type 1 series and also significantly present in myotonic dystrophy type 2, which additionally showed impaired focused attention, pointing towards frontal lobe dysfunction in both myotonic dystrophy types 1 and 2. These results are in line with earlier studies showing that impaired frontal lobe function is a predominant finding in myotonic dystrophies (Meola et al., 2003). The discrepancy regarding the extent of cognitive deficits between previous studies and our results might be at least partially explained by methodological differences as correction for fine motor performance. This is supported by findings of Gaul et al. (2006) who also found only subtle prefrontal lobe dysfunction when excluding patients with severe motor impairment and selecting more motor-independent tests. The clinical impression of cognitive decline particularly in adult patients with myotonic dystrophy type 1 may thus be partly unjustified and rather due to a combination of facial muscular weakness, depression and the highly underestimated, however frequent and treatable, symptoms of fatigue/increased daytime sleepiness. One further explanation for the presence of only minor neuropsychological deficits in our patient series might be a selection bias towards cognitively less severely affected individuals. The study participation lasted about 4–5 h in an outpatient setting, and patients with a serious mental impairment might have been more likely to refuse study participation. Third, there is some evidence that neuropsychological deficits in myotonic dystrophies might partially escape commonly applied neuropsychological test batteries due to still unclear reasons. This observation is primarily based on the personal experience of neurologists and neuropsychologists specialized in myotonic dystrophies. Consequently, a current international intention is to find a consensus on neuropsychological tests to be generally recommended for use in patients with myotonic dystrophy worldwide. Accordingly, the most sensitive and reliable, best validated, and most applicable tests are expected to be selected in the near future. Our neuropsychological test selection was a compromise resulting from our experience in patients with neurological/neurodegenerative disorders and the time constraints that were dictated by the study protocol (combined neuroimaging study). For practical reasons, we used (i) the German NeuroCogFX, which is validated in a variety of neurological disorders and has also been applied to other trinucleotide repeat disorders (Fliessbach et al., 2006; Hoppe et al., 2009; Klinke et al., 2010) and (ii) the Cerebraler Insuffizienztest (Lehrl and Fischer, 1997), which assesses attention and interference in a reliable and fast way compared with other frontal lobe function tests. However, some of the applied tests had not previously been systematically used in myotonic dystrophies. Thus, the selection of the neuropsychological test battery might have additionally influenced the neuropsychological profiling of our patients.
Relation of white matter integrity and depression
Depressive symptoms are well known in myotonic dystrophy types 1 and 2, but do not seem to be a prominent feature if DSM-IV (Diagnostic and Statistical Manual of Mental Disorders) criteria are applied (Meola and Sansone, 2007). Using the self-rating questionnaire BDI, Winblad et al. (2010) found signs of mostly mild depression in 32% of examined patients with myotonic dystrophy type 1, which is identical to our findings in myotonic dystrophy type 1 (32%) and type 2 (38%). However, it is still a matter of debate if depression might be a consequence of structural brain damage or rather a reactive adjustment disorder. We therefore investigated the interaction between fractional anisotropy reduction and BDI scores and found higher BDI scores associated with higher fractional anisotropy values in myotonic dystrophy type 1, whereas higher BDI scores were associated with lower fractional anisotropy values in myotonic dystrophy type 2. Regression analyses in myotonic dystrophy type 1 and type 2 revealed a decline of fractional anisotropy with increasing age and disease duration. Thus, our results implicate that depressed mood in myotonic dystrophy type 1 might be more pronounced in earlier disease stages, whereas depression is more likely to be found in later disease stages of myotonic dystrophy type 2. Our results are supported by recent data of Winblad et al. (2010): the authors found less depressive symptoms in patients with myotonic dystrophy type 1 with a longer disease duration and presence of white matter lesions, whereas patients with myotonic dystrophy type 1 without white matter lesions had more depressive symptoms (Winblad et al., 2010). If depressed mood was a consequence of structural brain affection, a continuous worsening of depression would be expected as myotonic dystrophy types 1 and 2 progress. Our results indicate that depression might be a reactive adjustment disorder rather than a consequence of structural brain damage in myotonic dystrophies. Myotonic dystrophy type 1 is more severe in general and leads to a more serious impairment in earlier life than myotonic dystrophy type 2. Therefore, patients with myotonic dystrophy type 1 are more likely to notice early limitations in everyday activities and develop reactive depression. As disease progresses, these patients may develop efficient coping strategies or may be less able to perceive their limitations due to cognitive deficits or personality changes. Since we found only minor neuropsychological deficits, the so called ‘lack of awareness’ may explain the presence of a less depressed mood in advanced disease stages (Meola and Sansone, 2007; Winblad et al., 2010). This condition has previously been linked to localized brain lesions in other disorders, affecting regions (prefrontal cortex, frontal and parietotemporal areas, thalamus) that also show structural abnormalities in myotonic dystrophy type 1 (Orfei et al., 2008; Winblad et al., 2010). Myotonic dystrophy type 2 is usually less severe, and patients may not notice serious limitations in everyday life in early disease stages. Symptoms worsen over time and may cause depression in advanced disease stages. However, we did not find direct correlations between BDI scores and age or disease duration in myotonic dystrophy type 1 or 2.
The use of additional depression scores would have been favourable, though was not practicable in the present comprehensive study setting, which took several hours and required careful consideration of patients' psychophysical limits as well as time constraints.
Relation of white matter integrity and increased daytime sleepiness/fatigue
Increased daytime sleepiness and fatigue are among the most frequent non-muscular symptoms in patients with myotonic dystrophy type 1 and type 2 (Hilton-Jones, 1997; van der Werf et al., 2003; Meola and Sansone, 2007; Laberge et al., 2009a, b; Tieleman et al., 2010). Weak oropharyngeal and respiratory muscles leading to obstructive sleep apnoea and alveolar hypoventilation have been regarded as causative factors of daytime sleepiness. However, there is increasing evidence that tiredness primarily results from CNS dysfunction rather than progressive respiratory weakness (van der Meché et al., 1994; Park and Radtke, 1995; Rubinsztein et al., 1998; Laberge et al., 2009a, b; Romigi et al., 2011; Yu et al., 2011). An association with the hypocretin neurotransmission system had been suggested. However, data regarding the role of hypocretin-1, a hypothalamic neuropeptide essential in the regulation of the sleep/wakefulness cycle and vigilance, are still controversial (Martínez-Rodriguez et al., 2003; Ciafaloni et al., 2008).
Fatigue, equivocally defined as a lack of energy and feeling of exhaustion (Shen et al., 2006), is a prominent and common complaint in myotonic dystrophy type 1 (60–80%; Kalkman et al., 2005; Meola and Sansone, 2007; Laberge et al., 2009b; Tieleman et al., 2010). The prevalence of fatigue in myotonic dystrophy type 2 has been investigated only very recently, finding a similar result of 66% (Tieleman et al., 2010). These data are in accordance with our findings, showing a prevalence of 68–70% fatigue in patients with myotonic dystrophy types 1 and 2. While our patients with myotonic dystrophy type 1 differed from controls in all sleepiness and fatigue scales, patients with myotonic dystrophy type 2 differed from controls only in PSQI and KFSS scores, not in daytime sleepiness scales. This again is in line with findings of Tieleman et al. (2010). Subjective sleep quality, as measured by the total PSQI score, was impaired in ∼40% of both patient groups, which might be an indicator of a disturbed sleep/awakening cycle in myotonic dystrophy types 1 and 2 and should be further examined by polysomnographic studies.
We performed correlation analyses to evaluate the relation between fatigue (KFSS score) and white matter integrity (fractional anisotropy values). Similar to results of regression analyses with BDI scores, we found that higher fractional anisotropy values were associated with more pronounced fatigue in myotonic dystrophy type 1. In contrast, lower fractional anisotropy values were associated with more pronounced fatigue in myotonic dystrophy type 2. In contrast to previous findings, anterior corpus callosum integrity did not correlate with fatigue in our study (Giubilei et al., 1999).
Damage to the reticular activating system of the upper brainstem and/or to its cortical projections has already been discussed as related to the chronic fatigue syndrome (Dickinson, 1997). Morphological brainstem changes in myotonic dystrophy type 1 include neurofibrillary tangles, Marinesco bodies, as well as a decrease of serotonergic neurons in raphe nuclei and catecholaminergic neurons in the medullary reticular formation (Ono et al., 1987, 1998a, b; Oyamada et al., 2006). Interestingly, sleep disturbances and apathy were more frequent in patients with myotonic dystrophy type 1 with fewer neurofibrillary tangles (Oyamada et al., 2006). We comparably depicted less fatigue with more pronounced brainstem affection in patients with myotonic dystrophy type 1. Thus, myotonic dystrophy type 1 specific brainstem changes might prevent the feeling of fatigue or again might result in a lack of self-awareness.
Laberge et al. (2009b) found higher depression scores, more muscular impairment, and higher number of CTG repeats in patients with myotonic dystrophy type 1 with fatigue and/or increased sleepiness. This in accordance with our results in myotonic dystrophy type 1, as we found higher KFSS scores correlating with both poorer motor performance and higher BDI scores. In contrast, we did not find a correlation of KFSS with BDI scores in myotonic dystrophy type 2, whereas higher KFSS scores correlated with poorer motor performance similar to myotonic dystrophy type 1 and to controls.
Thus, depressed mood and fatigue were closely related at least in myotonic dystrophy type 1. BDI and KFSS may overlap with regard to the target symptoms, and some questions of the BDI are known to target on symptoms of sleepiness and fatigue. In myotonic dystrophy type 2, we found similar directed correlations of fractional anisotropy values, BDI and KFSS scores. However, we did not find BDI and KFSS scores correlating with each other as shown in myotonic dystrophy type 1. Thus, one might conclude that the applied questionnaires are measuring partially overlapping, but not entirely identical conditions.
Polysomnographic studies as well as the use of further fatigue, anxiety and apathy scales like the Checklist Individual Strength or the Hospital Anxiety and Depression Scale would have been of interest but were not applicable in the current study setting. However, the present data warrant further investigations in the near future.
Relation of white matter integrity and age, disease duration and CTG repeat size
The pattern of affected fibre tracts in correlation analyses with age and disease duration was identical in our myotonic dystrophy type 1 but not the myotonic dystrophy type 2 group. Disease onset in general is earlier in myotonic dystrophy type 1, usually resulting in a parallel progression of disease and ageing. Consequently, the effect of age and disease duration cannot be easily separated in myotonic dystrophy type 1. However, since age and disease duration were not correlated in our patient groups, it is tempting to speculate that the effect of the disease itself on white matter structure resembles effects of ageing in myotonic dystrophy type 1. In myotonic dystrophy type 2, we found more frontally located areas with fractional anisotropy reduction that correlated with age but not with disease duration. This might be interpreted as a stronger effect of age in these distinct regions. Accelerated or premature ageing has been discussed in myotonic dystrophy type 1 (Toscano et al., 2005; Oyamada et al., 2006; Romigi et al., 2011), and age-specific translational dysfunctions have been described in myotonic dystrophy type 2 (Jin et al., 2009). Frontal-lobe accentuated white matter changes in myotonic dystrophy type 2, which correlated with age, may support this hypothesis since fractional anisotropy reduction in frontal regions had been described in normal ageing previously (Damoiseaux et al., 2009). A recent study investigated the time course of radial and axial diffusivity changes during ageing in the callosal body, which strikingly mirrors diffusivity changes present in our myotonic dystrophy type 1 and type 2 groups (Fig. 3; Lebel et al., 2010). The same study showed a sharp drop of fractional anisotropy values in anterior regions of the corpus callosum and a more substantial fractional anisotropy drop in outer sections of the callosal body with increasing age. While fractional anisotropy values were reduced along the entire corpus callosum in our myotonic dystrophy type 1 series, mainly anterior parts of the callosal body were affected in myotonic dystrophy type 2. These anterior callosal body fractional anisotropy reductions correlated with age and showed the peak fractional anisotropy loss with age in right outer sections. This might further underline similarities with normal ageing and might suggest an accelerated or more pronounced ageing process in patients with myotonic dystrophy types 2 and 1 compared with controls.
CTG repeat expansion sizes in blood are traditionally used as ‘biomarkers’ for disease severity in myotonic dystrophy type 1. Thus, there are a multitude of neuroimaging studies that have examined the relation of brain affection and CTG repeat lengths. However, results are highly controversial, and most studies failed to find any correlations (Kassubek et al., 2003; Antonini et al., 2004; Di Costanzo et al., 2008). This might be due to the marked somatic mosaicism of CTG repeat lengths. There are considerable tissue variations in repeat sizes with rather small expansions in blood and much larger expansions in heart or skeletal muscle tissue. Moreover, repeat sizes may increase throughout life even in post-mitotic tissues, which suggests that repeat lengths in blood might not correlate with repeat lengths in brain and cerebral affection. Nevertheless, a variety of neuroimaging studies did show more serious morphological cerebral changes with larger repeat expansions in adult patients (Ota et al., 2006; Romeo et al., 2010). Our present findings equally showed that larger CTG repeat sizes were associated with more severe white matter affection in several brain regions. Remarkably, the pattern of fibre tract degradation that was associated with larger CTG repeats was similar to the pattern that was associated with higher age and longer disease duration. These findings again may indicate that the effect of the disease itself on white matter structure resembles the effects of ageing in myotonic dystrophy type 1. Specific brain regions might be particularly susceptible to the disease effects that are reflected by CTG repeat lengths and disease duration.
Altogether, our data suggest that white matter affection is progressive over time in myotonic dystrophies. Despite this tempting speculation, cross-sectional data and correlation analyses, as obtained in our study, do not sufficiently allow analyses of the age- and disease duration-related impact on brain morphology in myotonic dystrophy types 1 and 2. Longitudinal MRI studies investigating the progress of grey and white matter changes over time and its role in clinical deterioration in both types of myotonic dystrophies are strongly required to address these issues appropriately in the future.
Funding
Maria von Linden-Support Programme, University of Bonn, Germany (to M.M., partial); Gerok Programme of the BONFOR Commission, University of Bonn, Germany (to J.-C. S.-B., partial) and Deutsche Forschungsgemeinschaft (DFG) (Heisenberg grant WE 4427/3-1 to B.W. and Heisenberg grant BE 2346/4-1 to R.C.B.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Preliminary data were presented in part at the 81st Annual Meeting of the German Neurological Society in Hamburg (Germany), 2008; the 13th International World Muscle Society Congress in Newcastle (UK), 2008; the 82nd Annual Meeting of the German Neurological Society in Nürnberg (Germany), 2009; the 19th Annual Meeting of the Scientific Advisory Board of the German Society of Muscle Disorders in Darmstadt (Germany), 2009; the 7th International Myotonic Dystrophy Consortium Meeting, Würzburg (Germany), 2009. We gratefully acknowledge the participation of all patients and healthy controls investigated in this study. We thank Beate Newport and Lotte Kühl for technical support with MRI acquisition.
Glossary
Abbreviations
- BDI
Beck Depression Inventory
- DTI
diffusion tensor imaging
- KFSS
Krupp's fatigue severity scale
- PSQI
Pittsburgh sleep quality index
- VBM
voxel-based morphometry
References
- Abe K, Fujimura H, Toyooka K, Yorifuji S, Nishikawa Y, Hazama T, et al. Involvement of the central nervous system in myotonic dystrophy. J Neurol Sci. 1994;127:179–85. doi: 10.1016/0022-510x(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Antonini G, Mainero C, Romano A, Giubilei F, Ceschin V, Gragnani F, et al. Cerebral atrophy in myotonic dystrophy: a voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2004;75:1611–3. doi: 10.1136/jnnp.2003.032417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–67. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3’ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caramia F, Mainero C, Gragnani F, Tinelli E, Fiorelli M, Ceschin V, et al. Functional MRI changes in the central motor system in myotonic dystrophy type 1. Magn Reson Imaging. 2010;28:226–34. doi: 10.1016/j.mri.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Catani M. Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Curr Opin Neurol. 2006;19:599–606. doi: 10.1097/01.wco.0000247610.44106.3f. [DOI] [PubMed] [Google Scholar]
- Ciafaloni E, Mignot E, Sansone V, Hilbert JE, Lin L, Lin X, et al. The hypocretin neurotransmission system in myotonic dystrophy type 1. Neurology. 2008;70:226–30. doi: 10.1212/01.wnl.0000296827.20167.98. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, et al. White matter tract integrity in aging and Alzheimer's disease. Hum Brain Mapp. 2009;30:1051–9. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JW, Ranum LP. RNA pathogenesis of the myotonic dystrophies. Neuromuscul Disord. 2005;15:5–16. doi: 10.1016/j.nmd.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Dhaenens CM, Schraen-Maschke S, Tran H, Vingtdeux V, Ghanem D, Leroy O, et al. Overexpression of MBNL1 fetal isoforms and modified splicing of Tau in the DM1 brain: two individual consequences of CUG trinucleotide repeats. Exp Neurol. 2008;210:467–78. doi: 10.1016/j.expneurol.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Dickinson CJ. Chronic fatigue syndrome–aetiological aspects. Eur J Clin Invest. 1997;27:257–67. doi: 10.1046/j.1365-2362.1997.1120664.x. [DOI] [PubMed] [Google Scholar]
- Di Costanzo A, Di Salle F, Santoro L, Bonavita V, Tedeschi G. T2 relaxometry of brain in myotonic dystrophy. Neuroradiology. 2001;43:198–204. doi: 10.1007/s002340000459. [DOI] [PubMed] [Google Scholar]
- Di Costanzo A, Santoro L, de Cristofaro M, Manganelli F, Di Salle F, Tedeschi G. Familial aggregation of white matter lesions in myotonic dystrophy type 1. Neuromuscul Disord. 2008;18:299–305. doi: 10.1016/j.nmd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Dineen RA, Vilisaar J, Hlinka J, Bradshaw CM, Morgan PS, Constantinescu CS, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–49. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Hoppe C, Schlegel U, Elger CE, Helmstaedter C. NeuroCogFX – A computer-based neuropsychological assessment battery for the follow-up examination of neurological patients. Fortschr Neurol Psychiatr. 2006;74:643–50. doi: 10.1055/s-2006-932162. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Horiguchi J, Ono C, Ohshita T, Takaba J, Ito K. Diffusion tensor imaging of cerebral white matter in patients with myotonic dystrophy. Acta Radiol. 2005;46:104–9. doi: 10.1080/02841850510015974. [DOI] [PubMed] [Google Scholar]
- Gaul C, Schmidt T, Windisch G, Wieser T, Müller T, Vielhaber S, et al. Subtle cognitive dysfunction in adult onset myotonic dystrophy type 1 (DM1) and type 2 (DM2) Neurology. 2006;67:350–2. doi: 10.1212/01.wnl.0000225180.27833.c1. [DOI] [PubMed] [Google Scholar]
- Ghanem D, Tran H, Dhaenens CM, Schraen-Maschke S, Sablonnière B, Buée L, et al. Altered splicing of Tau in DM1 is different from the foetal splicing process. FEBS Lett. 2009;583:675–9. doi: 10.1016/j.febslet.2008.12.065. [DOI] [PubMed] [Google Scholar]
- Giubilei F, Antonini G, Bastianello S, Morino S, Paolillo A, Fiorelli M, et al. Excessive daytime sleepiness in myotonic dystrophy. J Neurol Sci. 1999;164:60–3. doi: 10.1016/s0022-510x(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Miyazaki M, Murakawa K, Kawai H, Nishitani H, et al. Neuroimaging study of myotonic dystrophy. I. Magnetic resonance imaging of the brain. Brain Dev. 1995;17:24–7. doi: 10.1016/0387-7604(94)00096-g. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. The Beck Depressive Inventar (BDI) Bern: Hans Huber; 1995. [Google Scholar]
- Hilton-Jones D. Myotonic dystrophy–forgotten aspects of an often neglected condition. Curr Opin Neurol. 1997;10:399–401. doi: 10.1097/00019052-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Fließbach K, Schlegel U, Elger CE, Helmstaedter C. NeuroCog FX - computerized screening of cognitive functions in epilepsy patients. Epilepsy Behav. 2009;16:298–310. doi: 10.1016/j.yebeh.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkilä K. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3:52–9. doi: 10.1111/j.1365-2869.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Hund E, Jansen O, Koch MC, Ricker K, Fogel W, Niedermaier N, et al. Proximal myotonic myopathy with MRI white matter abnormalities of the brain. Neurology. 1997;48:33–7. doi: 10.1212/wnl.48.1.33. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mitani M, Kawamoto K, Futamura N, Funakawa I, Jinnai K, et al. Neuropathology does not correlate with regional differences in the extent of expansion of CTG repeats in the brain with myotonic dystrophy type 1. Acta Histochem Cytochem. 2010;43:149–56. doi: 10.1267/ahc.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Mankodi A, Swanson MS, Moxley RT, Thornton CA. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum Mol Genet. 2004;13:3079–88. doi: 10.1093/hmg/ddh327. [DOI] [PubMed] [Google Scholar]
- Jin J, Wang GL, Salisbury E, Timchenko L, Timchenko NA. GSK3beta-cyclin D3-CUGBP1-eIF2 pathway in aging and in myotonic dystrophy. Cell Cycle. 2009;8:2356–9. doi: 10.4161/cc.8.15.9248. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Breslau N, Roth T, Roehrs T, Rosenthal L. Psychometric evaluation of Daytime Sleepiness and Nocturnal Sleep onset Scales in a representative community sample. Biol Psychiatry. 1999;45:764–70. doi: 10.1016/s0006-3223(98)00111-5. [DOI] [PubMed] [Google Scholar]
- Kalkman JS, Schillings ML, van der Werf SP, Padberg GW, Zwarts MJ, van Engelen BG, et al. Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy, and HMSN-I. J Neurol Neurosurg Psychiatry. 2005;76:1406–9. doi: 10.1136/jnnp.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Hoffmann S, Rosenbohm A, Kurt A, Jurkat-Rott K, et al. Quantification of brain atrophy in patients with myotonic dystrophy and proximal myotonic myopathy: a controlled 3-dimensional magnetic resonance imaging study. Neurosci Lett. 2003;348:73–6. doi: 10.1016/s0304-3940(03)00740-7. [DOI] [PubMed] [Google Scholar]
- Klinke I, Minnerop M, Schmitz-Hübsch T, Hendriks M, Klockgether T, Wüllner U, et al. Neuropsychological features of patients with spinocerebellar ataxia (SCA) type 1, 2, 3 and 6. Cerebellum. 2010;9:433–42. doi: 10.1007/s12311-010-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum C, Reul J, Kress W, Grothe C, Amanatidis N, Klockgether T, et al. Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. J Neurol. 2004;251:710–4. doi: 10.1007/s00415-004-0408-1. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Kuo HC, Hsieh YC, Wang HM, Chuang WL, Huang CC. Correlation among subcortical white matter lesions, intelligence and CTG repeat expansion in classic myotonic dystrophy type 1. Acta Neurol Scand. 2008;117:101–7. doi: 10.1111/j.1600-0404.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- Laberge L, Bégin P, Dauvilliers Y, Beaudry M, Laforte M, Jean S, et al. A polysomnographic study of daytime sleepiness in myotonic dystrophy type 1. J Neurol Neurosurg Psychiatry. 2009a;80:642–6. doi: 10.1136/jnnp.2008.165035. [DOI] [PubMed] [Google Scholar]
- Laberge L, Dauvilliers Y, Bégin P, Richer L, Jean S, Mathieu J. Fatigue and daytime sleepiness in patients with myotonic dystrophy type 1: to lump or split? Neuromuscul Disord. 2009b;19:397–402. doi: 10.1016/j.nmd.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010;52:20–31. doi: 10.1016/j.neuroimage.2010.03.072. [DOI] [PubMed] [Google Scholar]
- Lehrl S, Fischer B. Kurztest für cerebrale Insuffizienz. Göttingen: Hogrefe; 1997. [Google Scholar]
- Leroy O, Wang J, Maurage CA, Parent M, Cooper T, Buée L, et al. Brain-specific change in alternative splicing of Tau exon 6 in myotonic dystrophy type 1. Biochim Biophys Acta. 2006;1762:460–7. doi: 10.1016/j.bbadis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–7. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- Machuca-Tzili L, Brook D, Hilton-Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve. 2005;32:1–18. doi: 10.1002/mus.20301. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. 2010;183:144–50. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rodríguez JE, Lin L, Iranzo A, Genis D, Martí MJ, Santamaria J, et al. Decreased hypocretin-1 (Orexin-A) levels in the cerebrospinal fluid of patients with myotonic dystrophy and excessive daytime sleepiness. Sleep. 2003;26:287–90. doi: 10.1093/sleep/26.3.287. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Boivin H, Meunier D, Gaudreault M, Bégin P. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology. 2001;56:336–40. doi: 10.1212/wnl.56.3.336. [DOI] [PubMed] [Google Scholar]
- Maurage CA, Udd B, Ruchoux MM, Vermersch P, Kalimo H, Krahe R, et al. Similar brain tau pathology in DM2/PROMM and DM1/Steinert disease. Neurology. 2005;65:1636–8. doi: 10.1212/01.wnl.0000184585.93864.4e. [DOI] [PubMed] [Google Scholar]
- Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007;36:294–306. doi: 10.1002/mus.20800. [DOI] [PubMed] [Google Scholar]
- Meola G, Sansone V, Perani D, Colleluori A, Cappa S, Cotelli M, et al. Reduced cerebral blood flow and impaired visual-spatial function in proximal myotonic myopathy. Neurology. 1999;53:1042–50. doi: 10.1212/wnl.53.5.1042. [DOI] [PubMed] [Google Scholar]
- Meola G, Sansone V, Perani D, Scarone S, Cappa S, Dragoni C, et al. Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM-1) and in proximal myotonic myopathy (PROMM/DM-2) Neuromuscul Disord. 2003;13:813–21. doi: 10.1016/s0960-8966(03)00137-8. [DOI] [PubMed] [Google Scholar]
- Minnerop M, Luders E, Specht K, Ruhlmann J, Schneider-Gold C, Schröder R, et al. Grey and white matter loss along cerebral midline structures in myotonic dystrophy type 2. J Neurol. 2008;255:1904–9. doi: 10.1007/s00415-008-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka T, Watanabe C, Kitamura J, Ishigame K, Nakamura S. Movement-related cortical potentials in myotonic dystrophy. Clin Neurophysiol. 2003;114:99–106. doi: 10.1016/s1388-2457(02)00325-5. [DOI] [PubMed] [Google Scholar]
- Modoni A, Silvestri G, Pomponi MG, Mangiola F, Tonali PA, Marra C. Characterization of the pattern of cognitive impairment in myotonic dystrophy type 1. Arch Neurol. 2004;61:1943–7. doi: 10.1001/archneur.61.12.1943. [DOI] [PubMed] [Google Scholar]
- Modoni A, Silvestri G, Vita MG, Quaranta D, Tonali PA, Marra C. Cognitive impairment in myotonic dystrophy type 1 (DM1) : a longitudinal follow-up study. J Neurol. 2008;255:1737–42. doi: 10.1007/s00415-008-0017-5. [DOI] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15:553–60. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Naka H, Imon Y, Ohshita T, Honjo K, Kitamura T, Mimori Y, et al. Magnetization transfer measurements of cerebral white matter in patients with myotonic dystrophy. J Neurol Sci. 2002;193:111–6. doi: 10.1016/s0022-510x(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Ogata A, Terae S, Fujita M, Tashiro K. Anterior temporal white matter lesions in myotonic dystrophy with intellectual impairment: an MRI and neuropathological study. Neuroradiology. 1998;40:411–5. doi: 10.1007/s002340050613. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Brighina F, La Bua V, Aloisio A, Buffa D, Fierro B. Magnetic stimulation study in patients with myotonic dystrophy. Electroencephalogr Clin Neurophysiol. 1997;105:297–301. doi: 10.1016/s0924-980x(97)00023-4. [DOI] [PubMed] [Google Scholar]
- Ono S, Inoue K, Mannen T, Kanda F, Jinnai K, Takahashi K. Neuropathological changes of the brain in myotonic dystrophy–some new observations. J Neurol Sci. 1987;81:301–20. doi: 10.1016/0022-510x(87)90105-5. [DOI] [PubMed] [Google Scholar]
- Ono S, Takahashi K, Jinnai K, Kanda F, Fukuoka Y, Kurisaki H, et al. Loss of catecholaminergic neurons in the medullary reticular formation in myotonic dystrophy. Neurology. 1998a;51:1121–4. doi: 10.1212/wnl.51.4.1121. [DOI] [PubMed] [Google Scholar]
- Ono S, Takahashi K, Jinnai K, Kanda F, Fukuoka Y, Kurisaki H, et al. Loss of serotonin-containing neurons in the raphe of patients with myotonic dystrophy: a quantitative immunohistochemical study and relation to hypersomnia. Neurology. 1998b;50:535–8. doi: 10.1212/wnl.50.2.535. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14:203–22. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Ota M, Sato N, Ohya Y, Aoki Y, Mizukami K, Mori T, et al. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci Lett. 2006;407:234–9. doi: 10.1016/j.neulet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Oyamada R, Hayashi M, Katoh Y, Tsuchiya K, Mizutani T, Tominaga I, et al. Neurofibrillary tangles and deposition of oxidative products in the brain in cases of myotonic dystrophy. Neuropathology. 2006;26:107–14. doi: 10.1111/j.1440-1789.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- Park JD, Radtke RA. Hypersomnolence in myotonic dystrophy: demonstration of sleep onset REM sleep. J Neurol Neurosurg Psychiatry. 1995;58:512–3. doi: 10.1136/jnnp.58.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo V, Pegoraro E, Ferrati C, Squarzanti F, Sorarù G, Palmieri A, et al. Brain involvement in myotonic dystrophies: neuroimaging and neuropsychological comparative study in DM1 and DM2. J Neurol. 2010;257:1246–55. doi: 10.1007/s00415-010-5498-3. [DOI] [PubMed] [Google Scholar]
- Romigi A, Izzi F, Pisani V, Placidi F, Pisani LR, Marciani MG, et al. Sleep disorders in adult-onset myotonic dystrophy type 1: a controlled polysomnographic study. Eur J Neurol. 2011 doi: 10.1111/j.1468-1331.2011.03352.x. Advance Access published on February 21, 2011, doi: 10.1111/j.1468-1331.2011.03352.x. [DOI] [PubMed] [Google Scholar]
- Rosman NP, Kakulas BA. Mental deficiency associated with muscular dystrophy. A neuropathological study. Brain. 1966;89:769–88. doi: 10.1093/brain/89.4.769. [DOI] [PubMed] [Google Scholar]
- Rubinsztein JS, Rubinsztein DC, Goodburn S, Holland AJ. Apathy and hypersomnia are common features of myotonic dystrophy. J Neurol Neurosurg Psychiatry. 1998;64:510–5. doi: 10.1136/jnnp.64.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant N, Sablonniere B, Schraen-Maschke S, Ghestem A, Maurage CA, Wattez A, et al. Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Hum Mol Genet. 2001;10:2143–55. doi: 10.1093/hmg/10.19.2143. [DOI] [PubMed] [Google Scholar]
- Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tieleman AA, Knoop H, van de Logt AE, Bleijenberg G, van Engelen BG, Overeem S. Poor sleep quality and fatigue but no excessive daytime sleepiness in myotonic dystrophy type 2. J Neurol Neurosurg Psychiatry. 2010;81:963–7. doi: 10.1136/jnnp.2009.192591. [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue pegboard, examiner's manual. Chicago: Science Research Associates, Inc.; 1987. [Google Scholar]
- Toscano A, Messina S, Campo GM, Di Leo R, Musumeci O, Rodolico C, et al. Oxidative stress in myotonic dystrophy type 1. Free Radic Res. 2005;39:771–6. doi: 10.1080/10715760500138932. [DOI] [PubMed] [Google Scholar]
- van der Meché FG, Bogaard JM, van der Sluys JC, Schimsheimer RJ, Ververs CC, Busch HF. Daytime sleep in myotonic dystrophy is not caused by sleep apnoea. J Neurol Neurosurg Psychiatry. 1994;57:626–8. doi: 10.1136/jnnp.57.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf S, Kalkman J, Bleijenberg G, van Engelen B, Schillings M, Zwarts M, Netherlands Fatigue Research Group The relation between daytime sleepiness, fatigue, and reduced motivation in patients with adult onset myotonic dystrophy. J Neurol Neurosurg Psychiatry. 2003;74:138–9. doi: 10.1136/jnnp.74.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermersch P, Sergeant N, Ruchoux MM, Hofmann-Radvanyi H, Wattez A, Petit H, et al. Specific tau variants in the brains of patients with myotonic dystrophy. Neurology. 1996;47:711–7. doi: 10.1212/wnl.47.3.711. [DOI] [PubMed] [Google Scholar]
- Vielhaber S, Jakubiczka S, Gaul C, Schoenfeld MA, Debska-Vielhaber G, Zierz S, et al. Brain (1)H magnetic resonance spectroscopic differences in myotonic dystrophy type 2 and type 1. Muscle Nerve. 2006;34:145–52. doi: 10.1002/mus.20565. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- Weber YG, Roebling R, Kassubek J, Hoffmann S, Rosenbohm A, Wolf M, et al. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology. 2010;74:1108–17. doi: 10.1212/WNL.0b013e3181d8c35f. [DOI] [PubMed] [Google Scholar]
- Winblad S, Jensen C, Månsson JE, Samuelsson L, Lindberg C. Depression in myotonic dystrophy type 1: clinical and neuronal correlates. Behav Brain Funct. 2010;6:25. doi: 10.1186/1744-9081-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski HM, Berry K, Spiro AJ. Ultrastructure of thalamic neuronal inclusions in myotonic dystrophy. J Neurol Sci. 1975;24:321–9. doi: 10.1016/0022-510x(75)90252-x. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Ward EE, Lim KO, Day JW. White matter abnormalities and neurocognitive correlates in children and adolescents with myotonic dystrophy type 1: a diffusion tensor imaging study. Neuromuscul Disord. 2011;21:89–96. doi: 10.1016/j.nmd.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Laberge L, Jaussent I, Bayard S, Scholtz S, Raoul M, et al. Daytime sleepiness and REM sleep characteristics in myotonic dystrophy: a case-control study. Sleep. 2011;34:165–70. doi: 10.1093/sleep/34.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.