Abstract
AIM: To examine the links between quality of sleep and the severity of intestinal symptoms in irritable bowel syndrome (IBS).
METHODS: One hundred and forty-two outpatients (110 female, 32 male) who met the Rome III criteria for IBS with no psychiatric comorbidity were consecutively enrolled in this study. Data on age, body mass index (BMI), and a set of life-habit variables were recorded, and IBS symptoms and sleep quality were evaluated using the questionnaires IBS Symptom Severity Score (IBS-SSS) and Pittsburgh Sleep Quality Index (PSQI). The association between severity of IBS and sleep disturbances was evaluated by comparing the global IBS-SSS and PSQI score (Pearson’s correlation and Fisher’s exact test) and then analyzing the individual items of the IBS-SSS and PSQI questionnaires by a unitary bowel-sleep model based on item response theory (IRT).
RESULTS: IBS-SSS ranged from mild to severe (120-470). The global PSQI score ranged from 1 to 17 (median 5), and 60 patients were found to be poor sleepers (PSQI > 5). The correlation between the global IBS-SSS and PSQI score indicated a weak association (r = 0.2 and 95% CI: -0.03 to 0.35, P < 0.05), which becomes stronger using our unitary model. Indeed, the IBS and sleep disturbances severities, estimated as latent variables, resulted significantly high intra-subject correlation (posterior mean of r = 0.45 and 95% CI: 0.17 to 0.70, P < 0.05). Moreover, the correlations between patient features (age, sex, BMI, daily coffee and alcohol intake) and IBS and sleep disturbances were also analyzed through our unitary model. Age was a significant regressor, with patients ≤ 50 years old showing more severe bowel disturbances (posterior mean = -0.38, P < 0.05) and less severe sleep disturbances (posterior mean = 0.49, P < 0.05) than older patients. Higher daily coffee intake was correlated with a lower severity of bowel disturbances (posterior mean = -0.31, P < 0.05). Sex (female) and daily alcohol intake (modest) were correlated with less severe sleep disturbances.
CONCLUSION: The unitary bowel-sleep model based on IRT revealed a strong positive correlation between the severity of IBS symptoms and sleep disturbances.
Keywords: Irritable bowel syndrome, Sleep disorders, Item response theory model, Bayesian model
INTRODUCTION
Irritable bowel syndrome (IBS) is quite prevalent in the general population (5%-20%) and is in fact the functional gastrointestinal disorder most frequently encountered in primary and secondary care[1,2]. IBS is characterized by abdominal discomfort, pain and changes in bowel habits (constipation and/or diarrhea)[3]. Visceral hypersensitivity, slowed gastrointestinal transit, and alterations in secretion activity are also often reported[4]. The direct and indirect costs of the syndrome are significant. IBS can have a serious impact on quality of life, and because it is often associated with other disorders, the patient may have to undergo expensive tests and treatments[5-7].
Sleep disturbances connected with gastrointestinal functional disorders, in particular IBS, have been reported[8-10], but no consistent abnormality in sleep patterns has been identified, apart from a significant increase in rapid-eye-movement (REM) sleep[11,12]. Many studies have associated sleep alterations with severity of IBS symptoms[12], but it is not yet known whether there is a direct pathophysiological relationship[13,14]. To date, a fundamental question remains - is sleep disturbance a cause or a consequence of IBS[15]?
Growing awareness of the importance of the “brain-gut axis” has led some gastroenterologists to investigate sleep as an independent factor in the pathogenesis and clinical expression of IBS[11,16].
The aim of the present study was to evaluate the association between the severity of sleep disturbances and intestinal symptoms, measured using two widely accepted questionnaires: the IBS Symptom Severity Score (IBS-SSS)[17] and the Pittsburgh Sleep Quality Index (PSQI)[18]. In addition, individual patient features were also analyzed to investigate their possible interaction with the severity of bowel and sleep disturbances and to exclude their potential confounding role.
MATERIALS AND METHODS
Study patients
Between October 2007 and September 2008, 142 patients who met the Rome III criteria for IBS[3] with no confounding psychiatric comorbidity (diagnosed according to DSM IV axis I criteria) were consecutively enrolled from outpatients attending the Gastrointestinal Unit of the University of Pisa. All patients gave their informed written consent as required by the University Ethics Committee for Clinical Studies. The cohort was comprised of 110 female and 32 male patients (median age: 38 years; range: 18-79 years), of whom, 52 had constipation as the predominant symptom (C-IBS), 48 had diarrhea as the predominant symptom (D-IBS), and 42 had mixed symptoms (M-IBS).
We also evaluated physical and life-habit variables in each patient (Table 1): (1) sex and age; (2) body mass index (BMI)[19]; (3) smoking; (4) alcohol intake[20]; (5) coffee intake; (6) physical activity; and (7) water intake.
Table 1.
Clinical subtypes, physical and life-habit variables of irritable bowel syndrome patients
| Item categories | Patients (%) | |
| IBS subtype | M-IBS | 29.6 |
| D-IBS | 33.8 | |
| C-IBS | 36.6 | |
| Age (yr) | ≤ 50 | 78.9 |
| > 50 | 21.1 | |
| Sex | Male | 22.5 |
| Female | 77.5 | |
| BMI (kg/m2) | ≤ 25 | 76.1 |
| > 25 | 23.9 | |
| Smoking | No | 74.6 |
| Yes | 25.4 | |
| Alcohol consumption | Non-/occasional drinkers | 82.4 |
| Light drinkers (< 30 g ethanol/d) | 17.6 | |
| Coffee consumption | < 2 cups/d | 55.6 |
| ≥ 2 cups/d | 44.4 | |
| Physical activity | No | 66.2 |
| Yes | 33.8 | |
| Water consumption | < 1.5 L/d | 61.3 |
| ≥ 1.5 L/d | 38.7 |
IBS: Irritable bowel syndrome; BMI: Body mass index.
IBS and sleep questionnaires
IBS symptoms were evaluated using the IBS-SSS questionnaire[17], which measured five separate items (Table 2): (1) presence and severity of abdominal pain or discomfort; (2) frequency of abdominal pain or discomfort; (3) presence and severity of abdominal distension; (4) degree of satisfaction with defecatory function; and (5) degree of interference of IBS symptoms with daily lifestyle. For each of the five items, the questions generated a score ranging from 0 to 100 that, for the purposes of analysis, was divided into three consecutive intervals: low, medium, and high (Table 2). The scores for the five items were summed to arrive at a global IBS-SSS (range: 0-500) and the IBS severity was then classified as mild (75-175), moderate (175-300) or severe (≥ 300).
Table 2.
Distribution of responses to the items in the irritable bowel syndrome Symptom Severity Score across categories (low, medium and high)
| IBS-SSS items | Category (score interval) | Patients (%) |
| Pain severity | Low: ≤ 40 | 31.0 |
| Medium: 40-70 | 43.7 | |
| High: > 70 | 25.4 | |
| Pain frequency | Low: ≤ 20 | 19.7 |
| Medium: 20-70 | 33.8 | |
| High: > 70 | 46.5 | |
| Distension | Low: ≤ 40 | 30.3 |
| Medium: 40-70 | 28.2 | |
| High: > 70 | 41.5 | |
| Dissatisfaction with defecatory function | Low: ≤ 40 | 12.7 |
| Medium: 40-70 | 26.8 | |
| High: > 70 | 60.6 | |
| Interference with daily life style | Low: ≤ 40 | 28.9 |
| Medium: 40-70 | 32.4 | |
| High: > 70 | 38.7 |
IBS-SSS: Irritable bowel syndrome Symptom Severity Score.
Sleep quality was evaluated using the PSQI[18], a self-administered questionnaire based on seven items: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep inducers, and daytime dysfunction. Each item was scored from 0 to 3, and the PSQI global index was calculated by summing these scores (range: 0-21); a global score > 5 identified poor sleepers.
In both questionnaires, lower values indicated lower severity, and higher values a more severe condition.
Statistical analysis
The association between the severity of IBS symptoms and sleep disturbances was first verified by directly comparing the global IBS-SSS and PSQI score, and then by evaluating the links between individual items in the two questionnaires.
For the first approach, we used both the Pearson correlation coefficient and the generalized Fisher exact test[21]. The Pearson correlation measured the linear dependence between global scores, whereas the Fisher exact test verified the association between IBS-SSS and PSQI through a contingency table.
We adopted a unitary bowel-sleep model based on item response theory (IRT) that enabled us to investigate directly the dependences between IBS-SSS and PSQI single items. Furthermore, we also evaluated the weight of patient features and life habits in affecting the severity of IBS and sleep disturbances.
By focusing on individual items as the unit of analysis rather than the global score, the IRT model could circumvent the problem of different patterns of responses generating identical global scores. To this end, the values of both IBS-SSS single items and patient features were transformed in categories as indicated in the Tables 1 and 2. At variance, the PSQI items were not transformed since each item could have only four values (0-3), thus PSQI single item categories corresponded to the item values.
The output of our IRT model was the estimate of two latent variables: one related to the severity of IBS, and the other to sleep disturbances. In order to evaluate the possible intra-subject associations between IBS and sleep disturbances, the latent variables for the two were jointly modeled using a bivariate normal distribution. The strength of this association was quantified by the covariance parameter. Specifically, we adopted the partial credit model[22], which could be used for the polytomous ordered categories for each item. The partial credit model postulated that the probability of a given response with respect to the next response was a function (logistic curve) of the severity of the subject’s symptoms and of structural parameters. The severity of symptoms, considered as a latent variable, was assumed to have a normal distribution[23]. Finally, we used a regression model to remove the effects of the patient features from the latent severities, and to identify the features with a significant effect on each latent severity variable.
In the partial credit model analysis, a sum-to-zero constraint was imposed on the structural parameters[24]. The model was estimated within a Bayesian framework by means of a Markov-chain Monte Carlo algorithm with Gibbs sampling (using WinBUGS 1.4)[25]. This estimation required that one specified the prior distributions of all parameters, but in the present study, no such a priori information was available; thus non-informative priors (i.e., parameter-flat a priori distributions) were used.
The Bayesian method enabled us to estimate the distribution of each parameter (posterior distribution), from which we could derive the central tendency (posterior mean) along with the Bayesian confidence (or Credibility) interval (CI).
In summary, the unitary bowel-sleep model yielded estimates for each patient of the latent severity of their IBS and sleep disturbances, with their covariance parameters.
Finally, the mean latent severity of IBS and sleep disturbances were correlated with patient features (including IBS subtypes). One category was maintained as the reference and we verified whether the regression coefficient (posterior mean), describing the change from the reference to every other category, was significantly different from zero.
RESULTS
Analysis of the global scores
Patients had IBS-SSS ranging from 120 to 470 (median 280): 10 subjects were classified as mild, 69 as moderate, and 63 as severe. In Table 2, the distribution of responses to the items in the IBS-SSS across categories (low, medium and high) is shown.
The sleep quality in our patients ranged from 1 to 17, with a median PSQI score of 5. Sixty patients were poor sleepers (global PSQI > 5). The distribution of the scores for the items in the PSQI is shown in Table 3.
Table 3.
Distribution of responses to Pittsburgh Sleep Quality Index items
| PSQI items | Item scores | Patients (%) |
| Subjective sleep quality | 0 | 14.1 |
| 1 | 62.7 | |
| 2 | 20.4 | |
| 3 | 2.8 | |
| Sleep latency | 0 | 52.8 |
| 1 | 28.2 | |
| 2 | 14.1 | |
| 3 | 4.9 | |
| Sleep duration | 0 | 43.7 |
| 1 | 35.9 | |
| 2 | 14.8 | |
| 3 | 5.6 | |
| Habitual sleep efficiency | 0 | 54.9 |
| 1 | 33.8 | |
| 2 | 7.7 | |
| 3 | 3.5 | |
| Sleep disturbances | 0 | 8.5 |
| 1 | 81.0 | |
| 2 | 9.9 | |
| 3 | 0.7 | |
| Use of sleeping medication | 0 | 81.0 |
| 1 | 3.5 | |
| 2 | 2.1 | |
| 3 | 13.4 | |
| Daytime dysfunction | 0 | 31.0 |
| 1 | 50.7 | |
| 2 | 13.4 | |
| 3 | 4.9 |
PSQI: Pittsburgh Sleep Quality Index.
The correlation between the severity of IBS and sleep disturbances was evaluated by comparing the global IBS-SSS and PSQI scores. Pearson’s r was found to be 0.2 (95% CI: 0.03 to 0.35), indicating a weak significant linear relationship between the two scores. Analysis of a contingency table for IBS-SSS vs PSQI categories using Fisher’s exact test failed to detect any association between the two indices (P = 0.18) (Table 4).
Table 4.
Contingency table for the global irritable bowel syndrome Symptom Severity Score and global Pittsburgh Sleep Quality Index score
|
Global PSQI score |
||
| Good | Poor | |
| Global IBS-SSS | ||
| Mild | 7 | 3 |
| Moderate | 44 | 25 |
| Severe | 31 | 32 |
IBS-SSS: Irritable bowel syndrome Symptom Severity Score; PSQI: Pittsburgh Sleep Quality Index.
Analysis of the latent severities
To complete the study of the questionnaires, the analysis was extended by modeling the responses to each single item with the partial credit models, and the regression analysis to determine the effect of the individual features on the estimated severity of IBS and sleep disturbances.
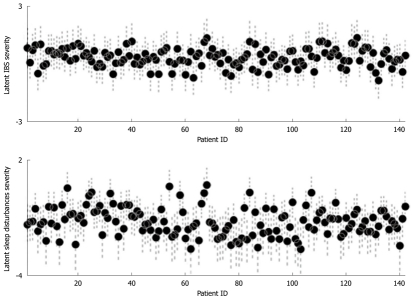
In Figure 1, the posterior means of the severity of IBS and sleep disturbances (expressed as latent variables) for the 142 patients are plotted (with their 95% CIs). The latent severity of sleep disturbances was a better discriminant between subjects than IBS, since it showed a more marked between-subject variability: all of the patients had a priori IBS symptoms, whereas the quality of their sleep was measured for the first time in this study.
Figure 1.
Irritable bowel syndrome latent severity in each patient, with 95% CI (top) and sleep disturbances latent severity in each patient, with 95% CI (bottom). Error bars indicate CI. ID indicates the label of each patient. IBS: Irritable bowel syndrome. CI: Confidence Interval.
Our unitary bowel-sleep model demonstrated a significant association between the severity of bowel symptoms and sleep disturbances. In contrast to the results of the global score comparison, a significant intra-subject correlation was found (posterior mean r = 0.45 and 95% CI: 0.17 to 0.70).
Figure 2 shows two scatter plots, on the left the IBS-SSS and PSQI global scores, whereas on the right, the two latent severities. The usage of the unitary bowel-sleep model provided a relationship between latent sleep disturbances severity and latent IBS severity. At variance, the usage of global scores did not provide any information about sleep and bowel dysfunction interactions. In the right panel of Figure 2, each black dot corresponds to a single patient and the dashed grey cross indicates the respective CI. Despite the variability between patients, the correlation between the two latent severities was evident, while the scatter plot on the left panel did not confirm any association between the global scores.
Figure 2.
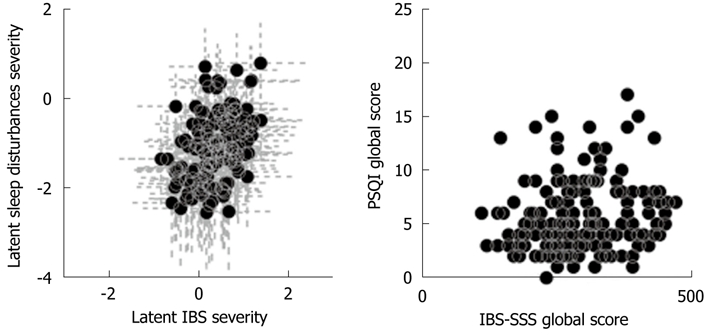
Scatter plot of the global Pittsburgh Sleep Quality Index score vs the global irritable bowel syndrome-Symptom Severity Score (left panel), and of sleep disturbances and irritable bowel syndrome latent severities (dots) (right panel). The grey lines indicate the 95% CI. CI: Confidence interval.
Furthermore, the unitary bowel-sleep model allowed us to identify a significant regression between IBS and sleep disturbances latent severities and patient features (Table 5).
Table 5.
For each patient, feature regression coefficients (posterior means) and 95% confidence interval for the latent severities of both irritable bowel syndrome and sleep disturbances are shown
| Patient features | Categories | IBS latent severity, coef (95% CI) | Sleep disturbances latent severity, coef (95% CI) |
| IBS subtype | Mixed | 0 | 0 |
| Diarrhoea | -0.10 (-0.45 to 0.24) | 0.02 (-0.42 to 0.46) | |
| Constipation | 0.32 (-0.05 to 0.69) | 0.16 (-0.30 to 0.65) | |
| Age (yr) | ≤ 50 | 0 | 0 |
| > 50 | -0.38 (-0.75 to -0.02)1 | 0.49 (0.02 to 0.96)1 | |
| Sex | Male | 0 | 0 |
| Female | 0.10 (-0.24 to 0.46) | -0.53 (-0.95 to -0.07)1 | |
| BMI (kg/m2) | ≤ 25 | 0 | 0 |
| > 25 | 0.04 (-0.32 to 0.38) | 0.09 (-0.33 to 0.52) | |
| Smoking | No | 0 | 0 |
| Yes | 0.07 (-0.27 to 0.41) | -0.04 (0.45 to 0.38) | |
| Alcohol consumption | Non-/occasional drinkers | 0 | 0 |
| Light drinkers | -0.22 (-0.60 to 0.16) | -0.60 (-1.08 to -0.12)1 | |
| Coffee consumption | < 2 cups/d | 0 | 0 |
| ≥ 2 cups/d | -0.31 (-0.60 to -0.01)1 | -0.08 (-0.44 to 0.26) | |
| Physical activity | No | 0 | 0 |
| Yes | -0.18 (-0.48 to 0.11) | -0.29 (-0.68 to 0.08) | |
| Water consumption | < 1.5 L/d | 0 | 0 |
| ≥ 1.5 L/d | 0.27 (-0.03 to 0.56) | 0.07 (-0.29 to 0.42) |
Significant differences between covariables. IBS: Irritable bowel syndrome. CI: Confidence interval; BMI: Body mass index.
The significance of a feature effect corresponded to a significant regression coefficient, and the sign of this coefficient indicated the direction of the relationship: a positive sign signified that the change of category (from the reference one to the other) came with a severity increase and vice versa; the 95% CI indicated the significance of the coefficient.
Among the individual features, age was a significant regressor for both latent severities; young patients (≤ 50 years old) showed more severe bowel disturbances (posterior mean = -0.38) and less severe sleep disturbances (posterior mean = 0.49) than older patients. Daily coffee intake was significantly correlated with severity of bowel disturbances (posterior mean = -0.31); subjects who consumed ≥ 2 cups per day showed less severe bowel disturbances. Sex and daily alcohol intake were found to be significantly correlated with severity of sleep disturbances; female patients exhibited less severe sleep disturbances than male patients, while non-drinkers showed more severe sleep disturbances than those with a moderate alcohol intake. No other life-habit variables were found to be significantly correlated with latent severity of IBS or sleep disturbances.
DISCUSSION
The aim of this study was to evaluate the mutual influences between sleep disturbances and IBS in a large sample of IBS patients. The study provides a detailed statistical analysis of the possible association between bowel disorders, sleep disturbances and life habits.
Our sample may be considered as representative of the IBS population in Italy (this was confirmed by the distribution of IBS subtypes, age and BMI in the cohort). Therefore, our results can be compared and discussed in the light of the results of other studies on IBS patients.
Despite the fact that 42% of the participants had a global PSQI score > 5, only a weak and ambiguous association between IBS-SSS categories and PSQI categories was evident by using standard statistical analysis. Indeed, the correlation coefficient between the global scores was weakly significant and the Fischer exact test did not yield any association.
Conversely a strong link was highlighted by applying the IRT model[26,27] and linear regression.
IRT provides a framework to detect the effect of a series of variables (such as the responses to items on psychological tests) on latent traits of interest (IBS and sleep disturbances). In standard data analyses, the patient’s raw score is calculated by summing the scores for the different items, without taking into account differences in the responses to specific items by different patients. It may arise that two patients have the same raw global score but different patterns of symptoms, or different raw global scores but similar symptom patterns. The inability to discriminate between the two possibilities can be resolved by using IRT model, which focuses on individual items rather than the global score. In addition, IRT allows one to introduce covariates (in our case age, BMI, and life habits) and analyze their effect on the parameters of interest.
The IRT model identified a latent link between sleep disturbances and IBS symptoms. These results showed that IBS patients suffered from a considerable degree of sleep impairment, and are in line with those of other studies[8,28,29]. In particular, a recent survey found that sleep disturbances were an independent predictor of IBS in nurses[8].
Through IRT analysis, we also determined that older age, male sex, and no alcohol intake were significant predictors of more severe sleep disturbances.
Our study found that female IBS patients complained with fewer sleep disturbances than males. This would appear to be inconsistent with studies in the general population, which show that females are more likely to suffer from sleep disorders[30], more susceptible to the effects of stress on sleep[31], and after menopause, run a higher risk of developing insomnia[30]. The present findings could be explained by the demographics of our sample, which were characterized by a relatively low mean age and hence a higher percentage of fertile women; indeed, IBS patients tend to be relatively young (< 50 years old)[3].
Evaluation of the predictive parameters for the latent severity of sleep disorders showed that age was positively correlated with sleep disturbances. These data come as no surprise, because aging is widely considered to be a triggering factor for insomnia[32-34].
Finally, this study showed that light alcohol intake was correlated with a lower latent severity of sleep disturbances. It has long been known that a little amount of alcohol consumed by healthy individuals before going to sleep shortens sleep latency, reduces REM sleep, and increases non-REM sleep[35-39]. Alcohol is frequently used as a form of self-medication by patients suffering from sleep disorders, especially insomnia[40].
More unexpectedly, we found that younger age and higher coffee intake were associated with less severe IBS symptoms. The first-line treatment for IBS usually consists of a change in lifestyle and diet, and drinking less coffee is often recommended, even though there are no studies demonstrating a link between the consumption of coffee and the severity of IBS symptoms. Sloots et al[41] have reported that the intake of 280 mL of coffee caused no change in rectal compliance or visceral sensibility. However, in our study, coffee drinkers actually consumed no more than two cups per day.
In conclusion, using IRT analysis we found that: (1) there was a positive association between the severity of IBS symptoms and sleep disturbances, and (2) some patient features were significant predictors of severity of IBS and sleep disturbances. These results are consistent with those of other studies[10,14]. The pathophysiological mechanism underlying this association is only partially understood, however. One possibility is that sleep disorders can induce visceral hyperalgesia, which increases the patient perception of gastrointestinal symptoms[8,15]. Several other factors could play a role, including endophenotypic traits such as those associated with stress susceptibility[42].
Further research is needed to clarify whether the association of IBS and sleep disturbances represents comorbidity or the expression of a single disturbance in the brain-gut axis.
ACKNOWLEDGMENTS
The authors are grateful to Lucia Giorgetti and Ilaria Luschi for their valuable help in carrying out the study.
COMMENTS
Background
Sleep disturbances connected with gastrointestinal functional disorders, in particular irritable bowel syndrome (IBS), have been reported and some studies have associated sleep alterations with the severity of IBS symptoms. It is not yet known whether it is a simple comorbidity or if there is a direct pathophysiological relationship. To date, a fundamental question has arisen: is sleep disturbance a cause or a consequence of IBS?
Research frontiers
Sleep disturbances represent a negative factor affecting the outcome of several medical and psychiatric conditions, therefore, growing awareness has led some gastroenterologists to investigate sleep as an independent factor also in the pathogenesis and clinical expression of IBS.
Innovations and breakthroughs
Item response theory (IRT) for the first time has been used to identify latent links between digestive symptoms and sleep quality. Furthermore, this unitary bowel-sleep model enabled us to evaluate better the weight of patient features and life habits in the severity of IBS symptoms and sleep disturbances.
Applications
The results of this study suggest: (1) the use of IRT models for uncovering latent links in clinical comorbidity; (2) the importance of studying sleep quality in functional digestive disorders; and (3) further surveys on the clinical mutual interaction between sleep disturbances and functional digestive disorders (i.e., if the treatment of sleep disturbances improves digestive symptoms and/or vice versa)
Terminology
IRT is a paradigm for the analysis of tests and questionnaires. IRT models are often referred to as “latent trait models”. The term latent is used to emphasize discrete item responses not directly observed but inferred from the manifest responses. IRT brings greater flexibility and provides more sophisticated information and is generally considered an improvement of classical test theory. The Bayesian method enables one to estimate the distribution of each parameter from which one could derive the central tendency.
Peer review
The authors have looked at sleep quality in patients with IBS to evaluate the relationship between digestive symptoms and sleep quality by using IRT analysis. The results are analyzed in detail and the statistical tool of IRT is used to obtain significant associations. The data are solid, the paper is interesting, has clinical significance and is well written.
Footnotes
Peer reviewer: Guang-Yin Xu, MD, PhD, Assistant Professor, Division of Gastroenterology, Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0655, United States
S- Editor Wu X L- Editor Kerr C E- Editor Zheng XM
References
- 1.Bellini M, Tosetti C, Costa F, Biagi S, Stasi C, Del Punta A, Monicelli P, Mumolo MG, Ricchiuti A, Bruzzi P, et al. The general practitioner’s approach to irritable bowel syndrome: from intention to practice. Dig Liver Dis. 2005;37:934–939. doi: 10.1016/j.dld.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Videlock EJ, Chang L. Irritable bowel syndrome: current approach to symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:665–685. doi: 10.1016/j.gtc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Spiller R. Clinical update: irritable bowel syndrome. Lancet. 2007;369:1586–1588. doi: 10.1016/S0140-6736(07)60726-0. [DOI] [PubMed] [Google Scholar]
- 5.Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Nyrop KA, Palsson OS, Levy RL, Korff MV, Feld AD, Turner MJ, Whitehead WE. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead WE, Palsson OS, Levy RR, Feld AD, Turner M, Von Korff M. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102:2767–2776. doi: 10.1111/j.1572-0241.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 8.Cremonini F, Camilleri M, Zinsmeister AR, Herrick LM, Beebe T, Talley NJ. Sleep disturbances are linked to both upper and lower gastrointestinal symptoms in the general population. Neurogastroenterol Motil. 2009;21:128–135. doi: 10.1111/j.1365-2982.2008.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperber AD, Tarasiuk A. Disrupted sleep in patients with IBS--a wake-up call for further research? Nat Clin Pract Gastroenterol Hepatol. 2007;4:412–413. doi: 10.1038/ncpgasthep0847. [DOI] [PubMed] [Google Scholar]
- 10.Robert JJ, Elsenbruch S, Orr WC. Sleep-related autonomic disturbances in symptom subgroups of women with irritable bowel syndrome. Dig Dis Sci. 2006;51:2121–2127. doi: 10.1007/s10620-006-9305-z. [DOI] [PubMed] [Google Scholar]
- 11.Orr WC. Gastrointestinal functioning during sleep: a new horizon in sleep medicine. Sleep Med Rev. 2001;5:91–101. doi: 10.1053/smrv.2000.0149. [DOI] [PubMed] [Google Scholar]
- 12.Heitkemper M, Jarrett M, Burr R, Cain KC, Landis C, Lentz M, Poppe A. Subjective and objective sleep indices in women with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:523–530. doi: 10.1111/j.1365-2982.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett M, Heitkemper M, Cain KC, Burr RL, Hertig V. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Dig Dis Sci. 2000;45:952–959. doi: 10.1023/a:1005581226265. [DOI] [PubMed] [Google Scholar]
- 14.Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26:747–752. doi: 10.1093/sleep/26.6.747. [DOI] [PubMed] [Google Scholar]
- 15.Maneerattanaporn M, Chey WD. Sleep disorders and gastrointestinal symptoms: chicken, egg or vicious cycle? Neurogastroenterol Motil. 2009;21:97–99. doi: 10.1111/j.1365-2982.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu JP, Song ZY, Xu Y, Zhang YM, Shen RH. [Probe into sleep quality in the patients with irritable bowel syndrome] Zhonghua Neike Zazhi. 2010;49:587–590. [PubMed] [Google Scholar]
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Douketis JD, Paradis G, Keller H, Martineau C. Canadian guidelines for body weight classification in adults: application in clinical practice to screen for overweight and obesity and to assess disease risk. CMAJ. 2005;172:995–998. doi: 10.1503/cmaj.045170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakabayashi I. Modification of the association of alcohol drinking with blood pressure by cigarette smoking. Blood Press. 2008;17:87–93. doi: 10.1080/08037050801915492. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson DB, Fan Y, Joe H. A remark on algorithm 643: FEXACT: an algorithm for performing Fisher's exact test in r x c contingency tables. ACM T Math Software. 1993;19:484–488. [Google Scholar]
- 22.Masters GN. A rasch model for partial credit scoring. Psychometrika. 1982;47:149–174. [Google Scholar]
- 23.Skrondal A, Rabe-Hesketh S. Latent variable modelling. Stat Methods Med Res. 2008;17:3–4. doi: 10.1177/0278364907081235. [DOI] [PubMed] [Google Scholar]
- 24.Gelman BA, Hill BJ. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press; 2006. [Google Scholar]
- 25.Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Stat Med. 2003;22:3687–3709. doi: 10.1002/sim.1586. [DOI] [PubMed] [Google Scholar]
- 26.Walls TA, Schafer JL. Models for intensive longitudinal data. New York: Oxford University Press; 2006. pp. 288–289. [Google Scholar]
- 27.Lord FM, Novick MR. Statistical theories of mental test score. Reading, MA: Addison-Welsley Publishing Company; 1968. [Google Scholar]
- 28.Zhen Lu W, Ann Gwee K, Yu Ho K. Functional bowel disorders in rotating shift nurses may be related to sleep disturbances. Eur J Gastroenterol Hepatol. 2006;18:623–627. doi: 10.1097/00042737-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Nojkov B, Hoogerwerf S, Chey WD. The effect of shift work on the prevalence and clinical impact of functional bowel disorders in nurses. Am J Gastroenterol. 2008;103:S469. [Google Scholar]
- 30.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 31.Gadinger MC, Fischer JE, Schneider S, Fischer GC, Frank G, Kromm W. Female executives are particularly prone to the sleep-disturbing effect of isolated high-strain jobs: a cross-sectional study in German-speaking executives. J Sleep Res. 2009;18:229–237. doi: 10.1111/j.1365-2869.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- 32.Sateia MJ, Nowell PD. Insomnia. Lancet. 2004;364:1959–1973. doi: 10.1016/S0140-6736(04)17480-1. [DOI] [PubMed] [Google Scholar]
- 33.Fetveit A. Late-life insomnia: a review. Geriatr Gerontol Int. 2009;9:220–234. doi: 10.1111/j.1447-0594.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 34.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10 Suppl 1:S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Yules RB, Freedman DX, Chandler KA. The effect of ethyl alcohol on man’s electroencephalographic sleep cycle. Electroencephalogr Clin Neurophysiol. 1966;20:109–111. doi: 10.1016/0013-4694(66)90153-2. [DOI] [PubMed] [Google Scholar]
- 36.Knowles JB, Laverty SG, Kuechler HA. Effects on REM sleep. Q J Stud Alcohol. 1968;29:342–349. [PubMed] [Google Scholar]
- 37.Rundell OH, Lester BK, Griffiths WJ, Williams HL. Alcohol and sleep in young adults. Psychopharmacologia. 1972;26:201–218. doi: 10.1007/BF00422697. [DOI] [PubMed] [Google Scholar]
- 38.MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young men. J Stud Alcohol. 1982;43:434–444. doi: 10.15288/jsa.1982.43.434. [DOI] [PubMed] [Google Scholar]
- 39.Williams DL, MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young women. J Stud Alcohol. 1983;44:515–523. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- 40.Brower KJ, Hall JM. Effects of age and alcoholism on sleep: a controlled study. J Stud Alcohol. 2001;62:335–343. doi: 10.15288/jsa.2001.62.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sloots CE, Felt-Bersma RJ, West RL, Kuipers EJ. Stimulation of defecation: effects of coffee use and nicotine on rectal tone and visceral sensitivity. Scand J Gastroenterol. 2005;40:808–813. doi: 10.1080/00365520510015872. [DOI] [PubMed] [Google Scholar]
- 42.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]