EXECUTIVE SUMMARY
In June 2009, the Assistant Secretary for Health (ASH), Dr. Howard Koh, asked the National Vaccine Advisory Committee (NVAC) “to develop recommendations for establishing a comprehensive, sustainable, national adult immunization program that will lead to vaccine-preventable disease reduction by improving adult immunization coverage levels.” The NVAC serves in an advisory capacity to the ASH, in his role as Director of the National Vaccine Program. During a nearly two-year period, the NVAC reviewed prior recommendations and reports on adult immunization from the last two decades and examined the current landscape of research devoted to barriers to vaccinating adults. Based on this analysis, the NVAC developed a white paper and recommendations that aimed to address unresolved issues in a novel way to protect adults in the United States from vaccine-preventable diseases through increased vaccination. The report and recommendations were presented to the NVAC by the Adult Immunization Working Group (AIWG) and approved on June 14, 2011. The report and recommendations have been transmitted to the ASH as an official report of the NVAC.
The NVAC identified a need for national leadership and coordination of adult immunization activities. Strong leadership and coordination for adult vaccination are critical, as health-care utilization by adults, when sought, is often spread across a variety of specialist and generalist physicians and nonphysician providers. This model contrasts with the childhood vaccination model, where vaccinations are often provided within a medical home by a limited range of physician providers. Additionally, developing a cohesive system for delivering routine vaccinations to adults can have a longer-term impact by providing a framework to rapidly and efficiently deliver vaccines during public health emergencies such as pandemics, utilizing all available vaccination venues in concert.
Through the review and development of recommendations, nine categories of barriers to adult immunization were identified and examined in detail:
Lack of coordination of adult immunization activities
Lack of public knowledge
Lack of provider recommendations for immunization
Financial impediments to vaccinations
Lack of access to, and utilization of, health-care services by adults
Lack of utilization of reminder or assessment systems
Racial/ethnic disparities
Health literacy
Concern about adverse events
To address these barriers, the NVAC developed three recommendations, which are summarized in this article. These recommendations address the need for national leadership for an adult immunization program, the identification of resources for this program, and the development of a strategic plan for the adult immunization program. Additionally, more specific gaps in adult immunization were addressed through the development of recommended focused activities. These activities are categorized as addressing (1) general infrastructure considerations, (2) the expansion of access to vaccination, (3) provider- or system-based interventions, (4) the increasing community demand for vaccinations, and (5) research needs.
SUMMARY OF RECOMMENDATIONS
The full text of the recommendations, with identified essential governmental and nongovernmental entities and proposed timelines for implementation, can be found starting on page 25.
Recommendation #1: national leadership for an adult immunization program
The Department of Health and Human Services (HHS) should develop and adequately support a coordinated and comprehensive National Adult Immunization Program, administratively led by the Secretary of HHS, operationally led by the Centers for Disease Control and Prevention (CDC), and closely linked to other governmental and nongovernmental organizations involved in adult immunization.
The Secretary of HHS should designate and empower the ASH as the central point person to coordinate adult immunization activities through HHS. This coordination should occur through either the appointment of an Interagency Adult Immunization Working Group or through creation of an Adult Immunization Working Group of the existing Interagency Vaccine Group (hereafter, the Interagency Working Group).
The Interagency Working Group would provide an annual report to the NVAC on progress toward meeting these recommendations and improving adult immunization uptake.
Recommendation #2: resources for an adult immunization program and action plan implementation
Appropriate resources (financial and infrastructure) should be allocated by the leadership of the National Adult Immunization Program in consultation with the Interagency Working Group to carry out the strategic action plan outlined in Recommendation #3. At a minimum, these resources will include staffing for a National Adult Immunization Program office at CDC, at levels sufficient to implement the components outlined after Recommendation #3.
Funding levels could be partially measured through CDC assessments of immunization grantees regarding their projected plans for implementing a widespread adult immunization program within their jurisdiction, with overall coordination through the Interagency Working Group described in Recommendation #1.
CDC and the National Vaccine Program Office (NVPO) should work to utilize this information, in conjunction with internal expert analyses, to estimate the costs of implementing the focused activities recommended in this report.
Recommendation #3: strategic plan for adult immunization
The Interagency Working Group created in Recommendation #1 should lead the development of a comprehensive National Strategic Plan for Adult Immunization (hereafter, National Strategic Plan) that incorporates input from a broad range of stakeholders (e.g., the public; health-care providers and organizations; federal, state, and local government; insurers, payers, and employers; and vaccine manufacturers), obtained through a collaborative effort of the NVPO and NVAC. To facilitate continuous refinement and action on the National Strategic Plan, ongoing input from nonfederal stakeholders (including traditional and nontraditional immunizing health-care providers and their representative professional organizations, health-care payers, employers, public advocacy groups, and vaccine manufacturers) should be regularly provided to the Interagency Working Group through routine public and stakeholder engagement sessions facilitated by the NVAC.
At a minimum, the National Strategic Plan should be designed to meet adult immunization goals specified in Healthy People 2020 and the National Vaccine Plan, and efforts of the National Adult Immunization Program to meet these goals should be included in routine NVAC progress reports related to Healthy People 2020 and the National Vaccine Plan.
The Interagency Working Group created in Recommendation #1 will be charged with routine evaluation of the contents of and progress toward the goals of the National Strategic Plan, with these evaluations serving as the basis of the Interagency Working Group annual report to the NVAC.
Summary of recommended activities for a comprehensive National Adult Immunization Program
The full text of the recommended activities, along with identified essential governmental and nongovernmental entities and proposed timelines for implementation, can be found starting on page 27.
- General infrastructure considerations
- Alignment of adult immunization goals across agencies
- Adult immunization activities in federal grant guidance
- Infrastructure development and coordination
- Quality measures for adult vaccination
- Expanding access to vaccination
- Ensuring a consistent and adequate supply of adult vaccines for the U.S.
- Increasing application of Section 317 funds used for adult immunization
- Developing and fostering innovative adult immunization partner organization networks
- Standardizing Medicaid vaccine administration reimbursement rates and mechanisms
- Provider- or systems-based interventions
- Education of providers on quality improvement/quality assurance activities
- Education of providers on standards of care and immunization practice
- Expansion of the adult immunization provider network
- Improving and expanding immunization information systems for adult vaccinations
- Education of vaccine providers and partners on health-care reform and immunization business practices
- Increasing community demand for vaccinations
- Development and implementation of an ongoing, comprehensive education and outreach campaign on adult vaccines, directed to both the public and providers
- Research needs
- Establishing costs of administering adult vaccines, and basing reimbursement of vaccine administration on these costs
- Continued collection and evaluation of adult immunization data
- Studying the economic benefits of adult immunization
- Studying the impact of differing medical care reimbursement systems on vaccine uptake
- Evaluation of health-care professional training
- Studying adult health-care providers to further examine provider vaccine stocking and administration practices and the relationship to vaccination coverage disparities
- Evaluation of the 2013–2014 Medicaid reimbursement modification
- Studying public and provider knowledge, attitudes, and practices related to adult vaccination after implementation of these recommendations
- Conducting a standardized evaluation of adult vaccination in nontraditional immunization venues
- Better understanding the impact of health literacy on vaccinations and vaccination disparities
- Researching the optimal use of social networking
- Researching state-level policies and practices
- Researching the development of new and improved vaccines and vaccine delivery systems
NATIONAL VACCINE ADVISORY COMMITTEE
Chair
Guthrie S. Birkhead, MD, MPH, New York State Department of Health, Albany, NY
Executive Secretary
Bruce G. Gellin, MD, MPH, National Vaccine Program Office, U.S. Department of Health and Human Services, Washington, DC
Public Members
Tawny Buck, Wasilla, AK
Richard D. Clover, MD, University of Louisville, School of Public Health and Information Sciences, Louisville, KY
Lisa A. Jackson, MD, MPH, Center for Health Studies, Group Health Cooperative, Seattle, WA
Philip S. LaRussa, MD, Columbia University, Department of Pediatrics, New York, NY
James O. Mason, MD, DrPH, Farmington, UT
Marie McCormick, MD, ScD, Harvard School of Public Health, Department of Society, Human Development, and Health, Boston, MA
Julie Morita, MD, Chicago Department of Public Health, Chicago, IL
Christine Nevin-Woods, DO, MPH, Pueblo City-County Health Department, Pueblo, CO
Walter A. Orenstein, MD, Bill and Melinda Gates Foundation, Seattle, WA
Amy Pisani, MS, Every Child by Two, Mystic, CT
Laura E. Riley, MD, Massachusetts General Hospital, Boston, MA
Litjen Tan, PhD, MS, American Medical Association, Chicago, IL
Thomas E. Stenvig, RN, MS, MPH, South Dakota State University College of Nursing, Brookings, SD
Representative Members
Seth Hetherington, MD, Genocea Biosciences, Cambridge, MA
Clement Lewin, PhD, MBA, Novartis Vaccines and Diagnostics, Cambridge, MA
Liaison Representatives
Wayne Rawlins, MD, MBA, America's Health Insurance Plans, Hartford, CT
Charlene Gallagher, RPh, JD, Advisory Committee on Childhood Vaccines, Berwyn, PA
Jose R. Romero, MD, U.S. Food and Drug -Administration, Vaccines and Related Biologics Products Advisory Committee, Little Rock, AR
Carol Baker, MD, Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices, Houston, TX
Claire Hannan, MPH, Association of Immunization Managers, Rockville, MD
Paul Jarris, MD, MBA, Association of State and Territorial Health Officials, Arlington, VA
Anne Bailowitz, MD, MPH, National Association of County and City Health Officials, Baltimore, MD
Keith Pritchard, Public Health Agency of Canada, Ottawa, ON
David Salisbury, CB, FRCP, FRCPCH, FFPHM, United Kingdom, Department of Health, London, UK
Vesta Richardson, MD, Health Ministry of Mexico, Mexico City, Mexico
Federal Ex Officio Members
Anne Schuchat, MD, Centers for Disease Control and Prevention, Atlanta, GA
Norman W. Baylor, PhD, U.S. Food and Drug Administration, Rockville, MD
Charlene Douglas, PhD, MPH, RN, Advisory Committee on Childhood Vaccines, Fairfax, VA
Paul D. Moore, PhD, Health Resources and Services Administration, Rockville, MD
Jeffrey A. Kelman, MD, MMSc, Centers for Medicare and Medicaid Services, Washington, DC
Richard Martinello, MD, U.S. Department of Veterans Affairs, West Haven, CT
Margaret McCluskey, RN, MPH, U.S. Agency for International Development, Washington, DC
Barbara Mulach, PhD, National Institutes of Health, Bethesda, MD
COL Renata J.M. Engler, MD, U.S. Department of Defense, Washington, DC
Rick Hill, DVM, MS, U.S. Department of Agriculture, Ames, IA
NATIONAL VACCINE ADVISORY COMMITTEE ADULT IMMUNIZATION WORKING GROUP
NVAC Members
Guthrie S. Birkhead, MD, MPH, New York State Department of Health, Albany, NYa
Julie Morita, MD, Chicago Department of Public Health, Chicago, ILb
Christine Nevin-Woods, DO, MPH, Pueblo City-County Health Department, Pueblo, COb
Richard D. Clover, MD, University of Louisville, School of Public Health and Information Sciences, Louisville, KY
Lisa A. Jackson, MD, MPH, Center for Health Studies, Group Health Cooperative, Seattle, WA
Clement Lewin, PhD, MBA, Novartis Vaccines and Diagnostics, Cambridge, MA
Laura E. Riley, MD, Massachusetts General Hospital, Boston, MA
Litjen Tan, PhD, MS, American Medical Association, Chicago, IL
NVAC Liaison Members
Richard Beigi, MD, MSc, University of Pittsburgh Medical Center, Pittsburgh, PA (American College of Obstetrics and Gynecology)
Thomas Koinis, MD, Oxford, NC (American Academy of Family Physicians)
Gregory Poland, MD, Mayo Clinic, Rochester, MN (American College of Physicians)
Wayne Rawlins, MD, MBA, Aetna, National Medical Services, Hartford, CT (America's Health Insurance Plans)
Alan Rosenberg, MD, Wellpoint, Inc., Chicago, IL (America's Health Insurance Plans)
Mitchel Rothholz, RPh, MBA, American Pharmacists Association, Washington, DC (American Pharmacists Association)
NVAC Ex Officio Members
Amy Groom, MPH, Indian Health Service, Albuquerque, NM
Jeffrey A. Kelman, MD, MMSc, Centers for Medicare and Medicaid Services, Washington, DC
Richard Martinello, MD, Veterans Health Administration, Washington, DC
Abigail Shefer, MD, Centers for Disease Control and Prevention, Atlanta, GA
Raymond Strikas, MD, Centers for Disease Control and Prevention, Atlanta, GA
Candace Swartwood, MPH, Centers for Disease Control and Prevention, Atlanta, GA
Staff
Mark Grabowsky, MD, U.S. Department of Health and Human Services, National Vaccine Program Office, Washington, DCc
Robert A. Bednarczyk, PhD, New York State Department of Health, Albany, NYd
Lauren Wu, MHS, U.S. Department of Health and Human Services, National Vaccine Program Office, Washington, DC
1. INTRODUCTION
Immunizations are one of the most effective public health interventions, reducing or eliminating the burden of many infectious diseases.1 The primary focus of vaccination programs has historically been directed to childhood immunizations, with near-record or record high immunization coverage levels achieved.2 For adults, chronic diseases have been the primary focus of preventive and medical health care, though there has been increased emphasis on preventing infectious diseases. However, the gains seen in childhood immunization coverage have not been mirrored for adult immunizations. Increased immunization of both children and adults can substantially reduce morbidity and mortality from infectious disease, as long as efforts to achieve high immunization coverage levels are coordinated and sustained.
In the U.S., many preventive health services, including but not limited to immunizations, are underutilized.3 The recommended list of preventive health services is continually increasing, with increased use of preventive services having been shown—both through individual reviews (e.g., U.S. Preventive Services Task Force [USPSTF]4 and Advisory Committee on Immunization Practices [ACIP] recommendations5) and larger, comprehensive modeling6—to reduce morbidity and mortality from preventable causes. However, with an increasingly long list of preventive services, health-care providers may be faced with having to choose which services they provide, rather than providing all recommended services.7,8
The organization of the childhood and adult immunization enterprises is very different. The childhood immunization program involves a universal schedule encompassing a limited age range and a relatively narrow network of provider types (primarily pediatricians and family physicians) for whom childhood vaccinations play a central role in their practice. The adult immunization enterprise is more complex, encompassing a wide variety of vaccines and a very diverse target population ranging from healthy young adults, to young adults and elderly people with chronic conditions, to those who are less likely to have a medical home and seek medical care in nontraditional settings. This diverse population is, in turn, served by an equally diverse network of health-care providers, including primary care providers (e.g., family physicians and internists), specialists, mid-level providers, and pharmacists in settings such as outpatient clinics, hospitals, public health clinics, travel medicine clinics, and rapid-access health-care clinics.
Additionally, vaccination recommendations for adults span the interface between adolescents and adults (human papillomavirus [HPV] and meningococcal vaccines) and include vaccines that are universally recommended (e.g., influenza), those that are recommended for certain age groups (e.g., herpes zoster), those that are targeted to individuals with specific risk factors (e.g., hepatitis A and B), travel vaccines (e.g., typhoid, yellow fever, and polio), and vaccines targeted toward particular combinations of age and risk factors (e.g., pneumococcal).
There is no coordinated public health infrastructure to support an adult immunization program as there is for children (i.e., the Section 317 Program9 and Vaccines for Children [VFC] program10) and little coordination among adult health-care providers in terms of vaccine provision.11 This lack of coordination was highlighted as a barrier to effective delivery of H1N1 influenza vaccine during the 2009–2010 H1N1 influenza vaccination program10 and remains a barrier to other routine adult immunizations.12 In addition to increasing routine vaccination delivery to adults, development of a comprehensive and sustainable adult immunization program would improve public health preparedness and emergency response capability (e.g., delivery of medical countermeasures and dissemination of information).
As a result of the general recognition that increasing adult immunization levels is challenging, multiple reports and recommendations have been issued to attempt to galvanize action.12–25 However, many of these reports acknowledge a lack of significant progress on this front, evidenced by the similarity of many of the prior recommendations during a two-decade span. Additionally, the landscape of medical care and preventive health services is currently changing through the implementation of the Affordable Care Act (ACA), which comprises the Patient Protection and Affordable Care Act of 201026 and the Healthcare and Education Reconciliation Act of 2010.27
This report reviews the current state of adult immunization in the U.S. and contains recommendations of the National Vaccine Advisory Committee (NVAC). The NVAC makes recommendations to the Director of the National Vaccine Program, which is currently the Assistant Secretary for Health (ASH), Department of Health and Human Services (HHS), for consideration of implementation options.
2. BACKGROUND
2.1. Vaccine-preventable disease burden among adults
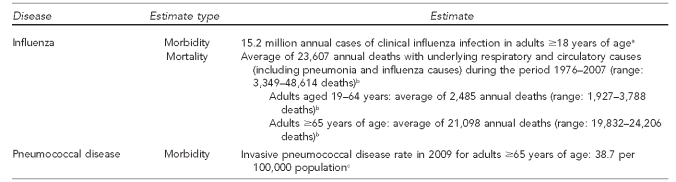
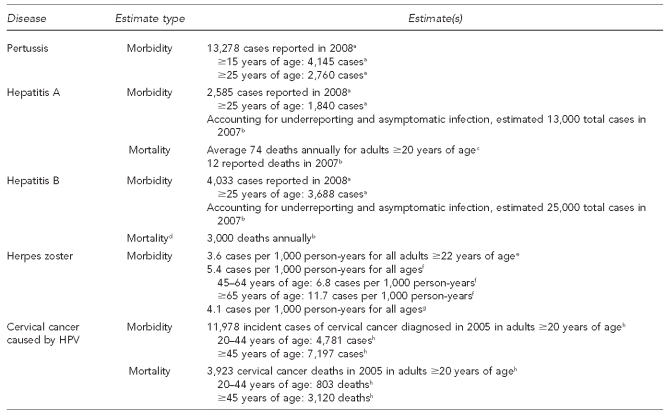
The burden of infectious disease in adults is often overshadowed by the burden of chronic disease. However, for some infectious diseases, this burden is substantial. Additionally, for some vaccine-preventable diseases, the benefit lies not just in prevention of the infection, but in prevention of the more clinically important conditions that can occur many years after the initial infection (e.g., liver cancer associated with hepatitis B infection and cervical cancer associated with HPV infection). The burden of disease related to influenza and pneumococcal infections in adults is summarized in Figure 1. The burden of disease related to infections other than influenza or pneumococcal is summarized in Figure 2.
Figure 1.
Estimates of influenza and pneumococcal disease burden in adults, United States, 2005–2008
aMolinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintrub E, et al. The annual impact of seasonal influenza in the U.S.: measuring disease burden and costs. Vaccine 2007;25:5086-96.
bEstimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010;59(33):1057-62.
cCenters for Disease Control and Prevention (US). Active Bacterial Core Surveillance report, Emerging Infections Program Network, Streptococcus pneumonia, 2009 [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/abcs/reports-findings/survreports/spneu09.pdf
Figure 2.
Estimates of disease burden in adults, excluding influenza and pneumococcal disease: United States, 2005–2008
aHall-Baker PA, Nieves E Jr, Jajosky RA, Adams DA, Sharp P, Anderson WJ, et al. Summary of notifiable diseases—United States, 2008. MMWR Morb Mortal Wkly Rep 2010;57(54):1-94.
bCenters for Disease Control and Prevention (US). Viral hepatitis statistics and surveillance [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/hepatitis/Statistics/index.htm
cVogt TM, Wise ME, Bell BP, Finelli L. Declining hepatitis A mortality in the United States during the era of hepatitis A vaccination. J Infect Dis 2008;197:1282-8.
dData stratified by age not available
eYawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic Proc 2007;82:1341-9.
fYih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 2005;5:68.
gJumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis 2005;191:2002-7.
hCenters for Disease Control and Prevention (US), National Program of Cancer Registries. United States cancer statistics: 1999–2006 incidence and mortality Web-based report [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/cancer/npcr/uscs/2007/download_data.htm
HPV = human papillomavirus
2.1.1. Influenza
One of the most commonly occurring vaccine-preventable diseases in adults is influenza. However, difficulty in estimating influenza morbidity and mortality is highlighted by differences in recently published estimates. Two previous publications related to annual underlying respiratory and circulatory deaths associated with influenza estimated 36,155 total deaths (32,651 deaths in individuals ≥65 years of age)28 and 36,171 total deaths (32,752 deaths in individuals ≥65 years of age).29 Another recent study estimated that annually, approximately 24.7 million individuals develop a clinical influenza infection, of whom 15.2 million (62%) are ≥18 years of age and 3.2 million (13%) are ≥65 years of age. The mortality estimate in this study was slightly higher than in other studies, at 41,009 annual deaths, of which 40,812 (99.5%) occurred in those ≥18 years of age and 36,016 (88%) occurred in those ≥65 years of age.30 The most recent influenza mortality study evaluated several influenza seasons and estimated an average of 23,583 annual influenza deaths, with 21,098 occurring in adults ≥65 years of age, although mortality estimates for individual influenza seasons were higher when the H3N2 strain was dominant31 and lower in other years. Regardless, it is clear that influenza leads to substantial morbidity, and mortality and widespread economic costs.
2.1.2. Invasive pneumococcal disease
Invasive pneumococcal disease is monitored nationally through the Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance system in place at 10 Emerging Infections Program sites. For 2009, the rate of invasive pneumococcal disease for adults ≥65 years of age was 38.7 per 100,000 population,32 which was substantially lower than the estimate for the year 2000 (58.1 per 100,000 population),33 and lower than the Healthy People 2010 objective of 42 per 100,000 population.32 Reductions in adult pneumococcal disease are mainly due to use of the pneumococcal conjugate vaccine in children, offering less opportunity for transfer of the organism from children to nearby adults.34 Additionally, a recent study estimated direct and indirect costs associated with the 1.98 million annual cases of Streptococcus pneumoniae infections in adults to be approximately $6.5 billion.35
2.1.3. Pertussis
Recent outbreaks of pertussis (whooping cough) in California36,37 and Michigan highlight the ongoing risk of pertussis infections among children and adults. In the first nine months of 2010, more than 4,200 cases of pertussis in California and 610 cases in Michigan.37 By comparison, in 2008 a total of 13,278 pertussis cases was reported nationally.38 While most of the reported cases in the more recent outbreaks were in infants and young children, adults whose immunity to pertussis has waned can often be asymptomatic carriers of Bordetella pertussis and can serve as vectors to spread disease to children who are too young to be fully vaccinated.39 In 2008, there were 4,145 reported pertussis cases in individuals aged 15 years and older, 2,760 of which were in individuals aged 25 years and older.38
2.1.4. Hepatitis A and B
CDC estimates that for 2007, there were 13,000 acute clinical cases of hepatitis A infection, with a total of 25,000 acute and asymptomatic hepatitis A infections, and 12 reported deaths following hepatitis A infection. For hepatitis B, CDC estimates that there were 13,000 acute clinical cases in 2007, with a total of 43,000 acute and asymptomatic hepatitis B infections.40 While there were 28 reported deaths following hepatitis B infection,38 CDC estimates that approximately 3,000 hepatitis B deaths occur annually.41
2.1.5. Herpes zoster (shingles)
Herpes zoster (shingles) is a condition caused by the reactivation of varicella (chickenpox virus) and is not a reportable condition in the U.S., making population-level estimates of incidence difficult to ascertain. However, results from studies conducted regionally in the U.S. (i.e., in Olmstead County, Minnesota;42 Massachusetts;43 and Washington State enrollees in the Group Health Cooperative Health Maintenance Organization44) and in other countries (i.e., Italy,45 United Kingdom,46 France,47 and Australia48) have been consistent. Generally, annual incidence of herpes zoster in adults is approximately four cases per 1,000 population, with increasing incidence observed with older age and an immunocompromised state.
While zoster-associated mortality is rare, the direct and societal costs related to the medical care of acute cases of shingles, treatment of post-herpetic neuralgia (e.g., emotional costs), and lost work time/disability support the cost-effectiveness of a widespread zoster vaccination program for adults.49
2.1.6. HPV-associated cancers
HPV is the most common sexually transmitted infection, with approximately 27% of U.S. women aged 14–59 years having a cervical or vaginal infection with at least one HPV strain. In a 2007 study, the highest prevalence of infection was seen among women aged 20–24 years (45%).50 Persistent infection with certain HPV strains is a necessary, but insufficient, cause of cervical cancer. HPV infection is also associated with other ano-genital cancers and some oropharyngeal cancers. Two HPV strains (HPV-16 and HPV-18) are responsible for approximately 70% of incident cervical cancer,51 and HPV (i.e., HPV-6 and HPV-11) causes approximately 90% of incident genital warts.52 Nationally, in 2005, there were nearly 12,000 new cases of cervical cancer reported (7.9 cases per 100,000 women), with 4,021 cervical cancer-related deaths (2.4 deaths per 100,000 women).53 Additionally, in 2007, there were 4,159 new cases of vulvar cancer, 1,149 cases of vaginal cancer, and 3,199 cases of anal cancer in women; and there were 1,118 cases of penile cancer and 1,942 cases of anal cancer in men.54,55
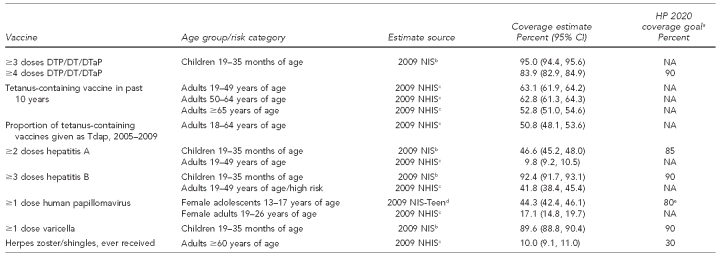
2.2. Vaccine coverage levels among adults
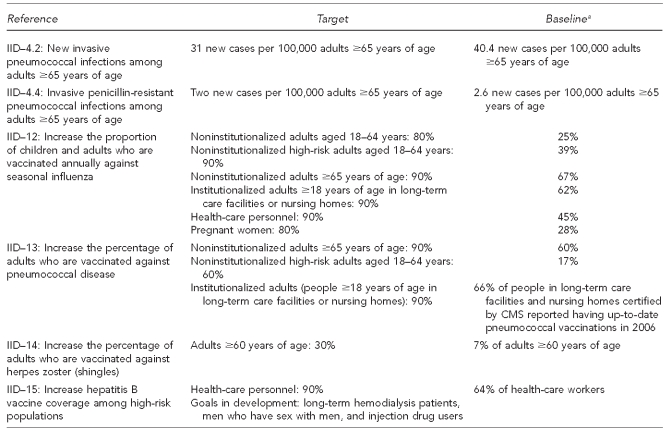
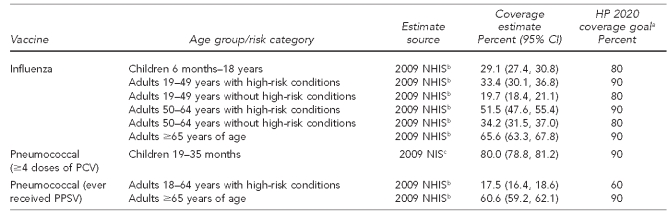
In comparison with pediatric vaccination uptake levels and existing Healthy People 2020 targets,56 which are shown in Figure 3, adult vaccination coverage levels are low. A comparison of childhood and adult vaccination levels for influenza and pneumococcal vaccine is presented in Table 1. A comparison of childhood and adult vaccination levels for vaccines other than those for influenza or pneumococcal disease is presented in Table 2.
Figure 3.
Healthy People 2020 goals related to adult immunization
aBaseline estimates are from 2008, unless otherwise specified.
IID = immunization and infectious diseases
CMS = Centers for Medicare and Medicaid Services
Table 1.
Influenza and pneumococcal vaccination uptake comparison for children and adults, United States, 2008–2009
aDepartment of Health and Human Services (US). Healthy People 2020 objectives [cited 2011 Jan 3]. Available from: URL: http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf
bCenters for Disease Control and Prevention (US). Vaccines and immunizations. Statistics and surveillance: 2009 adult vaccination coverage, National Health Interview Survey [cited 2011 Jan 3]. Available from: URL: http://www.cdc.gov/vaccines/stats-surv/nhis/2009-nhis.htm
cNational, state, and local area vaccination coverage among children aged 19–35 months—United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59(36):1171-7.
CI = confidence interval
HP = Healthy People
NHIS = National Health Interview Survey
PCV = pneumoccocal conjugate vaccine
NIS = National Immunization Survey
PPSV = pneumococcal polysaccharide vaccine
Table 2.
Vaccination uptake comparison for routinely recommended childhood and adult vaccines, excluding influenza and pneumococcal vaccines: United States, 2007–2009
aDepartment of Health and Human Services (US). Healthy People 2020 objectives [cited 2011 Jan 3]. Available from: URL: http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf
bNational, state, and local area vaccination coverage among children aged 19–35 months—United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59(36):1171-7.
cCenters for Disease Control and Prevention (US). Vaccines and immunizations. Statistics and surveillance: 2009 adult vaccination coverage, National Health Interview Survey [cited 2011 Jan 3]. Available from: URL: http://www.cdc.gov/vaccines/stats-surv/nhis/2009-nhis.htm
dNational, state, and local area vaccination coverage among adolescents aged 13–17 years—United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59(32):1018-23.
eAge group for HP 2020 coverage goal is 13–15 years.
CI = confidence interval
HP = Healthy People
DTP = Diphtheria and tetanus toxoids and whole-cell pertussis vaccine (child formulation)
DT = Diphtheria and tetanus toxoids vaccine (child formulation)
DTaP = Diphtheria and tetanus toxoids and acellular pertussis vaccine (child formulation)
NIS = National Immunization Survey
NA = not applicable
NHIS = National Health Interview Survey
Tdap = Tetanus and diphtheria toxoids and acellular pertussis vaccine (adult formulation)
2.2.1. Influenza vaccine
Seasonal (2009–2010) influenza vaccine uptake among all American adults aged 18 years and older was 40% (95% confidence interval [CI] 40, 41), with the highest seasonal influenza vaccination levels seen in those aged 65 years and older (70%, 95% CI 69, 72). In contrast, only 28% (95% CI 28, 29) of adults aged 18–49 years who were not in a high-risk group received a seasonal influenza vaccine.57 In general, estimates of seasonal influenza vaccination coverage for the 2009–2010 season were higher than estimates for the 2008–2009 influenza season (18–49 years of age without high-risk conditions: 22%, 95% CI 21, 24; ≥65 years of age: 67%, 95% CI 65, 69).58 Observation of the highest influenza vaccination levels among adults ≥65 years of age may be related to this subpopulation having a universal immunization recommendation the longest, particularly relative to adults aged 18–49 years who are not in a high-risk group.
For the 2009 influenza A (H1N1) vaccine, overall uptake among those aged six months and older in the U.S. was estimated at 27% (95% CI 27, 27), with more than one-third (34%, 95% CI 34, 35) of individuals in the initial target populations (i.e., pregnant women, health-care/emergency medical services workers, all individuals aged six months to 24 years, and adults aged 25–64 years with high-risk conditions) receiving the H1N1 vaccine.57
2.2.2. Pneumococcal vaccine
For vaccines against invasive pneumococcal disease, vaccination uptake among high-risk adults aged 19–64 years (18%) and adults ≥65 years of age (61%) is much lower than that of children, and is nearly 40 and 30 percentage points, respectively, lower than the Healthy People 2020 goals.
2.2.3. Tetanus, diphtheria, and acellular pertussis vaccine
While 95% of children up to 3 years of age have received at least three doses of either diphtheria and tetanus toxoids and whole-cell pertussis vaccine (child formulation), diphtheria and tetanus toxoids vaccine (child formulation), or diphtheria and tetanus toxoids and acellular pertussis vaccine (child formulation),2 about 63% of adults aged 19–64 years and 53% of adults ≥65 years of age have gotten a tetanus and diphtheria toxoids (Td) vaccine (adult formulation) booster in the past 10 years. Among those aged 19–64 years who received a Td booster between 2005 and 2009, approximately 51% had received this immunization in the form of the tetanus and diphtheria toxoids and acellular pertussis vaccine (adult formulation) (Tdap) vaccination to additionally boost immunity to pertussis.59
2.2.4. Hepatitis A and B vaccines
Documented uptake of at least three doses of the hepatitis B vaccine by high-risk adults (42%) and non-high-risk adults (34%)57 is low compared with uptake among children (92%).2 Receipt of at least two doses of hepatitis A vaccine was documented for approximately 10% of adults aged 19–49 years.59 However, this vaccine is not routinely recommended for all adults, but for targeted behavioral, medical, and occupational indications, as well as other specific populations, such as travelers to countries with a high or intermediate endemicity of hepatitis A virus infection.60
2.2.5. Herpes zoster (shingles) vaccine
While nearly 90% of children have received the varicella vaccine, only 10% of adults ≥60 years of age reported receipt of the herpes zoster/shingles vaccine in 2009.59 However, these numbers are not directly comparable due to differences in indication, financing mechanisms, and vaccine availability.61
2.2.6. HPV
In 2009, initiation of the three-dose HPV vaccine series was reported by 17% of females aged 19–26 years,59 much lower than the observed initiation in 13- to 17-year-old females (44%) or completion of the three-dose series in adolescents (27%).
2.2.7. Racial/ethnic disparities in immunization rates
For nearly all adult immunization measures tracked in the 2009 National Health Interview Survey (NHIS), vaccination coverage among non-Hispanic white people was higher than for either non-Hispanic black or Hispanic people. Adult white people consistently had the highest influenza immunization coverage among all age and risk categories, as well as having the highest coverage for vaccinations against pneumococcal disease, hepatitis A virus, herpes zoster, and HPV. While white adults had the highest tetanus-containing vaccination levels, black adults had the highest proportion of these vaccinations administered as Tdap during the period 2005–2009. Additionally, the highest hepatitis B immunization coverage was seen in black adults. Immunization coverage among Hispanic people was nearly always lowest among these three racial/ethnic categories.59 This finding contrasts with childhood coverage estimates, where Hispanic children are often immunized at equal or greater levels than their white counterparts, with the lowest coverage estimates often found among black children.2
2.3. Prior recommendations to improve adult immunization
During the past two decades, numerous reports and sets of recommendations to address the suboptimal immunization status of adults have been developed. These recommendations have been issued by a number of groups, including the NVAC in 1990,17 1994,18 1997,19 2000,24 and 2004;20 the Institute of Medicine;62 a 2010 collaboration among Trust for America's Health, the Infectious Diseases Society of America, and the Robert Wood Johnson Foundation;12 the 2007 and 2010 National Immunization Congress;13,63 and other groups of independent experts in the field working with federal agencies.22,25
In 2009, the NVAC issued a report directed to federal agencies' adult immunization programs.21 The current report represents the work of the NVAC on phase two of this process: a broad examination of the national adult immunization program.
3. RATIONALE
Given these prior efforts and the large number of reports and recommendations that have previously been put forth—often identifying the same issues, albeit years apart—what is the rationale for developing this report and the recommendations presented herein?
First, prior reports and recommendations have not resulted in sufficient improvements in the current status of adult immunization in the U.S. While the NVAC did not systematically analyze reasons for the lack of responsiveness to those prior recommendations, there seemed to be a lack of prioritization among the recommendations, a lack of specificity of actions required to meet the recommendations, a lack of coordination in advancing recommendations that were separate yet intricately intertwined, and a lack of clear definition of the groups responsible for implementing the recommendations.
Second, recent research has helped to better identify additional barriers to adult immunization (e.g., knowledge and attitudes of the public and providers, and access to health-care providers who administer vaccinations). This clearer understanding of the complexity of these barriers, and the interrelationships between them, will give the recommendations presented in this report more relevance. A more detailed examination of these barriers is presented in Section 5.
Third, the NVAC recently approved recommendations to address financial barriers to childhood and adolescent vaccination64 that can serve as a foundation for recommendations to address adult vaccine financing barriers. In addition, the ACA is anticipated to eliminate some financial barriers to adult immunization, primarily through the provision of health-care insurance to a larger proportion of the U.S. population and the requirement to eliminate cost-sharing for preventive services in new health plans.65 Although the full impact of this legislation has yet to be determined, these recommendations may provide an opportunity for clarifying immediate and future changes mandated in the ACA through the provision of guidance on the legislative interpretation during the implementation process.
Fourth, lessons learned from the recent, rapidly developed vaccine delivery system created by public health to manage vaccine allocation during the 2009 H1N1 pandemic make clear the need for public health infrastructure for all adult vaccines. The H1N1 vaccine distribution system was built upon the existing infrastructure for the childhood vaccination program.10 Elements of the childhood program that were essential to the H1N1 vaccine distribution system included the VFC vaccine distribution system and public health partnerships with professional organizations (e.g., American Academy of Pediatrics and American Academy of Family Physicians). These systems were leveraged for adult H1N1 immunizations, as there was no comparable system in place for adult immunization. Defaulting to a system designed to serve children, with ad hoc efforts to apply this system to adults, illustrates the need for an infrastructure that can provide immunization services across the age spectrum. The public health community's ability to communicate with health-care providers and optimize delivery of medical countermeasures is dependent on both continued investment in childhood vaccination and new investment in an adult-centric vaccination program.
Finally, prior recommendations have provided the NVAC with a road map of what has and has not been successful, and which recommendations have the greatest opportunity for success. This road map has been evaluated by a wide variety of stakeholders with broad expertise. It is hoped that, given this input—as well as utilizing the expertise and leadership of federal offices addressing vaccination policy, minority health disparities, health literacy issues, and current trends in communications—the process described in this report will result in recommendations that can lead to truly transformative changes in the way that adult immunizations are perceived and provided in our society. The passage of the ACA in 2010 has provided some evidence that this type of large-scale change is possible, giving the NVAC hope for the implementation of these recommendations.65
4. METHODS
To address the issue of increasing adult immunization levels, the NVAC convened the Adult Immunization Working Group (AIWG) in 2008. The first phase of the AIWG's work was to evaluate and make recommendations on federal adult immunization activities. This task was completed with the approval of a report and recommendations in June 2009.21
Following this report, the NVAC moved to a broader evaluation of the adult immunization infrastructure in the U.S. Additionally, following a September 2009 request from the ASH that the NVAC review and provide recommendations related to racial/ethnic disparities in influenza vaccination, the AIWG incorporated this task as part of the work on phase two of its charge. Membership of the AIWG was supplemented with additional NVAC members and liaisons to related professional organizations and stakeholders. The charge to the AIWG for phase two, the current undertaking, is “to develop recommendations for establishing a comprehensive, sustainable, national adult immunization program that will lead to vaccine-preventable disease reduction by improving adult immunization coverage levels.”
Initial AIWG activities included two literature reviews. The first was designed to identify studies of barriers to adult immunization that have been published in the medical and public health literature. These barriers are summarized later in this report. The second was designed to identify previous recommendations and reports related to adult immunization. These reports were reviewed to identify recommendations, which were then summarized and organized by theme. Prior recommendations were matched to barriers to adult immunization, and AIWG members were asked to rank the key recommendations within each category of barrier and identify additional gaps or barriers that were not previously denoted. The highest priority recommendations and newly identified gaps then served as the basis for the development of recommendations for this report.
Following completion of the draft report, it was released for public comment, and two stakeholder engagement meetings were held: one in Denver, Colorado, and one in Chicago, Illinois. A wide variety of stakeholders were brought together to discuss the draft report and offer both verbal and written comments. These comments were consulted and, where appropriate, the report draft was revised to address these comments.
Following the stakeholder engagement meetings, attendees were asked to complete a prioritization survey of the focused activities directed to research needs. The summary of this survey is in the Appendix.
RAND Corporation conducted an evaluation of the prior work of the NVAC. Recommendations were made to the NVAC on how to increase its effectiveness, with an emphasis on developing recommendations that would be directed to specific partners, actionable, and contain timelines for implementation and monitoring the status of recommendations, as well as progress on the development and execution of implementation plans.66 The RAND recommendations were consulted during the development of these recommendations. Consistent with the RAND recommendations, an implementation plan should be developed by the National Vaccine Program Office (NVPO) and CDC with progress routinely tracked via reports to the NVAC.
5. RESULTS AND FINDINGS: BARRIERS TO ADULT IMMUNIZATION
5.1. Overview
There is a wide range of barriers to ensuring appropriate immunization of adults. The barriers to vaccinating a 19-year-old female college student with HPV and meningococcal conjugate vaccines are different from the barriers to providing annual influenza vaccinations to the elderly. Financial barriers to receipt of herpes zoster vaccine by a 60-year-old with private health insurance may be different from the financial barriers to receipt of herpes zoster vaccine by a 65-year-old with Medicare coverage. Nonetheless, many of the barriers to immunization apply across the spectrum of age, health conditions, and life situations of adults.
Recent research findings have provided greater insight into the complexity of understanding these barriers. Surveys of the public and health-care providers have been supplemented by research into health disparities and the effect of health literacy on utilization of preventive services. However, one of the major difficulties in addressing barriers to adult immunization is that these barriers do not often fall into neatly organized categories.
For example, a person's lack of knowledge about needing a specific vaccine may be due, in part, to the individual's physician(s) not recommending the vaccine. The physician may not be knowledgeable about current recommendations or unable to take the time to discuss immunizations with the individual. Stakeholder feedback indicated that a possible cause of this barrier may be the lack of a billing and reimbursement payment system to compensate for these types of discussions, particularly if a vaccine is not ultimately delivered. Additionally, reminder systems are not as commonly used for adults as they are for children. If the person has a number of physicians, such as a primary care physician and specialists who are not connected through electronic medical records or immunization information systems (IIS), there may be little ability for the different physicians to know if another provider has already recommended or provided vaccination.
5.2. Barrier: lack of coordination of adult immunization activities
In comparison with the resources allocated to childhood immunization in the U.S., adult immunization activities are underresourced on both financial and programmatic levels. The funding for vaccine purchase and provision through the VFC program and the associated vaccine delivery infrastructure have no equivalent system in adult immunization. While immunization grantees often have some staff support to address adult immunization topics, these are not always dedicated, full-time positions.15,16 A wide variety of federal efforts either directly or indirectly impact adult immunization (e.g., CDC activities, Department of Veterans Affairs [DVA] and Department of Defense [DoD] activities, Centers for Medicare and Medicaid Services [CMS] activities, health information technology (health IT) initiatives, and minority health and health literacy outreach programs). Coupled with an equally diverse group of nonfederal stakeholders (e.g., state and local government/public health agencies, health-care providers and organizations, health-care payers, manufacturers, the public, and advocacy groups), a multidisciplinary approach to addressing barriers to, and increasing rates of, adult immunization within an organized structure of coordination and leadership is necessary. However, such an approach is not apparent with current efforts in place to promote adult immunizations. This lack of coordination is evident in the previously mentioned adult immunization recommendations, which have attempted to address isolated issues or barriers without addressing larger, underlying issues of -coordination and leadership. Additionally, during an Institute of Medicine workshop on the response to the H1N1 influenza pandemic, the lack of an adult immunization infrastructure, similar to that of the VFC program, was cited as a challenge to effective distribution of H1N1 influenza vaccine to adults.10
Moving beyond coordination of efforts at the federal and state government level, it is important to acknowledge that many adults do not have a single medical home, and care received at multiple locations may not always be coordinated. Failure to properly leverage these opportunities (e.g., travel medicine clinics that screen for receipt of other adult immunizations, or pharmacists who vaccinate diabetics for influenza also inquiring about the person's most recent hemoglobin A1c test) with appropriate bidirectional communication between providers can lead to unresolved gaps in medical care and preventive services.
5.3. Barrier: lack of public knowledge
Increasing people's awareness of vaccination recommendations and needs is a key step toward increasing immunization coverage. Adults are often unaware of the availability of specific vaccines or of the potential risk of acquiring a disease that can be prevented by vaccination, a situation that is potentially compounded by the perception that immunizations are specifically designed and targeted for children. Childhood immunization rates are high, in part because of the use of school-entry mandates, but also because of the information provided to parents by health-care providers. While children may not be directly aware of the risks of the diseases they are being vaccinated against, their parents generally are.
Numerous studies have found that adults are not aware of their vulnerability to vaccine-preventable diseases nor are they aware of the availability of vaccines to prevent the infections. In one study of influenza and pneumococcal vaccination practices among people who indicated a prior history of coronary heart disease (CHD) or stroke, only 57% had received the influenza vaccine in the previous influenza season, and 48% had ever received the pneumococcal vaccine. Approximately 65% of people knew that their diagnosis of CHD or stroke put them in a high-risk category for which vaccination was recommended. Sixty-eight percent intended to receive the influenza vaccine in the upcoming influenza season; of those who did not plan to get the vaccine, nearly a quarter indicated they would not get vaccinated because they were not in a high-risk group.67 Intention to get vaccinated was not stratified by awareness of being in a high-risk group.
While awareness of individual high-risk status is important, two recent studies have identified gaps in adults' awareness of infectious diseases and related vaccines. A telephone survey of 2,002 adults documented high awareness of influenza (96%) and tetanus (90%) vaccines, while only 65% were aware of the pneumococcal vaccine. While most adults surveyed knew of the tetanus vaccine, only 36% knew a booster was recommended every 10 years, and only 27% knew when they were due for their next tetanus booster. Among the most commonly cited reasons for not receiving either the tetanus, influenza, or pneumococcal vaccines were that (1) the individual was healthy and did not need the vaccine, (2) the individual was concerned about side effects, and (3) the individual's doctor did not say that the individual needed the vaccine.68
A 2008 telephone survey by the National Foundation for Infectious Diseases (NFID) (n=1,005) found that only 49% of respondents knew that influenza could be prevented through vaccination, with awareness of vaccines to prevent infection from HPV, mumps, diphtheria, rubella, shingles, meningitis, and pertussis identified in fewer than 10% of respondents.69 In the 1996 Medicare Current Beneficiary Survey, 35% of the nearly 15,000 Medicare beneficiaries reported not getting the influenza vaccine in the past year, and nearly 20% of those unvaccinated indicated they did not know the vaccine was needed.70 Similar results were seen for Medicare beneficiaries across influenza seasons spanning 1997–2001.71
In addition to providers educating their patients about immunization and vaccine-preventable diseases, education through social networks may help increase adults' knowledge about the need for vaccination. Social networks, as used in this report, include Internet-based/electronic information dissemination systems and other social and community networks. A variety of networks can be used, including faith-based and community organizations, individual trusted leaders (e.g., individual clergy or tribal elders in the American Indian/Alaska Native populations), and peer networks with strong, trusted central voices.
Prior research has shown that outreach on preventive services, such as cancer screening education, through faith-based organizations and individual faith communities is acceptable72 and effective in increasing uptake of these services,73 as well as immunization services.74 The importance of these linkages is implicitly acknowledged through the 2009 H1N1 pandemic planning documents related to outreach through community and faith-based organizations.75
The concept of “core transmitters” is most commonly used in sexually transmitted disease -epidemiology, -relating to those individuals who are central to widespread transmission of disease. This concept of networking and core transmitters to pass information on to the wider network was a major component of stakeholder feedback on how to better educate adults about immunization, by using those trusted voices that are central to a social community to pass information on to the wider network. However, the evidence base for the best practices in this area is lacking and should be examined.
5.4. Barrier: lack of provider recommendations for immunization
A key finding of the aforementioned 2008 NFID survey was that 87% of respondents indicated they would be very likely or somewhat likely to get a vaccine if the vaccination was recommended by their doctor, with 55% indicating they would only get a vaccine if their doctor recommended it.69
While the results obtained from the Racial and Ethnic Adult Disparities in Immunization Initiative were concerned primarily with examining disparities, the interaction between an individual's attitude toward immunization and the presence of a health-care provider's recommendation was also documented in that study by Lindley et al. The highest vaccination levels were found for Medicare beneficiaries with a positive attitude toward vaccination and a provider's recommendation (93% of white people vs. 79% of African Americans). While coverage rates were lower, adults with negative attitudes toward vaccination who received a provider's recommendation (68% of white people vs. 41% of African Americans) had much higher immunization rates than adults with negative attitudes toward vaccination who did not receive a provider's recommendation (33% of white people vs. 13% of African Americans).76
A sample of 200 health-care providers (100 physicians and 100 physician assistants, nurse practitioners, and registered nurses) were asked for reasons they believe adults may not receive tetanus, influenza, or pneumococcal vaccines. An estimated 50%–70% (by provider type and vaccine type) of health-care providers cited people's confusion/lack of awareness of the vaccination schedule, 53%–60% cited the person not receiving a recommendation from the provider, and 62%–83% cited the person's lack of knowledge about illness prevention. While providers indicated a high frequency of discussing vaccination during routine health-care visits, this discussion occurred less frequently than during acute care visits. Additionally, more than 50% of providers indicated they did not routinely inform their patients of the consequences of missing recommended vaccinations.68 Another potential issue may be with providers for whom immunization is not a focus of their practice, particularly when presented with patients who have conditions that may make immunization recommendations less straightforward to follow (e.g., pregnant women).77,78
Due to time limitations in the provider practice, owing to the number of patients, number of recommended preventive services to potentially address, and emphasis on acute care, preventive services such as immunizations are often not addressed by providers during medical encounters.7,8 It has been estimated that for a practice with a patient population of 2,500, with similar demographics to the U.S. population, providers would need to dedicate more than seven hours a day to addressing USPSTF-recommended preventive services and ACIP-recommended immunizations.8
Providers may not discuss vaccinations with their patients due to the lack of monitoring of vaccination practices and lack of appropriate incentives. While recently published standards for adult immunization have called for routine assessments of coverage using the Assessment, Feedback, Incentives and eXchange of information (AFIX) methodology,25 this assessment is not required of immunization programs by CDC. Health-care provider professional organizations also do not have systematic assessment or quality improvement (QI) programs to assess their members' provision of adult immunization services in their practices, although the American College of Physicians does offer a continuing medical education credit related to an adult immunization course.79 Other immunization assessments are limited, with a Healthcare Effectiveness Data and Information Set (HEDIS) measure only for influenza and pneumococcal vaccination for adults,80 and Physician Quality Reporting System (formerly the Physician Quality Reporting Initiative [PQRI]) measures for influenza vaccination in adults aged ≥50 years and adults with end-stage renal disease, pneumococcal vaccination in adults aged ≥65 years, and hepatitis A vaccination in people with hepatitis B or hepatitis C infection.81 The NVAC has recommended additional PQRI measures related to vaccinations against pneumococcal disease, herpes zoster, and tetanus-containing vaccines, including a one-time Tdap booster.21
Another reason that providers may not recommend immunization is because of the lack of vaccine inventory in stock. A recent study identified that while many adult physicians stock at least some vaccines, the full complement of recommended adult vaccines was not always kept in stock. While Freed et al.82 did not find that many adult providers were planning to move away from immunization practices, many providers indicated that they did not plan to increase their immunization services. During the stakeholder engagement meetings held by the AIWG, stakeholders identified the need for standards of care that include the provision of immunization services and the ability to refer people to locations where immunizations can be obtained if the health-care provider does not stock that particular vaccine. In addition, stakeholders felt that setting up an adult immunization system in a provider venue is also a barrier to engaging in the vaccine business. Providers need to be aware of available resources, either through their related professional organizations or through groups such as the Immunization Action Coalition, which has produced a step-by-step guide for setting up a new adult immunization system.83
5.5. Barrier: financial impediments to vaccinations
5.5.1. General financial barriers
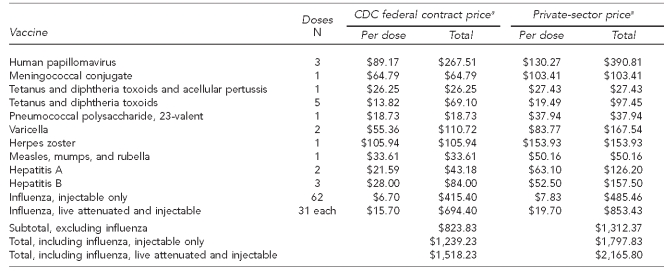
Fully vaccinating a 19-year-old female, including all catch-up vaccines from childhood and adolescence, would require delivery of a total of 20 non-influenza vaccine doses, for a total cost of $1,312, if purchased at the private-sector price. These individual vaccines range in cost from $19 for a dose of Td to $154 for a dose of herpes zoster (shingles) vaccine. Due to individual differences in provider ordering practices, however, these costs may be higher, as was seen in a survey of pediatric immunization inventory costs.84 Additionally, annual influenza vaccination can range from $8 to $20 per dose per year85 (Table 3). This cost does not include other applicable costs (e.g., vaccine administration fees and office visits). The impact of provider practice expenses for adult immunizations has not been documented as well as it has been documented for pediatric vaccinations,84,86 leaving some unanswered questions about adult providers' activities related to vaccine purchase, particularly concerning potential financial loss related to whether patients have public or private health-care coverage.87
Table 3.
Cost to deliver all ACIP-recommended adult immunizations for those aged 19–80 years in the U.S.
aCenters for Disease Control and Prevention (US). Pediatric/Vaccines for Children program price list [cited 2010 Nov 2]. Available from: URL: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm
ACIP = Advisory Committee on Immunization Practices
CDC = Centers for Disease Control and Prevention
While the costs to vaccine recipients for recommended adult immunizations are spread over the individual's lifespan, immunization providers are more directly impacted by the costs to purchase and maintain vaccine inventories. The NVAC previously made recommendations64 on immunization business practices that also apply for adult immunization. Specifically, vaccine manufacturers and third-party vaccine distributors should work with providers on an individual basis to reduce the financial burden for maintaining vaccine inventories, including extended payment periods or deferred payment until the vaccine has been administered and reimbursed, and for medical providers to participate in pools of vaccine purchasers to obtain volume ordering discounts, through joining or forming purchasing collaboratives or through regional vaccine purchasing contracts held by professional medical organizations on behalf of the providers.
However, costs for administering influenza vaccine to adults (including costs related to labor, overhead, and supplies) have been estimated, with per-shot costs of $13.87 to $46.27, which far exceeds the average Medicare payment for influenza vaccine administration.88 The highest costs of administration were seen for smaller, solo providers, while larger corporate provider practices were able to use economies of scale to reduce costs associated with influenza vaccine administration. In these cases, increased utilization of large-scale, lower-cost providers (e.g., retail pharmacies) may be appropriate to reduce the burden of vaccine administration costs. However, additional drivers of this variation need to be examined and understood so that appropriate evidence-based administration reimbursements can be consistently applied to adult immunizations. While the ACA calls for new health plans to cover administration of ACIP-recommended vaccines without cost-sharing, there is no comparable section of the legislation to address variable, and potentially inadequate, vaccination administration reimbursement rates. A survey of pediatric providers found that financial losses were associated with a greater proportion of patients who had publicly funded health insurance compared with private insurance.87
This finding may explain why approximately 25% of physicians who do not provide influenza or pneumococcal vaccinations cited inadequate reimbursement as the reason for not providing these vaccinations.89 In one study of 200 providers regarding perceptions of why adults are not routinely vaccinated against influenza, pneumococcal disease, or tetanus, providers often perceive that patients do not receive vaccines because of inadequate insurance coverage (61%–79%, by provider type and vaccine type) or because the vaccine is too expensive (43%–62%).68 However, it is worth noting that in 2008, America's Health Insurance Plans (AHIP) found that, for adult immunizations, the majority of their enrollees (80%–83%, with variation by vaccine type) had first-dollar coverage (coverage without cost-sharing for patients) for ACIP-recommended vaccines.90 AHIP also found that 84% of high-deductible health plans compatible with health savings accounts provide coverage for recommended preventive services without requiring enrollees to meet their plan deductibles, with 100% of these plans providing first-dollar coverage for adult immunizations.91 Additionally, as discussed in more detail in Section 5.5.2, the ACA calls for non-grandfathered health insurance plans to cover immunizations without cost-sharing, which may impact these cost coverage estimates.
In contrast with the findings of physicians' surveys, financial barriers are not as frequently identified as reasons for not receiving vaccines among surveys of patients. In one study, only 4% of adults with a history of a high-risk condition (e.g., CHD or stroke) who did not plan to receive influenza vaccine cited cost as a barrier.67 In a survey of 2,002 adults, only 14%–17% indicated that a particular vaccine (i.e., tetanus, influenza, or pneumococcal) was not received because insurance failed to cover the vaccination. In this same study, most individuals (67%–72% of adults, by vaccine type) indicated a willingness to pay $25–$30 out of pocket to receive a vaccine.68
However, in another survey of 1,005 adults, 22% of respondents reported not getting vaccines because they had to pay for the vaccine, and 26% had not gotten vaccines because they were too expensive.67 This survey did not measure these concerns by specific vaccine type. This caveat is especially important for the herpes zoster (shingles) vaccine, which is covered under Medicare Part D (rather than Part B) where payment for vaccination services can require (1) a provider to administer the vaccine and bill the patient, which can be submitted for reimbursement; (2) the vaccine to be administered in a pharmacy where it can be directly billed to Medicare Part D; (3) the provider's and patient's Medicare Part D carrier to register on and participate in the Web-based portal for payment (TransactRx); or (4) a provider to administer the vaccine with a pharmacy billing Part D directly through a collaborative agreement. These procedural steps may negatively impact vaccination levels for vaccines covered under Medicare Part D.61 Additionally, stakeholders reported ambiguity around Medicare Part D coverage when “permissive” vaccination recommendations were in place (e.g., Tdap for adults ≥65 years of age), as the lack of a routine immunization recommendation may not always trigger coverage. There have been numerous calls for coverage of all ACIP-recommended vaccines under Medicare Part B, rather than Part D, including a prior NVAC adult immunization recommendation.21 As required in Section 4204(e) of the ACA, this issue is currently under review by the U.S. Government Accountability Office, with a final report anticipated in 2011.
While the cost of the vaccine or its administration may be a barrier to immunization, a tangential barrier related to the financing of adult immunizations is the lack of comprehensive data on the financial benefit of adult immunization. In a 2005 study, it was estimated that during the lifetime of a single-year U.S. birth cohort, utilization of recommended pediatric immunizations would result in direct cost savings of $9.9 billion and indirect societal cost savings of $43.3 billion.92 While cost-effectiveness studies are conducted for individual immunizations and reported in ACIP recommendations, no study similar to the comprehensive pediatric schedule study examining the direct and societal financial impacts of the full complement of adult immunizations has been performed.
5.5.2. Potential impacts of the ACA
The requirement that all new health plans, and health plans that lose their grandfathered status, provide preventive services, including ACIP-recommended vaccinations, with no cost-sharing should reduce or eliminate many of the patient's financial barriers to vaccination that have existed, including issues of underinsurance. The ACA does not address providers' financial barriers to maintaining an immunization inventory. The requirement for preventive services without cost-sharing does not bind grandfathered health plans, leaving some individuals at risk of underinsurance, though mid-range estimates are that by 2013, 51% of all employer-based health plans (66% of small-employer and 45% of large-employer plans) will lose grandfathered status.93 While this requirement clearly addresses services provided by in-network providers, the extension of coverage to nontraditional immunizers who may be considered out of network is less clear.
Some key provisions of the ACA are related to Medicaid coverage. The first provision is the potential for an estimated 18 million new Medicaid enrollees with the revised Medicaid eligibility requirements in the ACA. Increased adult participation in Medicaid will require additional state funds to support Medicaid services, which may impact the allocation of funds available for other immunization services in the states. The second provision is the increase in reimbursement for immunization services covered by Medicaid, during 2013–2014, to the associated rates in Medicare. The impact of the two-year increase in reimbursement levels will need to be examined to identify the extent to which these changes in reimbursement impact immunization levels. The third provision is the 1% Federal Medical Assistance Percentages (FMAP) increase for offering preventive services without cost-sharing. The FMAP increase may lead to additional coverage of immunization services without cost-sharing, but the state-specific decisions for coverage may lead to unresolved inequities.
One additional effect of the ACA provision for preventive services without cost-sharing is the potential for reducing levels of underinsurance for childhood vaccinations, which are currently addressed, in part, through Section 317 grant funding for non-Medicaid-eligible children.65 As such, the majority of Section 317 funds for vaccine purchase or infrastructure are dedicated to serving children, with a related lack of use for adult immunization activities. If a smaller proportion of the Section 317 funds provided to immunization grantees is used for childhood vaccinations following implementation of the ACA, then more funds may be available for purchase of vaccines for adults, particularly with the provision in ACA Section 4204(a)(1)(1), which allows states to purchase vaccines for adults at the federal contract price. Additionally, some stakeholders indicated a desire to use Section 317 infrastructure funds for developing an adult immunization infrastructure, but it is unclear whether infrastructure-specific funds will remain at current levels. Barriers to local public health departments utilizing this ordering process or transfer of vaccine from state purchase supplies to local public health departments are still being examined.
Through the ACA, Medicare will now cover an annual wellness check with the development of a Personalized Prevention Plan, a review of preventive services including immunizations that are recommended for the individual Medicare beneficiary.
5.6. Barrier: lack of access to, and utilization of, health-care services by adults
Whereas children typically undergo a routine series of well-child visits with a well-defined group of providers (e.g., pediatricians and family practitioners), the utilization of health-care services among adults is divided among a wider variety of providers, for a larger variety of health concerns, including more acute care concerns or use of preventive services other than immunizations (e.g., cancer screening). In 2007, 959 million out of an estimated 1.2 billion health-care visits to physicians' offices, hospital outpatient departments, or hospital emergency departments were made by individuals aged 18 years and older. Of these 959 million visits, 799 million (83%) were to physicians' offices, with 410 million of these (51%) consisting of visits to primary care generalists (i.e., nonspecialized general/family practice, internal medicine, obstetric/gynecology, or pediatric physicians).94
As a proportion of total outpatient medical encounters, physicians' office visits were most common among adults aged 65 years and older (89% of total outpatient health-care visits in this age group) and least common among adults aged 18–44 years (77% of total visits in this age group). Similarly, specialist office visits were most common in adults aged 65 years and older (59% of all office visits) and lowest among 18- to 44-year-olds (35% of all office visits).94
In all adult age groups, women were more likely than men to have visited a generalist (18–44 years of age: 71% women vs. 52% men; 45–64 years of age: 50% women vs. 46% men; ≥65 years of age: 45% women vs. 37% men). The increase among adults aged 18–44 years was due primarily to visits to obstetrician/gynecologists, which accounted for 30% of physicians' visits in that group.94 This is an important consideration, as obstetrician/gynecologists may not routinely provide immunizations, though they may be a young woman's sole point of medical contact.
Alternatively, while there appears to be a high level of health-care service utilization by adults, approximately 24% of 18- to 44-year-olds reported no health-care visits in 2007, along with 15% of 45- to 64-year-olds and 7% of adults ≥65 years of age.94 Part of the reason for the lack of health-care utilization among adults, particularly younger adults, may be the lack of emphasis on routine well-adult checkups as opposed to acute care services.95 Szilagyi et al. documented that for physicians who do not provide influenza or pneumococcal vaccinations, approximately 44% indicated it was due to the focus on urgent concerns during the health-care visit, as opposed to routine preventive care.89
With many adults not having a medical home—defined as “an approach to providing comprehensive primary care for children, youth, and adults. [The medical home] is a care setting that facilitates partnerships between individual patients, and their personal physicians, and when appropriate, the patient's family”96—there is a greater need for immunizing adults in alternative venues, including employer-based vaccination clinics, pharmacies, or other nontraditional immunization venues.
With a decentralized distribution system for H1N1 influenza vaccine, states use a variety of venues and distribution points for provision of H1N1 vaccine to adults.10 This variety highlights the need for adult immunization services to be offered in health-care venues beyond traditional primary care providers, including obstetrician/gynecologists, subspecialists, and pharmacists. However, with increasing opportunities for health-care provision in settings other than the primary care medical home, accessibility of immunization records and other medical records must be optimized. Among providers who reported not administering pneumococcal vaccine to adults, 36% cited not knowing their patients' immunization history and 21% cited difficulties in identifying high-risk patients who are in need of vaccination.89 Providers' perceptions of reasons why adults do not get tetanus, influenza, or pneumococcal vaccines included people not making regular well-patient visits (73%–88%, by provider type and vaccine type), lack of an effective reminder system (62%–77%), people not going to the same physician regularly (59%–73%), and not enough time during the office visit (27%–40%).68
A 2006 review of state-level nonphysician immunization practice found that pharmacists in 23 of 51 jurisdictions (50 states and the District of Columbia) could administer immunizations within the purview of their state license or under standing orders.97 However, recent information from the American Pharmacists Association indicated that pharmacists in all states could provide immunization services, though differences in regulations were documented, including differences in the age of people that can be immunized by a pharmacist, which immunizations can be provided, and the requirement for physician prescriptions or standing orders.
5.7. Barrier: lack of utilization of reminder or assessment systems
While the use of reminder systems to improve immunization uptake has been recommended by both the Task Force on Community Preventive Services98 and the Cochrane Collaboration,99 there is not widespread use of IIS, also known as immunization registries, or reminder/recall systems for tracking adult immunizations and reminding adults of vaccinations they are due to receive. In the 2009 CDC IIS Annual Report, 43 of 51 jurisdictions reported having IIS that captured vaccines administered from childhood through adulthood, with approximately 13% of the U.S. population ≥19 years of age having at least one adult immunization recorded in IIS (state-specific levels ranged from <1% to 66% of adults). A 2008 CDC study of state IIS legislation and mandates found only 17 of 51 jurisdictions had immunization reporting mandates, with 44 jurisdictions having a state IIS opt-out system. One common theme from adult immunization stakeholders was the need for greater standardization in these regulations and databases to allow for more consistent electronic record transfer, including bidirectional information exchange among all immunization providers (e.g., physicians, pharmacists, and travel medicine clinics).
While CDC has metrics for measuring childhood participation in IIS,100 and Healthy People 2020 has goals for both childhood and adolescent participation in IIS,56 there are no comparable routinely tracked measures or goals for adults.
The HHS Office of the National Coordinator for Health Information Technology (ONC) is leading national efforts around health IT, including implementing the Health Information Technology for Economic and Clinical Health (HITECH) Act.101 The HITECH Act provides for funding opportunities to advance health IT, such as the meaningful use of electronic health records (EHRs) to help clinicians provide higher quality and safer care for their patients. Through the Medicare and Medicaid EHR Incentive Programs, CMS is providing incentive payments to eligible health-care professionals who adopt certified EHR technology and achieve meaningful use. This eligibility includes an option within a menu of choices to report into -immunization registries and link this information with EHRs. ONC is also working to ensure that health IT activities throughout the U.S. align through the state-level Health Information Exchange Consensus Project.102
5.8. Barrier: racial/ethnic disparities
Differences in adult vaccine coverage and access to preventive services including immunizations persist among racial/ethnic minority groups. However, in some studies that found no racial/ethnic differences in receipt of immunizations and preventive services, only regular patients engaged in primary care were included in the samples, and it may be that access to regular health care is a factor affecting vaccination coverage rates.103,104 Additionally, not all disparities are racial/ethnic in nature, but can be related to socioeconomic status.
As mentioned previously, studies that have examined the factors that may be associated with these racial/ethnic differences have found that access to preventive services differs between white and black adult patients. Physicians serving black Medicare beneficiaries reported greater difficulties in obtaining access to high-quality subspecialists, high-quality diagnostic imaging, and nonemergency admission to the hospital for their patients.105 In addition, in each racial/ethnic minority group, the prevalence of potential missed opportunities to vaccinate for influenza and pneumococcal disease during medical encounters was higher than the prevalence of potential vaccine refusal among adults ≥65 years of age, with a higher proportion of missed opportunity visits for black (27%) and Hispanic (20%) people compared with non-Hispanic white people (16%).106 However, while adjusting for access and type of health care does reduce the vaccination coverage disparities among racial/ethnic minority groups, nonwhite Hispanic adults still have lower rates, pointing to the need to determine and address other factors.
Understanding the impact of culture, attitudes toward health care and health-care providers, and concerns about immunization safety is also intimately linked with racial/ethnic disparities in preventive health-care use. Although provider recommendation is one of the strongest factors influencing vaccine uptake, there is a strong mistrust of government and the health-care system in certain racial/ethnic minority groups.107 While reference is often made to the Tuskegee Syphilis Experiment affecting modern racial/ethnic differences in medical care trust, some researchers have found that this mistrust may exist for factors other than concern over the Tuskegee Syphilis Experiment.108 One area in which this mistrust may be addressed is through outreach regarding vaccination from trusted groups, such as faith-based organizations. One recent randomized trial in San Francisco, California, area churches with >50% racial/ethnic minority composition documented an increased uptake of influenza and pneumococcal vaccines when offered through a church-based vaccine education and provision intervention, compared with attendees of churches without the intervention. Ninety-three percent of participants indicated they believed that vaccines should be administered in churches.74
Another possible reason for racial/ethnic disparities in immunization rates may be a magnification of vaccine supply and availability issues in areas with larger racial/ethnic minority populations. One recent study found that general increases in influenza vaccine availability were associated with a reduction in racial/ethnic vaccination disparities, while general influenza vaccine supply disruptions had an impact on worsening disparities in coverage.109
Reduction of disparities requires a multicomponent program that includes more than culturally sensitive education and availability of vaccine without financial barriers. As discussed previously, “core transmitters” (i.e., trusted community leaders) are essential for the provision of information about vaccines and vaccine-preventable diseases and immunization in areas where subpopulations have disparate immunization uptake.
5.9. Barrier: health literacy
A number of studies have also examined the relationship between individuals' health literacy and use of preventive health services. Health literacy is the degree to which individuals have the capacity to obtain, process, and understand basic health information and services to make appropriate health decisions.110 Studies have found that lower health literacy is independently associated with poorer health status111,112 and lower use of preventive health services.112,113 Importantly, health literacy has been found to partially mediate the association between race/ethnicity and receipt of vaccinations or use of preventive health services.114,115 Interventions that address low health literacy have the potential to reduce the racial/ethnic disparities that exist in adult vaccination, but they may not be the only solution.
The majority of the literature on adults has examined racial/ethnic differences in vaccine coverage for seasonal influenza and pneumococcal vaccines in adults ≥65 years of age, partially because this is the age group for which these vaccines have been universally recommended for the longest time, and because of quality reporting requirements such as those in Medicare and HEDIS. Less is known about the relationship between health literacy and racial/ethnic disparities in vaccine coverage for other adult groups, such as younger healthy adults and younger adults with chronic conditions. However, the evidence that exists is powerful enough to make improving health literacy a national health priority, as evidenced by the publication of the National Action Plan to Improve Health Literacy.116
5.10. Barrier: concern about adverse events
The most commonly cited adverse event that adults are concerned with is the mistaken belief that influenza vaccination can cause clinical influenza infection.67,68,70,76,117–119 General concern about the safety of vaccines has also been expressed; for example, the NFID survey of adults found that 35% of those who did not get at least one recommended vaccine claimed that vaccines are not safe.69 Furthermore, in a survey of 2,002 adults, 34% indicated they were skeptical of receiving any type of vaccine.
With increasing numbers of vaccines recommended for routine use in adults, across a wider range of ages (e.g., HPV vaccine recommended for young adults), safety concerns about new vaccines have been documented120,121 and need to be taken into account when counseling people about vaccination. Additionally, coverage of vaccines under the National Vaccine Injury Compensation Program (NVICP) is restricted to vaccines routinely recommended for use in pediatric or adolescent populations. While the NVICP may cover vaccines administered to adults, this coverage is not explicit in the program.122
People's concerns about vaccine safety are presented in this report for background purposes only. The NVAC currently has a Vaccine Safety Working Group (VSWG) evaluating and making recommendations on the broader national and federal vaccine safety infrastructure, and the NVAC is leaving the development of recommendations related to vaccine safety to the VSWG.
6. CONCLUSIONS
6.1. Overview
As stated previously, there have been many recommendations to improve the state of adult immunization in the U.S. While this report does not provide a large number of new details regarding what needs to be done, it does present a new way of organizing the effort, highlighting the need for a robust adult immunization infrastructure to support routine adult immunization services and deliver vaccines in the event of a pandemic or provide emergency drugs in an outbreak situation. Readiness to address routine issues in adult medical care also leads to enhanced readiness during emergency situations.
This report highlights specific steps that need to be taken, with identification of the federal and nonfederal entities that will be responsible for carrying out each recommended action.
6.2. Coordination of adult immunization activities
The lack of a coordinated national adult immunization program has hindered the ability to achieve adequate adult immunization coverage in the U.S. Without a clear mandate to improve adult immunization, in the form of a coordinated effort that encompasses the expertise and resources of federal and nonfederal partners, true improvements to adult immunization coverage levels will not be made. Whereas gains in childhood immunization were partly made through the use of school-entry mandates, a coordinated system including the VFC program helped achieve higher rates of childhood vaccine coverage.
Thus, the NVAC concludes, in the area of coordination, that there is a need for national leadership and coordination of adult immunization activities, in a top-down fashion as has been developed for pediatric vaccination efforts, involving both governmental (at the federal, state, and local levels) and nongovernmental stakeholders. Once appropriate leadership is in place, plans must be developed, refined, and supported with adequate resources to create an adult vaccination infrastructure to fit the needs of the extremely heterogeneous adult and provider population.
6.3. Lack of public knowledge
People's lack of awareness of the need for adult immunization indicates a need for concerted outreach and communication efforts. While there have been both general and topic-specific adult immunization outreach programs, there remains a gap in understanding vaccination recommendations among adults. Thus, the NVAC concludes, in the area of public knowledge, that there is a need for dedicated, ongoing educational outreach to adults about adult immunization.
6.4. Lack of provider recommendations for immunization
Health-care providers' lack of emphasis on immunizing adults may be due to many factors, including limited time during medical encounters; emphasis on acute medical complaints during the medical visit; or providers' knowledge, attitudes, or practice infrastructure regarding adult immunization. Throughout stakeholder engagement meetings, quality initiatives and outcome measurement were routinely discussed as a means to increase provider recommendations and provision of immunization, while acknowledging that the diverse adult patient population makes one-size-fits-all quality measures difficult to implement.
Thus, the NVAC concludes, in the area of provider recommendations, that:
There is a need for dedicated, ongoing educational outreach to adult health-care providers regarding vaccination recommendations and standards for immunization practices. This outreach is needed both for traditional providers of adult immunization (e.g., family practitioners and internists) as well as nontraditional immunizers (e.g., obstetrician/gynecologists and other subspecialists, pharmacists, and travel medicine clinics).
There is a lack of appropriate standards for assessing adult health-care providers' immunization practices, resulting in a lack of measurable outcomes for QI initiatives.
6.5. Financial issues
Costs associated with immunization services have often been cited as a major barrier in prior reports on adult immunization; as a result, many prior recommendations have addressed vaccine financing as a priority. Reducing client out-of-pocket costs has been recommended by the Task Force on Community Preventive Services as a means to improve vaccination uptake.123 However, even individuals with health insurance that fully covers immunization services are not fully vaccinated. The significance of this barrier, particularly in light of the passage of the ACA, needs to be more fully understood.
It has been estimated that implementation of the ACA will reduce the number of adults without health insurance coverage. Combining this estimated increase in coverage with the requirement that new health plans, and plans that lose their grandfathered status, cover ACIP-recommended immunization without cost--sharing should result in patients having greater access to adult immunizations without additional cost.65 However, providing health-care coverage to individuals does not necessarily mean they will seek care or have adequate access to providers. Additionally, the impact of an increasing number of patients on the provision of services during an office visit is unknown. The initial provisions of the ACA are currently being implemented, and it will take some time to identify the changes related to the provision of preventive services. Once these provisions of the ACA are implemented, health-care providers will need to be made aware of their impact and how best to utilize them within the scope of clinical and preventive practice.
While the ACA requires the provision of immunizations without cost-sharing, it is yet to be determined how vaccine administration costs will be addressed and the extent to which differences in vaccine purchase prices may be handled during reimbursement. Payment for time spent counseling on the benefits and risks of vaccines should be examined.
The potential reduction in underinsured children following implementation of ACA legislation calling for immunization coverage without cost-sharing may make more Section 317 funds available for use by state adult immunization programs.65 Additionally, there is the possibility to recoup the costs of vaccination provided to adults with insurance coverage in local public health departments through a current CDC demonstration project to bill insured individuals immunized in public health departments. While this project may ultimately return some funds to the immunization system, there needs to be careful accounting of the cost and effort required to recoup these funds and the potential impact based on the extent to which adult immunizations are provided in local public health departments. As this demonstration project is currently ongoing, no specific conclusions or recommendations are being made on this topic.
As Section 317 was conceived to address adult immunizations as well as childhood immunizations, there needs to be a concerted effort to ensure adequate allocation of Section 317 funds to adult immunization programs. While it is unclear how widespread this effect will be, the provision in the ACA allowing states to purchase vaccines for adults at the federal contract price could lead to an increase in state-provided safety nets for adult immunization, using vaccines purchased with available Section 317 funds.
Thus, the NVAC concludes, in the area of financial barriers, that:
There is a lack of evidence-based data on (1) the variation of purchase costs for adult immunizations and (2) adult immunization administration costs.
There is uncertainty with regard to the implementation of the ACA, and physicians will need to be made aware of the impact of, and how to implement, these regulations.
There needs to be a focused effort to ensure that Section 317 grant funds, which were at least partially intended for use in adult immunization provision, are made available and used in -sufficient ways to deliver immunizations to un- and underinsured adults.
6.6. Access to, and utilization of, health-care services
Health-care utilization among adults is very fragmented, with adults consulting a wide variety of generalist and specialist providers. There is a relative lack of well-adult visits to generalist providers compared with well-child visits. The focus among adults on addressing acute conditions during medical encounters is a factor in both physicians not recommending preventive services and people not requesting preventive services.
In addition to the potential direct benefits of the ACA related to individual coverage of preventive services, including immunizations, the availability of grant funding for demonstration projects to increase immunization levels offers an opportunity to develop an evidence base for future interventions and programs to improve adult immunization.
Thus, the NVAC concludes, in the area of access to and utilization of health-care services, that
Adults see a large number and variety of health-care providers, spanning both traditional and nontraditional immunizers. Coordinated, targeted outreach to these diverse providers is necessary to ensure optimal adult immunization practices and uptake.
There are many opportunities for outreach to adults regarding immunization, through both health-care- and non-health-care-related organizations (e.g., disease advocacy organizations and societal and cultural organizations) and social media. These opportunities need to be fully utilized to ensure that all avenues of outreach to adults are explored.
6.7. Lack of utilization of reminder or assessment systems
With the wide range of venues and providers through which adults seek health care, interconnectedness is important to ensure that vaccination opportunities are not missed or that adults are not over-immunized. Additional expansion of adults covered through EHRs—through efforts such as the Medicare and Medicaid EHR Incentive Programs, which began January 3, 2011—needs to be monitored to ensure sufficient and adequate use of these systems. This monitoring is especially important for the expanded network of adult immunization providers. Additionally, with the current and pending requirements for IIS reporting based on the proportion of Medicare and Medicaid beneficiaries seen in a practice, and the projected expansion of Medicaid enrollment following full implementation of the ACA, barriers to providers and systems meeting these requirements must be examined and addressed.
Thus, the NVAC concludes, in the area of utilization of reminder or assessment systems, that:
There is inadequate use of EHRs, particularly IIS, to track adult immunization practices across primary care, subspecialty, and nontraditional providers.
Reminder systems, both as part of IIS and stand-alone systems, are not widely used to inform adults about possible upcoming vaccination needs.
While there is concern regarding the potential loss of a comprehensive medical home through widespread immunization outside of primary care providers, for many adults there is no primary medical home, and all points of medical care must be able to communicate quickly and efficiently regarding immunizations. EHRs and IIS will be critical in coordinating adult immunization activities across diverse provider groups and patients.
With nationwide health IT initiatives led by ONC, it will be important to collaborate with the many partners involved in implementing the HITECH Act and related projects to monitor progress and address barriers to achieving widespread use of standardized health IT systems.
6.8. Racial/ethnic disparities and health literacy
While the NVAC acknowledges that health literacy is only one component of racial/ethnic disparities in immunization coverage, the main conclusion involving appropriate leveraging of existing federal efforts is very similar for these two barriers, and they are presented in a combined fashion in this report to reduce redundancy in identified conclusions. Federal initiatives related to addressing racial/ethnic disparities and deficiencies in health literacy are ongoing. Reducing racial/ethnic disparities in adult immunization rates in the U.S. will require a multifaceted approach that addresses access to health care, improving healthy literacy, culturally appropriate provider and public education, working with community and faith-based organizations, and transparent communication about vaccine safety.
Thus, the NVAC concludes, in the area of racial/ethnic disparities and health literacy, that there are opportunities for coordination across ongoing federal initiatives for addressing disparities and health literacy that should not be missed.
6.9. General research needs
Recent steps to better understand adult immunization uptake (e.g., 2007 National Immunization Survey–Adult Module, 2009 NHIS, and H1N1 influenza data-collection programs) have offered an increasingly detailed examination of adult immunization coverage, providing the baseline for future assessments of adult immunization.
Thus, the NVAC concludes, in the area of research needs, that:
Recent increases in data collection and assessment related to adult immunization are critical to understanding the current and future impact of adult immunization. However, there are remaining gaps in this knowledge that can be addressed through targeted research activities.
Implementation of these recommendations will require continued evaluation to identify their impact on addressing barriers to adult immunization.
6.10. Addressing the multifactorial nature of adult immunization barriers
While the previously identified barriers are presented in a discrete fashion, there are many areas in which they interact with each other. The challenge posed by this multifactorial nature indicates that no single solution or program will substantially improve adult immunization rates. As demonstrated by Traeger et al.,124 the wide application of a variety of approaches, in a coordinated manner, can greatly improve adult immunization rates. While Traeger et al.'s evaluation was performed in a relatively limited population of approximately 16,000 people served by the Indian Health Service (IHS) Whiteriver Service Unit on the White Mountain Apache Tribe Fort Apache Indian Reservation, it highlights how a concerted application of medical chart review, electronic management of immunization data, reminder/recall notification systems, standing orders and immunizations prior to inpatient discharge, immunization counseling in a variety of settings, and removal of financial barriers (i.e., IHS services are provided to American Indians/Alaska Natives at no charge) was able to exceed Healthy People 2010 pneumococcal vaccination goals, with maintenance of these immunization rates for multiple years. This study also highlights the importance of coordinated leadership to accomplish a single goal—increasing adult immunization rates to reduce morbidity and mortality.124
6.11. Measures of success
The NVAC proposes three key measures for the success of a comprehensive, national adult immunization program. The first measure is making progress toward, and ultimately achieving and maintaining, Healthy People 2020 adult immunization targets, which encompass vaccinations against influenza, pneumococcal disease, herpes zoster, and hepatitis B. The second measure is observing consistent increased uptake of ACIP-recommended vaccines for adults that are not addressed through the Healthy People 2020 goals (including immunizations against HPV; tetanus, diphtheria, and pertussis; and meningococcal disease). The third measure is the reduction of racial/ethnic disparities in adult immunization from levels currently identified. Routine measurement of adult immunization coverage levels through continued use of the NHIS should allow for direct comparisons of coverage over time.
7. RECOMMENDATIONS
The NVAC proposes three recommendations, all of which address the central issues of leadership of, resources for, and the strategic plan to guide adult immunization in the U.S.
Supplemental to these three recommendations are focused activities recommended to be included as part of the National Adult Immunization Program described in the three strategic recommendations. The focused activities of the National Adult Immunization Program include strengthening the public health infrastructure, expanding access to vaccination, implementing provider- or system-based interventions, increasing community demand for vaccinations, and meeting research needs. Specific activities related to vaccine safety and injury compensation infrastructure for adult vaccines are not addressed in this report, as the NVAC VSWG is currently completing a review and developing recommendations on the U.S. vaccine safety system.
Per the findings from the RAND Corporation evaluation of the NVAC,66 these recommendations were developed and phrased in a manner that would be as effective and directed as possible. Additionally, prioritization is evident from the timelines presented for each of the recommendations and program components.
Note that “providers” as used in these recommendations include all immunization providers (e.g., physicians, pharmacists, and nurses) and related staff in their offices and locations. “Health-care payers” include not just commercial health insurers, but also employers and any other organizations that pay health-care costs.
Given the large number of recommendations related to research needs (see pages 32–35), stakeholder engagement meeting attendees and AIWG members were asked to complete a prioritization ranking of the research components. A detailed description of the ranking methods and findings is presented in the Appendix.
Recommendation #1: national leadership for an adult immunization program
HHS should develop and provide adequate resources for a coordinated and comprehensive National Adult Immunization Program as part of a comprehensive national immunization program, administratively led by the Secretary of HHS, operationally led by CDC, and closely linked to nongovernmental organizations involved in adult immunization.
The Secretary of HHS should designate and empower the ASH as the central point person to coordinate adult immunization activities through HHS. This coordination should occur through either the appointment of an Interagency AIWG or through creation of an AIWG of the existing Interagency Vaccine Group (hereafter referred to as the Interagency Working Group). The Interagency Working Group should comprise representatives from HHS agencies and offices (e.g., CDC, the Food and Drug Administration [FDA], the National Institutes of Health [NIH], IHS, CMS, the Health Resources and Services Administration [HRSA], NVPO, the Office of Disease Prevention and Health Promotion [ODPHP], the Office of Healthcare Quality [OHQ], the Office of Minority Health [OMH], and the ONC) and related non-HHS departments and agencies (e.g., the DVA and the DoD) that would meet regularly to review the contents of and progress toward the strategic plan for adult immunization (see Recommendation #3).
The Interagency Working Group should have subcommittees on issues including, but not limited to, integrating vaccination into other adult preventive care, expanding access to vaccination, provider- or systems-based interventions, increasing community demand for vaccinations, and research. The Interagency Working Group would provide an annual report to the NVAC on progress toward meeting these recommendations and improving adult immunization uptake.
This National Adult Immunization Program should refer to the U.S. childhood immunization program for guidance on methodology and infrastructure development, while acknowledging inherent differences in the populations addressed (e.g., use of school-entry mandates for children). This program should be designed, at a minimum, to meet the adult immunization goals of the National Vaccine Plan and Healthy People 2020, while seeking to increase uptake of all ACIP routinely recommended vaccines for adults.
Directed to the following governmental entities:
HHS: Interagency Working Group, CDC, FDA, NIH, IHS, CMS, HRSA, NVPO, ODPHP, OHQ, OMH, and ONC; DoD; DVA; and the White House Office of Faith-Based and Neighborhood Partnerships
Directed to the following nongovernmental entities:
None
Timeline:
The Interagency Working Group should be established within six months of adoption of these recommendations. A preliminary report on the organization of the group and plans to move forward should be provided to the NVAC within six months of establishment.
Recommendation #2: resources for an adult immunization program and action plan implementation
Appropriate resources (financial and infrastructure) should be allocated by the leadership of the National Adult Immunization Program in consultation with the Interagency Working Group (see Recommendation #1) to carry out the strategic action plan outlined in Recommendation #3. At a minimum, these resources will include staffing for a National Adult Immunization Program office at CDC, at levels sufficient to implement the components outlined after Recommendation #3.
Funding levels could be partially measured through CDC assessments of immunization grantees regarding their projected plans for implementing a widespread adult immunization program within their jurisdiction, with overall coordination through the Interagency Working Group described in Recommendation #1.
CDC and the NVPO should work to use this information, in conjunction with internal expert analyses, to estimate the costs of implementing the components referenced in Section 8 of this report.
Directed to the following governmental entities:
HHS: Interagency Working Group, CDC, FDA, NIH, IHS, CMS, HRSA, NVPO, ODPHP, OHQ, OMH, and ONC; DoD; DVA; the White House Office of Faith-Based and Neighborhood Partnerships; and the Congressional Budget Office (analysis of appropriations)
Directed to the following nongovernmental entities:
None
Timeline:
Assessment of the funding needs for the National Adult Immunization Program should be identified by the Interagency Working Group created in Recommendation #1 by the start of fiscal year 2013, with allocation of these funds by the start of fiscal year 2014.
Recommendation #3: strategic plan for adult immunization
Recommendations #1 and #2, if carried out, would go a long way toward establishing an ongoing strategic plan. Further efforts to define the plan would focus on specific issues within the topic area framework described previously (e.g., expanding access to vaccination, implementing provider- or systems-based interventions, increasing community demand for vaccinations, and conducting research).
The Interagency Working Group created in Recommendation #1 should lead the development of a comprehensive National Strategic Plan for Adult Immunization (hereafter, National Strategic Plan)incorporating input from a broad range of stakeholders (e.g., the public; health-care providers and organizations; federal, state, and local government; insurers, payers, and employers; and vaccine manufacturers), as obtained through a collaborative effort of the NVPO and NVAC. To facilitate continuous refinement and action on the National Strategic Plan, there should be ongoing consultation between nonfederal liaisons (including traditional and nontraditional immunizing health-care providers and their representative professional organizations, health-care payers, employers, public advocacy groups, and vaccine manufacturers) and the Interagency Working Group, including, but not limited to, routine public and stakeholder engagement sessions facilitated by the NVAC.
This plan should be typified by elements of transformation and innovation in rapidly making progress to achieve immunization goals. The goals of this plan should be aligned with the National Vaccine Plan, and, at a minimum, should be designed to meet adult immunization goals specified in Healthy People 2020. Furthermore, efforts of the National Adult Immunization Program to meet these goals should be included in routine NVAC progress reports related to Healthy People 2020 and the National Vaccine Plan.
The Interagency Working Group created in Recommendation #1 will be charged with routine evaluation of the contents of and progress toward the goals of the National Strategic Plan, with these evaluations serving as the basis of the Interagency Working Group annual report to the NVAC.
The main components of the plan should include mechanisms to address, at a minimum, the Recommended Components of an Adult Immunization Program (see Section 8).
Directed to the following governmental entities:
HHS: Interagency Working Group, CDC, FDA, NIH, IHS, CMS, HRSA, NVPO, ODPHP, OHQ, OMH, and ONC; DoD; DVA; CDC immunization grantees; state and local government and public health agencies; state Medicaid agencies; and the White House Office of Faith-Based and Neighborhood Partnerships
Directed to the following nongovernmental entities:
The public, vaccine manufacturers, health-care providers, health-care professional organizations, health-care payers, public health organizations, and employers
Timeline:
National Strategic Plan created within one to three years of adoption of these recommendations
8. FOCUSED ACTIVITIES FOR A COMPREHENSIVE NATIONAL ADULT IMMUNIZATION PROGRAM
Following are recommended activities to address barriers or needs regarding the National Adult Immunization Program, as identified in this report. These activities should be developed and implemented in accordance with the National Strategic Plan identified in Recommendation #3. This is not intended to be an all-inclusive list; rather, these are the key elements required to develop this program.
- General infrastructure considerations
-
Alignment of adult immunization goals across agenciesEfforts should be taken to ensure that adult immunization recommendations and vaccination goals coming from multiple different sources (e.g., ACIP, Healthy People 2020, and the National Vaccine Plan) are harmonized to ensure alignment of priorities.Directed to the following governmental entities: HHS: Interagency Working Group, CDC, and NVPO; and CDC immunization granteesDirected to the following nongovernmental entities: NoneTimeline:One to three years following adoption of these recommendations
-
Adult immunization activities in federal grant guidanceFederal grant guidances (e.g., CDC and HRSA) should contain adult immunization activities as requirements. CDC grants to state and local immunization programs should include funding for a full-time adult immunization coordinator in these grantee locations. These coordinators would be responsible for implementing the components of the adult immunization program as described in this report, including, but not limited to, (1) prioritizing adult vaccines to be purchased using Section 317 funds; (2) coordinating distribution of vaccines to traditional and nontraditional providers of adult vaccines; (3) reaching out to health-care institutions and traditional and nontraditional providers; (4) educating traditional and nontraditional providers about implementing proven strategies to increase vaccination coverage; (5) reaching out to non-health-care employers (particularly small and medium-size businesses) on ways to increase employee vaccination rates by on-site or nearby vaccination clinics; and (6) integrating vaccination into medical care, particularly for high-risk adults, through inclusion of vaccination in disease management checklists and programs such as the initial and annual Medicare prevention benefit. Programmatic plans to define required and recommended activities for these coordinators should be developed through the program and resource requests described in Recommendation #2, including updates to immunization program guides (e.g., the CDC Immunization Program Operations Manual) as necessary.Directed to the following governmental entities: HHS: CDC and HRSA; CDC immunization grantees and HRSA granteesDirected to the following nongovernmental entities: Nongovernmental public health organizationsTimeline: One to three years following adoption of these recommendations
-
Infrastructure development and coordinationCDC should work with governmental partners and appropriate private-sector and nongovernmental organization partners to develop and coordinate appropriate infrastructure for delivery of adult vaccines through the expanding network of adult vaccinators (see Focused Activities 2.c and 3.c) and to coordinate vaccine purchasing contracts that can be utilized by states (see Focused Activity 2.b). This program should address subsequent components in this report, including outreach to existing and newly identified immunization providers to encourage and facilitate vaccine ordering and distribution. Funding of state adult immunization programs, through the resources described in Recommendation #2, could help incentivize the development of this infrastructure.Directed to the following governmental entities: HHS: CDC; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: The public, health-care providers, health-care professional organizations, health-care payers, and vaccine manufacturersTimeline:Three to five years following adoption of these recommendations
-
Quality measures for adult vaccinationExisting quality measures for adult immunization developed through different organizations (e.g., health-care provider professional organizations, accrediting bodies, National Committee for Quality Assurance, National Quality Forum, and state and federal governmental agencies) should be evaluated with a goal of developing more standardized and harmonized metrics wherever possible, acknowledging situations where inherent differences in metrics must be maintained. These metrics should be developed to contain established goals, targets, and appropriate incentives. Goals should not be limited to vaccine uptake, but should also include identification and elimination of barriers to vaccination.Directed to the following governmental entities: HHS: CDC and CMS; and state Medicaid agenciesDirected to the following nongovernmental entities: National Committee for Quality Assurance, National Quality Forum, health-care providers, health-care professional organizations, and health-care payersTimeline:One to three years following adoption of these recommendations
-
- Expanding access to vaccination
-
Ensure a consistent and adequate supply of adult vaccines for the U.S.The barriers to having multiple suppliers for each vaccine licensed and recommended for routine use in adults in the U.S. should be examined. In advance of potential vaccine shortages, stockpiles of vaccines and ancillary supplies for adult vaccines should be considered as methods for minimizing the impact of vaccine shortages. Plans for tracking adherence to changes in vaccine use recommendations during acute and long-term vaccine shortages should be developed, with appropriate communication plans in the event of a shortage.Directed to the following governmental entities: HHS: CDC, FDA, and the Biomedical Advanced Research and Development Authority (BARDA)Directed to the following nongovernmental entities: Vaccine manufacturersTimeline:One to three years following adoption of these recommendations
-
Increase application of Section 317 funds used for adult immunizationWith the potential for savings in Section 317 spending for childhood vaccinations through implementation of the ACA, these available funds should be targeted to adult immunization efforts. State and local governments receiving Section 317 grant funding should identify needs in adult immunization in their jurisdiction, and utilize Section 317 funds, to the extent possible, to address these adult immunization gaps. This use of funds could include the purchase of vaccines for targeted, high-risk populations (e.g., hepatitis A and B vaccine for men who have sex with men and injection drug users, and Tdap and influenza vaccine for postpartum parents), as well as the purchase of vaccines for a wider application of a vaccination safety net for underserved, underinsured, and uninsured adults. State-level vaccine purchase may occur at the federal contract price, per ACA Section 4204(a)(1)(1).Directed to the following governmental entities: HHS: CDC; and CDC immunization granteesDirected to the following nongovernmental entities: Section 317 CoalitionTimeline:Three to five years following adoption of these recommendations.
-
Develop and foster innovative adult immunization partner organization networksExisting partnerships between public health and interested stakeholders to encourage/provide adult vaccination services should be enhanced, and new partnerships should be developed and utilized. Examples of these partnerships include work with nontraditional immunization providers (e.g., pharmacists), organizations targeted to specific conditions (e.g., American Diabetes Association), groups or organizations working with specific populations (e.g., organizers of gay pride celebrations to encourage awareness/uptake of hepatitis B vaccination), groups that may not have regularly provided vaccinations but could be incorporated into education/vaccination campaigns (e.g., vaccine-friendly complementary and alternative medicine providers), and employers who currently run, or are considering developing, on-site or nearby vaccination clinics. Efforts to foster these partnerships may potentially be funded, in part, through immunization pilot project grant funding opportunities specified in Section 4204(m) of the ACA as well as the Community Transformation Grants specified in Section 4201 of the ACA.Directed to the following governmental entities: HHS: CDC, Interagency Working Group, and NVPO; CDC immunization grantees; state and local government/public health agencies; and the White House Office of Faith-Based and Neighborhood PartnershipsDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, health-care payers, disease- and population-specific advocacy groups, public health organizations, and employersTimeline:One to three years following adoption of these recommendations
-
Standardize Medicaid vaccine administration reimbursement rates and mechanismsAn updated Medicaid vaccine administration reimbursement rate for all states (including appropriately set floor and updated ceiling values) should be established that is sufficient to cover all costs of vaccine administration. These rates should be based on appropriate cost studies to define the costs of adult immunization services and evaluation of the 2013–2014 Medicaid reimbursement modification described later in this report. Once updated, these rates should be published annually, with additional updates reflected as necessary. Mechanisms for efficient Medicaid billing, such as roster billing and development of sample billing programs for provider offices, should be widely disseminated.Directed to the following governmental entities: HHS: CDC, CMS, and NVPO; CDC immunization grantees; state and local government/public health agencies; and state Medicaid agenciesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, and health-care payersTimeline:At least five years following adoption of these recommendations
-
- Provider- or systems-based interventions
-
Provider education: QI/quality assurance (QA) activitiesQI/QA activities for health-care providers undertaken by state and local government agencies and insurers should be further developed and refined. These should include the development and dissemination of evidence-based vaccination program models to health-care providers and the provision of vaccination information and educational resources to providers who primarily serve racial/ethnic minority groups. Appropriate methods to incentivize provider recommendations for, provision of, and recordkeeping related to adult immunization (e.g., standing orders, influenza vaccination thresholds, and EHR/IIS use) should be studied, implemented, and evaluated.Directed to the following governmental entities: HHS: CDC, CMS, ONC, OMH, and ODPHP; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: Health-care providers, health care professional organizations, and health-care payersTimeline:One to three years following adoption of these recommendations
-
Provider education: standards of care and immunization practiceStandards of care for immunization services125 (and related publications25,126), as well as best practices and business cases (including information on vaccines covered under Medicare Part B and Part D), should be widely communicated to providers, along with appropriate educational components and resources. Dissemination of best practices in this manner will work to ensure the appropriate and timely provision of vaccination services through evaluation of adherence to these standards of care, which should be regularly done with appropriately aligned incentives in place. Additional techniques for encouraging immunization, especially for adults for whom additional interventions (e.g., motivational interviewing) could be appropriate, should be incorporated. Provider education should also include information about the Vaccine Injury Compensation Program, particularly for specialty physicians who may not understand the coverage under this program.Directed to the following governmental entities: HHS: CDC, CMS, and HRSA; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, and health-care payersTimeline:One year following adoption of these recommendations for dissemination of materials; one to three years following adoption of these recommendations for evaluation
-
Expand the adult immunization provider networkNontraditional immunization providers should be encouraged to provide immunization education and services, particularly when providing services or medications related to comorbidities that are indications for vaccination, with the existing barriers to people and the reimbursement of vaccinations identified and removed. These providers include, but are not limited to, generalist obstetrician/gynecologists, subspecialists, pharmacists, “adult immunization stations” in children's hospitals, “essential community providers” in ACA-created exchanges, and school-located vaccination clinics that can serve adults as well as their children. While underserved areas (e.g., urban areas, areas with a substantial racial/ethnic vaccination disparity, and rural populations) are a main focus, this component should not be limited to these areas only. Connections among traditional providers, public health, and these nontraditional/complementary immunization providers should be established, including provider education activities and cross-linking with EHR and IIS, to leverage these providers as a resource to the public, while also maintaining the strengths of the medical home, when present. Efforts should be made to link providers so that if a vaccine is not available in one venue, appropriate referrals can be given. The initiative to expand the provider network to include nontraditional providers must be supported by an education program focused on the ACA (see Section 3.e) and must address potential issues with payment for immunizations received out of network. Adequate financial resources should be allocated to educate nontraditional providers about the new ACA-based policies and procedures for immunization billing and reimbursement.Directed to the following governmental entities: HHS: CDC, CMS, and ONC; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, pharmacy organizations, community and occupational health immunizers, health-care payers, employers, the Association of State and Territorial Health Officials (ASTHO), the National Association of County and City Health Officials (NACCHO), and pharmaciesTimeline:One to three years following adoption of these recommendations
-
Improve and expand IIS for adult vaccinationsExisting IIS that include childhood immunizations should be expanded to routinely include information for adult vaccinations, with appropriate standards for interoperability and data exchange with providers of adult vaccines. IIS should have the capability to link to EHRs, be accessible to providers in other states and federal departments (e.g., DoD and DVA), and generate reminder lists and notices to individuals who are due for immunizations. Appropriate training and education on IIS should be given to health-care providers, with development and dissemination of appropriate educational materials and toolkits. With the current efforts by ONC to improve and standardize health IT, collaboration among partners will be critical to achieve national goals and address barriers. This collaboration should also include nontraditional providers in health IT initiatives, given the related recommendations on expansion of the adult immunization provider network. The expanded number of health-care providers offering preventive services anticipated from implementation of the ACA should also be considered within the context of health IT initiatives.Directed to the following governmental entities: HHS: CDC, ONC, and CMS; and state and local government/public health agenciesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, health-care payers, community health centers, Association of Immunization Managers, American Immunization Registry Association, ASTHO, and NACCHOTimeline:Three to five years following adoption of these recommendations
-
Educate vaccine providers and partners on health-care reform and immunization business practicesEducation about the provisions of the ACA that are relevant to adult immunization and about best business practices related to immunization services (e.g., inventory management, billing, addressing denied claims, and reduction of barriers related to in-network vs. out-of-network billing reimbursement) should be provided to the public, health-care providers and related organizations, health-care payers, employers, and other key stakeholders. Such a comprehensive educational initiative will help to remove obstacles to adult vaccination that currently exist due to providers' and/or payers' misunderstandings about the immunization-related provision of the ACA and about general immunization business practices that may reduce financial barriers to maintaining a vaccine inventory in providers' offices (see Recommendations #7, #8, #9, #10, #11, and #13 in the 2008 NVAC recommendations on pediatric and adolescent vaccine financing64). Health-care exchanges created through the ACA should also include education on immunization business practices.Directed to the following governmental entities: HHS: CDC; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: The public, health-care providers, health-care professional organizations, health-care advocacy organizations, health-care payers, and employersTimeline:Within one year following adoption of these recommendations
-
- Increasing community demand for vaccinations
-
Develop and implement an ongoing, comprehensive education and outreach campaign on adult vaccines, directed to both the public and providersA widespread education campaign should be developed and implemented to increase the valuation given by the public and providers to adult vaccination. In addition to utilizing scientific, medical, health-care payer, and public health communications expertise, experts in behavioral sciences (e.g., cognitive psychology, anthropology, and sociology) should be consulted during the development of this continual campaign. The National Adult Immunization Program office at CDC should be funded to hire staffers dedicated to media/public education programs regarding immunization. Additional components of this campaign should include vaccine availability and recommendations, information about the diseases that can be prevented, vaccine safety and vaccine myths, and where to obtain vaccines (including complementary providers). This campaign should use a variety of sources, including electronic media (e.g., www-- .vaccines-.gov), social media, television, national and community print and radio media, and information in immunization provider venues and other outlets frequented by adults. Information about health literacy obtained by the HHS ODPHP should be shared with health-care provider organizations to increase awareness about limitations in health literacy and to ensure the development of culturally and linguistically appropriate materials. Recommendations from this initiative should be clearly communicated to providers to improve communication between providers and the people they serve. One area of emphasis should be during National Adult Immunization Month.Directed to the following governmental entities: HHS: Interagency Working Group, CDC, OMH, ODPHP, and NVPO; CDC immunization grantees; state and local government/public health agencies; and the White House Office of Faith-Based and Neighborhood PartnershipsDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, health-care payers, academia, behavioral scientists, community and faith-based organizations, media, and vaccine manufacturersTimeline:One to three years following adoption of these recommendations
-
- Research needs
-
Establish costs of administering adult vaccines, and base reimbursement of vaccine administration on these costsThe cost of administering adult vaccinations, in addition to existing studies on influenza vaccine, should be examined and defined based on evidence-based, realistic estimates of cost and time, in a variety of settings (e.g., provider offices, pharmacies, and Federally Qualified Health Centers). These estimates should include all components that go into vaccine administration, including vaccine storage, handling, alarms, inventory management, loss insurance, staffing, data entry into immunization registries, and counseling. Reimbursement for adult vaccine administration should be guided by these cost studies.Directed to the following governmental entities: HHS: CDC, CMS, HRSA, and NVPO; state and local government; and state Medicaid agenciesDirected to the following nongovernmental entities: Health-care payers, health-care providers, and health-care professional organizationsTimeline:One to three years following adoption of these recommendations
-
Continued collection and evaluation of adult immunization dataImplementation of 2009 NVAC adult immunization recommendations calling for increased resources to evaluate adult immunization coverage data should be critically evaluated, with progress reports provided to key governmental partners (e.g., CDC and state governments). Evaluation should entail annual assessment of adult immunization levels through the NHIS, as well as monitoring trends in adult vaccine-preventable disease levels to identify the impact of adult vaccinations on morbidity and mortality. Where possible, examination of state-level data should be conducted. In the absence of benchmarks for adult immunization data in IIS, the IIS Annual Report should continue to track the level of adult immunizations captured in IIS.Directed to the following governmental entities: HHS: CDC; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: The public, health-care providers, health-care professional organizations, health-care payers, and employersTimeline: Ongoing
-
Study of the economic benefits of adult immunizationA study of the economic impacts of adult immunization, modeled after a similar evaluation of childhood vaccines92 and including the impact on health-care costs and societal costs (including employment and productivity), should be conducted.Directed to the following governmental entities: HHS: CDCDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, and academiaTimeline:One to three years following adoption of these recommendations
-
Study of the impact of differing medical care reimbursement systems on vaccine uptakeAn evaluation of the impact of differing medical care reimbursement systems (e.g., public vs. private insurance) on achieving pre-specified adult immunization benchmarks (e.g., approaching Healthy People 2020 goals) should be conducted, particularly in light of recent findings from pediatric providers indicating that financial losses due to inadequate reimbursement may affect decisions to offer vaccinations in their practices.127Directed to the following governmental entities: HHS: CDC and CMS; and state Medicaid agenciesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, academia, and health-care payersTimeline:At least five years following adoption of these recommendations
-
Provider education: health-care professional trainingAn evaluation of the depth and breadth of education and related certification testing on vaccine-preventable diseases, vaccination science, recommended vaccination schedules, and adverse event identification and reporting in health-care provider training (including training for physicians, nurses, medical assistants, physicians assistants, nurse practitioners, and pharmacists) should be performed. Following this evaluation, recommendations on improvements to the educational process at all levels to better incorporate vaccination in practice should be developed and promulgated.Directed to the following governmental entities: HHS: CDC and NVPODirected to the following nongovernmental entities: Medical boards; health-care professional organizations; schools of medicine, nursing, and other professions; continuing education (e.g., continuing medical education, continuing nursing education, and continuing professional education sources, such as provider organizations); and hospitalsTimeline:At least five years following adoption of these recommendations
-
Study of adult health-care providers to further examine provider vaccine stocking and administration practices and the relationship to vaccination coverage disparitiesFollowing the recent study of provider vaccine stocking and administration prayctices,82 additional targeted examinations of providers in areas with large racial/ethnic or socioeconomic disparities in adult immunization coverage should be conducted, to identify potential barriers to reducing these disparities.Directed to the following governmental entities: HHS: CDCDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, and academiaTimeline:One to three years following adoption of these recommendations
-
Evaluation of 2013–2014 Medicaid reimbursement modificationA study of the potential differences in vaccination patterns or trends among Medicaid beneficiaries during the 2013–2014 Medicaid reimbursement rate modification legislated in ACA should be conducted. This study should involve a detailed examination of adult immunization rates among Medicaid beneficiaries through 2015, to generate baseline immunization coverage data, and to allow comparisons during the time of the Medicaid reimbursement adjustment and after completion of the two-year pilot period.Directed to the following governmental entities: HHS: CDC and CMSDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, academia, and health-care payersTimeline:Three to five years following adoption of these recommendations
-
Study public and provider knowledge, attitudes, and practices related to adult vaccination after implementation of these recommendationsFollowing development of the National Adult Immunization Program and implementation of the key components of the program, as described in this report, studies should be undertaken to evaluate the types of policy interventions developed. The long-term goal will be to identify public and provider attitudes toward vaccination, with appropriate comparisons to surveys conducted prior to implementation of these recommendations. One model for rapidly assessing attitudes includes the routine public opinion polling conducted in the United Kingdom. Specific study topics should include, but not be limited to, knowledge about vaccines and vaccine-preventable diseases, perceptions among the public and providers regarding who is responsible for ensuring vaccination, acceptance of vaccination, barriers to uptake of vaccination, safety concerns related to vaccination, and providers' actual vaccination practices. Detailed examination by age, race/ethnicity, and other exposure to vaccines (e.g., parents of children whose vaccination status is known) should be included.Directed to the following governmental entities: HHS: CDC, CMS, HRSA and the Agency for Healthcare Research and Quality (AHRQ)Directed to the following nongovernmental entities: Health-care providers, health-care professional organizations, and academiaTimeline:Three to five years following adoption of these recommendations
-
Standardized evaluation of adult vaccination in nontraditional immunization venuesStudies and demonstration programs should be undertaken to evaluate adult vaccination services provided in settings complementary to the medical home (e.g., pharmacy-based vaccinations, mass vaccination clinics, college/university clinics, employee health services, and targeted community events [e.g., community festivals, religious events, and community center events]). These evaluations should include assessments of both potential increases in overall vaccine coverage as well as shifts in location of vaccination. These evaluations should be informed by the core metrics presented in the 2000 NVAC report, “Adult Immunization Programs in Nontraditional Settings: Quality Standards and Guidance for Program Evaluation.”24 Potential mechanisms for funding these evaluations and demonstration projects that should be considered are the immunization pilot project grant funding opportunities specified in Section 4204(m) of the ACA as well as the Community Transformation Grants specified in Section 4201 of the ACA.Directed to the following governmental entities: HHS: CDCDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, health-care payers, and academiaTimeline:Three to five years following adoption of these recommendations
-
Better understand the impact of health literacy on vaccinations and vaccination disparitiesThe impact of health literacy on racial/ethnic disparities in vaccination levels should be further examined. Current efforts by the HHS OMH and ODPHP should be coordinated to ensure that efforts are not being duplicated and that appropriate expertise is being used across offices and initiatives. Findings from this research should be used to ensure development of culturally and socially appropriate materials in the communications program recommended in Focused Activity 4.a.Directed to the following governmental entities: HHS: Interagency Working Group, CDC, OMH, ODPHP; White House Office of Faith-Based and Neighborhood Partnerships; and the NIH Institute on Minority Health and Health DisparitiesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, and academiaTimeline:Three to five years following adoption of these recommendations
-
Research into optimal use of social networkingThe use and impact of social networking, including, but not limited to, social media, on education, outreach, and adult vaccination seeking, should be further examined. This research should include the identification and effectiveness of using core transmitters in social networks as a point of dissemination of adult immunization messages.Directed to the following governmental entities: HHS: CDC, OMH, and ODPHP; and the White House Office of Faith-Based and Neighborhood PartnershipsDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, and academiaTimeline:Three to five years following adoption of these recommendations
-
Research into state-level policies and practices related to adult immunization provisionState-specific policy differences related to adult immunization, including use of IIS (e.g., ability to record adult immunization, differences in opt-in/opt-out provisions, legal mandates for data entry, and use by nontraditional vaccinators), as well as policies for vaccine administration by nontraditional vaccinators (e.g., use of standing orders, and which health-care providers are authorized to vaccinate), should be examined.Directed to the following governmental entities: HHS: CDC and ONC; CDC immunization grantees; and state and local government/public health agenciesDirected to the following nongovernmental entities: Health-care providers, health-care professional organizations, academia, Association of Immunization Managers, and American Immunization Registry AssociationTimeline:Three to five years following adoption of these recommendations.
-
Research into developing new and improved vaccines and vaccine delivery systemsDevelopment of new adult vaccines and improvements to existing adult vaccines should be undertaken to ensure the supply and availability of vaccines that are efficacious and effective in the adult population. Development of methods to accurately and more rapidly assess immunogenicity and vaccine efficacy in adult populations should occur concomitantly, to facilitate this type of vaccine development.Directed to the following governmental entities: HHS: NIH, FDA, BARDA, and CDCDirected to the following nongovernmental entities: Academia and vaccine manufacturersTimeline:The next decade following adoption of these recommendations
-
10. Appendix
Prioritization survey of NVAC Adult Immunizaton Working Group members and stakeholder groups on research recommendations for adult immunization, April–May 2011, Chicago, Illinois
Stakeholders who participated in the April 8, 2011, stakeholder meeting in Chicago, Illinois, suggested that the research needed to be prioritized to identify the top areas where more research is needed. Prioritization could also assist groups in adult immunization program planning, where resources are limited. The Adult Immunization Working Group (AIWG) of the National Vaccine Advisory Committee (NVAC) discussed the suggestion during a conference call on April 12, 2011, and agreed to survey organizations that had attended either of the two stakeholder meetings. A SurveyMonkey Web-based survey was designed and e-mailed to roughly 80 stakeholder groups (SurveyMonkey.com, LLC). Participants were asked to rank each research item from 1 to 5, with 1 being highest priority and 5 being lowest priority. The survey was open April 21–28, 2011, and 18 responses were received.
The results of the survey were shared with the AIWG and discussed during the group's May 3, 2011, conference call. The AIWG decided to reopen the survey to the stakeholder groups for a higher response rate, and to open the survey to AIWG members for input. The same SurveyMonkey survey was used and was open May 3–13, 2011.
Please note that the research needs in the survey were those that were in version 2.0 of the report, which was open for public comment, plus two additional items proposed by stakeholders. The final version of the report reflects changes that were made subsequent to public comment and the stakeholders' meetings, and the final research needs may differ from those in the survey.
Survey results.
 |
| 4. Prioritization results: all respondents (n=37) |
|---|
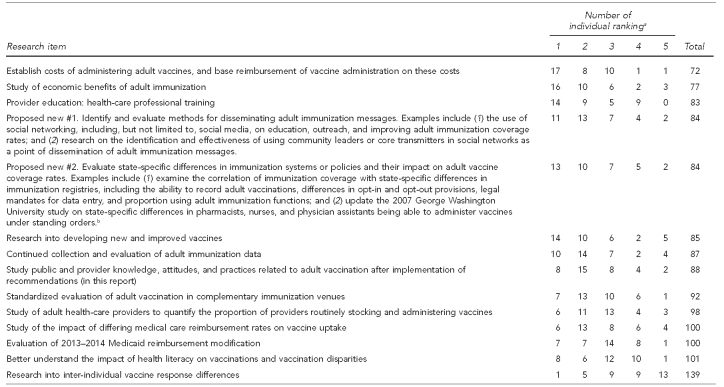 |
| 5. Prioritization results: stakeholders only (n=25) |
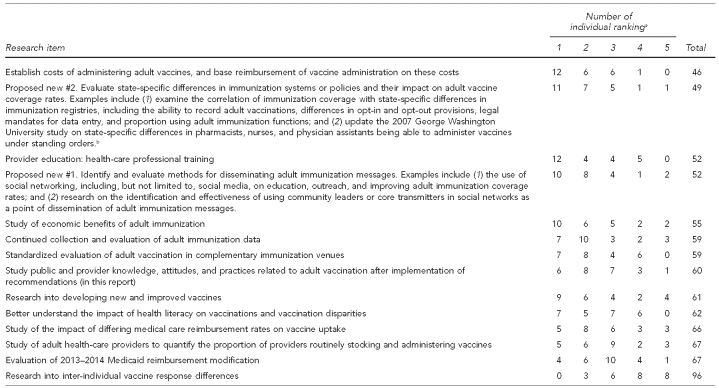 |
| 6. Prioritization results: Adult Immunization Working Group members only (n=12) |
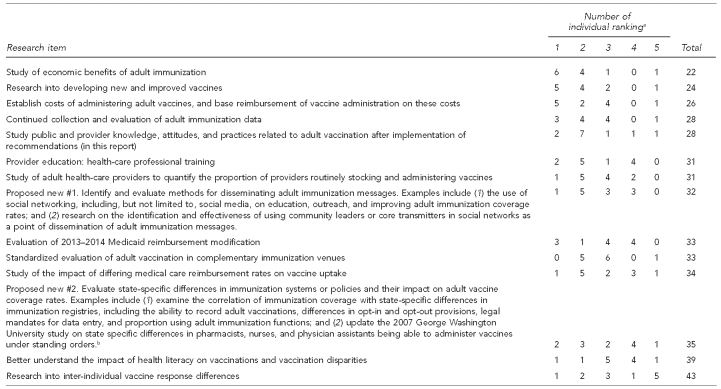 |
a1 = highest priority, 5 = lowest priority
bGeorge Washington University School of Public Health and Health Services. Standing orders project [cited 2011 Jul 28]. Available from: URL: http://www.gwumc.edu/sphhs/departments/healthpolicy/immunization/2010-Standing-Orders/index.cfm
Footnotes
NVAC Chair
Adult Immunization Working Group Co-Chair
National Vaccine Program Office Designated Federal Official
Current affiliation: Emory University, Rollins School of Public Health, Atlanta, GA
9. REFERENCES
- 1.Roush SW, Murphy TV Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298:2155–63. doi: 10.1001/jama.298.18.2155. [DOI] [PubMed] [Google Scholar]
- 2.National state and local area vaccination coverage among children aged 19–35 months—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(36):1171–7. [PubMed] [Google Scholar]
- 3.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 4.Preventive Services Task Force (US) Recommendations. [cited 2010 Oct 20]. Available from: URL: http://www.uspreventiveservicestaskforce.org/recommendations.htm.
- 5.Centers for Disease Control and Prevention (US) Advisory Committee for Immunization Practices recommendations. [cited 2010 Oct 20]. Available from: URL: http://www.cdc.gov/vaccines/pubs/ACIP-list.htm.
- 6.Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600–9. doi: 10.1016/j.amepre.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Pollak KI, Krause KM, Yarnall KS, Gradison M, Michener JL, Østbye T. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245. doi: 10.1186/1472-6963-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarnall KS, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (US), National Center for Immunization and Respiratory Diseases. Immunization grant program (section 317) 2007. [cited 2011 Jan 3]. Available from: URL: http://www.cdc.gov/ncird/progbriefs/downloads/grant-317.pdf.
- 10.Stroud C, Nadig L, Altevogt BM, editors. Washington: National Academies Press; 2010. Institute of Medicine, Board on Health Sciences Policy, Forum on Medical and Public Health Preparedness for Catastrophic Events. The 2009 H1N1 influenza vaccination campaign: summary of a workshop series. [Google Scholar]
- 11.Rambhia KJ, Watson M, Sell TK, Waldhorn R, Toner E. Mass vaccination for the 2009 H1N1 pandemic: approaches, challenges, and recommendations. Biosecur Bioterror. 2010;8:321–30. doi: 10.1089/bsp.2010.0043. [DOI] [PubMed] [Google Scholar]
- 12.Trust for America's Health, Infectious Diseases Society of America, Robert Wood Johnson Foundation. Adult immunization: shots to save lives. Washington: Trust for America's Health; 2010. [Google Scholar]
- 13.Centers for Disease Control and Prevention (US) 2007 National Immunization Congress: adult and adolescent immunization. Summary. [cited 2010 Mar 1]. February 28–March 1, 2007. Available from: URL: http://www.strategichealthpolicy.com/portals/0/articles/2007_National_Immunization_Congress.pdf.
- 14.High KP. Overcoming barriers to adult immunization. J Am Osteopath Assoc. 2009;109(6 Suppl 2):S25–8. [PubMed] [Google Scholar]
- 15.Hinman AR, Orenstein WA. Adult immunization: what can we learn from the childhood immunization program? Clin Infect Dis. 2007;44:1532–5. doi: 10.1086/519543. [DOI] [PubMed] [Google Scholar]
- 16.Infectious Diseases Society of America, National and Global Public Health Committee, Immunization Work Group. Actions to strengthen adult and adolescent immunization coverage in the United States: policy principles of the Infectious Diseases Society of America. Clin Infect Dis. 2007;44:e104–e8. doi: 10.1086/519541. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services (US), National Vaccine Program Office, National Vaccine Advisory Committee. Report of the Subcommittee on Adult Immunization. Washington: HHS; 1990. [Google Scholar]
- 18.Department of Health and Human Services (US), National Vaccine Program Office, National Vaccine Advisory Committee. Washington: HHS; 1994. Adult immunization: a report by the National Vaccine Advisory Committee. [Google Scholar]
- 19.Department of Health and Human Services (US), National Vaccine Program Office, National Vaccine Advisory Committee. Washington: HHS; 1997. Adult immunization action plan: report of the Workgroup on Adult Immunization. [Google Scholar]
- 20.Department of Health and Human Services (US), National Vaccine Program Office, National Vaccine Advisory Committee. Washington: HHS; 2004. Strengthening the nation's influenza vaccination system: an NVAC assessment. [Google Scholar]
- 21.Department of Health and Human Services (US), National Vaccine Program Office, National Vaccine Advisory Committee. Washington: HHS; 2009. Recommendations for federal adult immunization programs regarding immunization delivery, assessment, research, and safety monitoring. [Google Scholar]
- 22.Orenstein WA, Mootrey GT, Pazol K, Hinman AR. Financing immunization of adults in the United States. Clin Pharmacol Ther. 2007;82:764–8. doi: 10.1038/sj.clpt.6100401. [DOI] [PubMed] [Google Scholar]
- 23.Partnership for Prevention. Strengthening adult immunization: a call to action. Washington: Partnership for Prevention; 2005. [Google Scholar]
- 24.Postema AS, Breiman RF National Vaccine Advisory Committee. Adult immunization programs in nontraditional settings: quality standards and guidance for program evaluation. MMWR Recomm Rep. 2000;49(RR-1):1–13. [PubMed] [Google Scholar]
- 25.Poland GA, Shefer AM, McCauley M, Webster PS, Whitley-Williams PN, Peter G, et al. Standards for adult immunization practices. Am J Prev Med. 2003;25:144–50. doi: 10.1016/s0749-3797(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 26.2010. Healthcare and Education Reconciliation Act. Pub. L. No. 111-52, 124 Stat. 1029.
- 27.2010. Patient Protection and Affordable Care Act. Pub. L. No. 111-48, 124 Stat. 119.
- 28.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 29.Thompson WW, Weintraub E, Dhankhar P, Cheng PY, Brammer L, Meltzer MI, et al. Estimates of U.S. influenza-associated deaths made using four different methods. Influenza Other Respi Viruses. 2009;3:37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintrub E, et al. The annual impact of seasonal influenza in the U.S.: measuring disease burden and costs. Vaccine. 2007;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–62. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (US) Active Bacterial Core Surveillance report, Emerging Infections Program Network, Streptococcus pneumonia. 2009. [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/abcs/reports-findings/survreports/spneu09.pdf.
- 33.Centers for Disease Control and Prevention (US) Active Bacterial Core Surveillance report, Emerging Infections Program Network, Streptococcus pneumonia. 2000. [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/abcs/reports-findings/survreports/spneu00.pdf.
- 34.Lexau CA, Lynfield R, Danila R, Pilishvili T, Facklam R, Farley MM, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 35.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 36.Notes from the field: pertussis—California, January–June 2010. MMWR Morb Mortal Wkly Rep. 2010;59(26):817. [Google Scholar]
- 37.Centers for Disease Control and Prevention (US) Pertussis (whooping cough): outbreaks. [cited 2010 Sep 24]. Available from: URL: http://www.cdc.gov/pertussis/outbreaks.html.
- 38.Hall-Baker PA, Nieves E, Jr, Jajosky RA, Adams DA, Sharp P, Anderson WJ, et al. Summary of notifiable diseases—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;57(54):1–94. [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (US) Pertussis. In: Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Epidemiology and prevention of vaccine-preventable diseases. 11th ed. Washington: Public Health Foundation; 2009. pp. 199–216. [Google Scholar]
- 40.Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ. 2009;58(3):1–27. [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention (US) Viral hepatitis statistics and surveillance. [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/hepatitis/Statistics/index.htm.
- 42.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 43.Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health. 2005;5:68. doi: 10.1186/1471-2458-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 45.Gialloreti L, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137:38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez Chiappe S, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, et al. Herpes zoster: burden of disease in France. Vaccine. 2010;28:7933–8. doi: 10.1016/j.vaccine.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 48.Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, MacIntyre CR. Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine. 2009;27:520–9. doi: 10.1016/j.vaccine.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-5):1–30. quiz CE32-34. [PubMed] [Google Scholar]
- 50.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 51.Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–15. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 52.Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33:2058–63. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (US), National Program of Cancer Registries. United States cancer statistics: 1999–2006 incidence and mortality Web-based report. [cited 2010 Aug 25]. Available from: URL: http://www.cdc.gov/cancer/npcr/uscs/2007/download_data.htm.
- 54.Centers for Disease Control and Prevention (US) Sexually transmitted diseases. Genital human papillomavirus (HPV) infection—fact sheet. [cited 2010 Sep 23]. Available from: URL: http://www.cdc.gov/std/HPV/STDFact-HPV.htm.
- 55.Centers for Disease Control and Prevention (US), National Program of Cancer Registries. United States cancer statistics: 2007 cancer types grouped by race and ethnicity. [cited 2011 Apr 29]. Available from: URL: http://apps.nccd.cdc.gov/uscs/cancersbyraceandethnicity.aspx.
- 56.Department of Health and Human Services (US) Healthy People 2020 objectives. [cited 2011 Jan 3]. Available from: URL: http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf.
- 57.Centers for Disease Control and Prevention (US) Seasonal influenza. Final estimates for 2009–2010 seasonal influenza and influenza A (H1N1) 2009 monovalent vaccination coverage—United States, August 2009 through May 2010. [cited 2010 Oct 20]. Available from: URL: http://www.cdc.gov/flu/professionals/vaccination/coverage_0910estimates.htm.
- 58.Influenza vaccination coverage among children and adults—United States, 2008–2009 influenza season. MMWR Morb Mortal Wkly Rep. 2009;58(39):1091–5. [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention (US) Vaccines and immunizations. Statistics and surveillance: 2009 adult vaccination coverage, National Health Interview Survey. [cited 2011 Jan 3]. Available from: URL: http://www.cdc.gov/vaccines/stats-surv/nhis/2009-nhis.htm.
- 60.Recommended adult immunization schedule—United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1–4. [PubMed] [Google Scholar]
- 61.Hurley LP, Lindley MC, Harpaz R, Stokley S, Daley MF, Crane LA, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med. 2010;152:555–60. doi: 10.7326/0003-4819-152-9-201005040-00005. [DOI] [PubMed] [Google Scholar]
- 62.Institute of Medicine, Division of Health Care Services and Division of Health Promotion and Disease Prevention, Committee on Immunization and Finance Policies and Practices. Calling the shots: immunization finance policies and practices. Washington: National Academies Press; 2000. [PubMed] [Google Scholar]
- 63.Shen AK, Sobzcyk E, Buchanan A, Wu L, Duggan-Goldstein S. Second National Immunization Congress 2010: addressing vaccine financing for the future in the U.S. Hum Vaccin. 2011;7:12–8. doi: 10.4161/hv.7.1.13949. [DOI] [PubMed] [Google Scholar]
- 64.Lindley MC, Orenstein WA, Shen AK, Rodewald LE, Birkhead GS National Vaccine Advisory Committee Vaccine Financing Working Group. Washington: Department of Health and Human Services (US); 2009. [cited 2011 Apr 20]. Assuring vaccination of children and adolescents without financial barriers: recommendations from the National Vaccine Advisory Committee. Also available from: URL: http://www.hhs.gov/nvpo/nvac/nvacfwgreport.pdf. [Google Scholar]
- 65.Stewart A. Health reform and vaccines: review of federal legislation. The Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act. Presented at a meeting of the National Vaccine Advisory Committee; 2010 Jun 2–3; Washington, DC. [cited 2011 Jan 3]. Also available from: URL: http://www.hhs.gov/nvpo/nvac/meetings/pastmeetings/stewart060210.ppt. [Google Scholar]
- 66.Ringel J, Adelson M, Harris K, Khodyakov D, Lurie N. Santa Monica (CA): RAND Corporation; 2009. Improving the impact and effectiveness of the National Vaccine Advisory Committee. [Google Scholar]
- 67.Madjid M, Alfred A, Sahai A, Conyers JL, Casscells SW. Factors contributing to suboptimal vaccination against influenza: results of a nationwide telephone survey of persons with cardiovascular disease. Tex Heart Inst J. 2009;36:546–52. [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008;121(7 Suppl 2):S28–35. doi: 10.1016/j.amjmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 69.National Foundation for Infectious Diseases. Bethesda (MD): National Foundation for Infectious Diseases; 2008. National survey on adult vaccination reports low consumer awareness of vaccines and the risks of vaccine-preventable diseases. [Google Scholar]
- 70.Reasons reported by Medicare beneficiaries for not receiving influenza and pneumococcal vaccinations—United States, 1996. MMWR Morb Mortal Wkly Rep. 1999;48(39):886–90. [PubMed] [Google Scholar]
- 71.Influenza vaccination and self-reported reasons for not receiving influenza vaccination among Medicare beneficiaries aged ≥65 years—United States, 1991–2002. MMWR Morb Mortal Wkly Rep. 2004;53(43):1012–5. [PubMed] [Google Scholar]
- 72.Matthews AK, Berrios N, Darnell JS, Calhoun E. A qualitative evaluation of a faith-based breast and cervical cancer screening intervention for African American women. Health Educ Behav. 2006;33:643–63. doi: 10.1177/1090198106288498. [DOI] [PubMed] [Google Scholar]
- 73.Luque JS, Tyson DM, Markossian T, Lee JH, Turner R, Proctor S, et al. Increasing cervical cancer screening in a Hispanic migrant farmworker community through faith-based clinical outreach. J Low Genit Tract Dis. 2011;15:200–4. doi: 10.1097/LGT.0b013e318206004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daniels NA, Juarbe T, Moreno-John G, Pérez-Stable EJ. Effectiveness of adult vaccination programs in faith-based organizations. Ethn Dis. 2007;17(1 Suppl 1):S15–22. [PubMed] [Google Scholar]
- 75.Department of Health and Human Services (US) H1N1 flu: a guide for community and faith-based organizations. [cited 2011 May 1]. Available from: URL: http://www.pandemicflu.gov/professional/community/cfboguidance.html.
- 76.Lindley MC, Wortley PM, Winston CA, Bardenheier BH. The role of attitudes in understanding disparities in adult influenza vaccination. Am J Prev Med. 2006;31:281–5. doi: 10.1016/j.amepre.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 77.Ahluwalia IB, Singleton JA, Jamieson DJ, Rasmussen SA, Harrison L. Seasonal influenza vaccine coverage among pregnant women: pregnancy risk assessment monitoring system. J Womens Health (Larchmt) 2011;20:649–51. doi: 10.1089/jwh.2011.2794. [DOI] [PubMed] [Google Scholar]
- 78.Broughton DE, Beigi RH, Switzer GE, Raker CA, Anderson BL. Obstetric health care workers' attitudes and beliefs regarding influenza vaccination in pregnancy. Obstet Gynecol. 2009;114:981–7. doi: 10.1097/AOG.0b013e3181bd89c2. [DOI] [PubMed] [Google Scholar]
- 79.American College of Physicians. Running a practice. Quality improvement. Adult immunization. [cited 2011 Jan 7]. Available from: URL: http://www.acponline.org/running_practice/quality_improvement/library/immun.htm.
- 80.National Committee for Quality Assurance. Healthcare effectiveness data and information set and quality measurement. National Quality Forum-endorsed measures: list of measures. [cited 2011 Jan 7]. Available from: URL: http://www.ncqa.org/LinkClick.aspx?fileticket=p6Oa24wNQ6Y%3d&tabid=59&mid=1604&forcedownload=true.
- 81.Department of Health and Human Services (US), Centers for Medicare and Medicaid Services. 2010 Physician Quality Reporting Initiative measures list. [cited 2011 Jan 7]. Available from: URL: https://www.cms.gov/PQRI/Downloads/2010_PQRI_MeasuresList_111309.pdf.
- 82.Freed GL, Clark SJ, Cowan AE, Coleman MS. Primary care physician perspectives on providing adult vaccines. Vaccine. 2011;29:1850–4. doi: 10.1016/j.vaccine.2010.12.097. [DOI] [PubMed] [Google Scholar]
- 83.Immunization Action Coalition. Adult vaccination guide. [cited 2011 Jul 28]. Available from: URL: http://www.immunize.org/guide.
- 84.Freed GL, Cowan AE, Gregory S, Clark SJ. Variation in provider vaccine purchase prices and payer reimbursement. Pediatrics. 2009;124(Suppl 5):S459–65. doi: 10.1542/peds.2009-1542E. [DOI] [PubMed] [Google Scholar]
- 85.Centers for Disease Control and Prevention (US) Pediatric/Vaccines for Children program price list. [cited 2010 Nov 2]. Available from: URL: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm.
- 86.Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics. 2009;124(Suppl 5):S492–8. doi: 10.1542/peds.2009-1542H. [DOI] [PubMed] [Google Scholar]
- 87.Coleman MS, Lindley MC, Ekong J, Rodewald L. Net financial gain or loss from vaccination in pediatric medical practices. Pediatrics. 2009;124(Suppl 5):S472–91. doi: 10.1542/peds.2009-1542G. [DOI] [PubMed] [Google Scholar]
- 88.Coleman MS, Fontanesi J, Meltzer MI, Shefer A, Fishbein DB, Bennett NM, et al. Estimating medical practice expenses from administering adult influenza vaccinations. Vaccine. 2005;23:915–23. doi: 10.1016/j.vaccine.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 89.Szilagyi PG, Shone LP, Barth R, Kouides RW, Long C, Humiston SG, et al. Physician practices and attitudes regarding adult immunizations. Prev Med. 2005;40:152–61. doi: 10.1016/j.ypmed.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 90.America's Health Insurance Plans. 2008 adult immunization survey: practices and policies of private health insurance plans. 2009. [cited 2011 Feb 24]. Available from: URL: http://www.ahip.org/content/default.aspx?docid=32403.
- 91.America's Health Insurance Plans, Center for Policy and Research. A survey of preventive benefits in health savings account plans. 2007. Jul, [cited 2011 Feb 24]. Available from: URL: http://www.ahipresearch.org/pdfs/HSA_Preventive_Survey_Final.pdf.
- 92.Zhou F, Santoli J, Messonnier ML, Yusuf HR, Shefer A, Chu SY, et al. Economic evaluation of the 7-vaccine routine childhood immunization schedule in the United States, 2001. Arch Pediatr Adolesc Med. 2005;159:1136–44. doi: 10.1001/archpedi.159.12.1136. [DOI] [PubMed] [Google Scholar]
- 93.Department of Treasury, Department of Labor, Department of Health and Human Services (US) Group health plans and health insurance: coverage relating to status as a grandfathered health plan under the Patient Protection and Affordable Care Act: interim final rule and proposed rule. Federal Register. 2010;75:34538–70. [PubMed] [Google Scholar]
- 94.National Center for Health Statistics (US) Health, United States, 2009: with special feature on medical technology. Hyattsville (MD): NCHS; 2010. [PubMed] [Google Scholar]
- 95.Hoppe RB. The well adult. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990. pp. 1022–7. [PubMed] [Google Scholar]
- 96.American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint principles of the patient-centered medical home. 2007. [cited 2011 May 31]. Available from: URL: http://www.acponline.org/advocacy/where_we_stand/medical_home/approve_jp.pdf.
- 97.Stewart A, Cox M, Rosenbaum S. The epidemiology of U.S. immunization law: translating Centers for Disease Control and Prevention immunization guidelines into practice: state laws related to the use of standing orders covering immunization practice. 2007. [cited 2011 May 9]. Available from: URL: http://www.gwumc.edu/sphhs/departments/healthpolicy/immunization/National-standing-orders-report-Final.pdf.
- 98.The Guide to Community Preventive Services. Universally recommended vaccinations: client reminder and recall systems. [cited 2011 Jan 5]. Available from: URL: http://www.thecommunityguide.org/vaccines/universally/clientreminder.html.
- 99.Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Progress in immunization information systems—United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(1):10–2. [PubMed] [Google Scholar]
- 101.2009. HITECH Act. Pub. L. No. 111-115, 123 Stat. 226.
- 102.Department of Health and Human Services (US), Office of the National Coordinator for Health Information Technology. The state-level health information exchange (SLHIE) consensus project. [cited 2011 Aug 30]. Available from: URL: http://healthit.hhs.gov/portal/server.pt/community/healthit_hhs_gov__state_level_hie/1242.
- 103.Appel A, Everhart R, Mehler PS, MacKenzie TD. Lack of ethnic disparities in adult immunization rates among underserved older patients in an urban public health system. Med Care. 2006;44:1054–8. doi: 10.1097/01.mlr.0000228017.83672.c3. [DOI] [PubMed] [Google Scholar]
- 104.Williams RL, Flocke SA, Stange KC. Race and preventive services delivery among black patients and white patients seen in primary care. Med Care. 2001;39:1260–7. doi: 10.1097/00005650-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 105.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 106.Singleton JA, Santibanez TA, Wortley PM. Influenza and pneumococcal vaccination of adults aged ≥65: racial/ethnic differences. Am J Prev Med. 2005;29:412–20. doi: 10.1016/j.amepre.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 107.Burnett M, Genao I, Wong WF. Race, culture, and trust: why should I take a shot if I'm not sick? Ethn Dis. 2005;15(2 Suppl 3):S3–16. [PubMed] [Google Scholar]
- 108.Brandon DT, Isaac LA, LaVeist TA. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc. 2005;97:951–6. [PMC free article] [PubMed] [Google Scholar]
- 109.Yoo BK, Kasajima M, Phelps CE, Fiscella K, Bennett NM, Szilagyi PG. Influenza vaccine supply and racial/ethnic disparities in vaccination among the elderly. Am J Prev Med. 2011;40:1–10. doi: 10.1016/j.amepre.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 110.Department of Health and Human Services (US), Office of Disease Prevention and Health Promotion. Healthy People 2010. 2nd ed. 2 vols. Washington: Government Printing Office (US); 2000. With understanding and improving health and objectives for improving health. [Google Scholar]
- 111.Sentell TL, Halpin HA. Importance of adult literacy in understanding health disparities. J Gen Intern Med. 2006;21:862–6. doi: 10.1111/j.1525-1497.2006.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sudore RL, Mehta KM, Simonsick EM, Harris TB, Newman AB, Satterfield S, et al. Limited literacy in older people and disparities in health and healthcare access. J Am Geriatr Soc. 2006;54:770–6. doi: 10.1111/j.1532-5415.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 113.Scott TL, Gazmararian JA, Williams MV, Baker DW. Health literacy and preventive health care use among Medicare enrollees in a managed care organization. Med Care. 2002;40:395–404. doi: 10.1097/00005650-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 114.Bennett IM, Chen J, Soroui JS, White S. The contribution of health literacy to disparities in self-rated health status and preventive health behaviors in older adults. Ann Fam Med. 2009;7:204–11. doi: 10.1370/afm.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Howard DH, Sentell T, Gazmararian JA. Impact of health literacy on socioeconomic and racial differences in health in an elderly population. J Gen Intern Med. 2006;21:857–61. doi: 10.1111/j.1525-1497.2006.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Department of Health and Human Services (US), Office of Disease Prevention and Health Promotion. National action plan to improve health literacy. Washington: HHS (US); 2010. [Google Scholar]
- 117.Mayo AM, Cobler S. Flu vaccines and patient decision making: what we need to know. J Am Acad Nurs Pract. 2004;16:402–10. doi: 10.1111/j.1745-7599.2004.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 118.Schneider EC, Cleary PD, Zaslavsky AM, Epstein AM. Racial disparity in influenza vaccination: does managed care narrow the gap between African Americans and whites? JAMA. 2001;286:1455–60. doi: 10.1001/jama.286.12.1455. [DOI] [PubMed] [Google Scholar]
- 119.Bardenheier BH, Wortley PM, Winston CA, Washington ML, Lindley MC, Sapsis K. Do patterns of knowledge and attitudes exist among unvaccinated seniors? Am J Health Behav. 2006;30:675–83. doi: 10.5555/ajhb.2006.30.6.675. [DOI] [PubMed] [Google Scholar]
- 120.Zimet GD. Understanding and overcoming barriers to human papillomavirus vaccine acceptance. Curr Opin Obstet Gynecol. 2006;18(Suppl 1):S23–8. doi: 10.1097/01.gco.0000216317.10690.8f. [DOI] [PubMed] [Google Scholar]
- 121.Zimet GD, Weiss TW, Rosenthal SL, Good MB, Vichnin MD. Reasons for non-vaccination against HPV and future vaccination intentions among 19-26-year-old women. BMC Womens Health. 2010;10:27. doi: 10.1186/1472-6874-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Department of Health and Human Services (US), Health Resources and Services Administration, National Vaccine Injury Compensation Program. Covered vaccines. [cited 2010 Mar 1]. Available from: URL: http://www.hrsa.gov/vaccinecompensation/covered_vaccines.htm.
- 123.The Guide to Community Preventive Services. Universally recommended vaccines: reducing client out-of-pocket costs for vaccinations. 2008. [cited 2011 Jan 5]. Available from: URL: http://www.thecommunityguide.org/vaccines/universally/clientoutofpocketcosts.html.
- 124.Traeger MS, Say KR, Hastings V, Yost D. Achievement of Healthy People 2010 objective for adult pneumococcal vaccination in an American Indian community. Public Health Rep. 2010;125:448–56. doi: 10.1177/003335491012500314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Centers for Disease Control and Prevention (US) Appendix D: vaccine administration. In: Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and prevention of vaccine-preventable diseases. 12th ed. “The pink book.”. Washington: Public Health Foundation; 2011. [cited 2011 Aug 30]. Also available from: URL: http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/D/vacc_admin.pdf. [Google Scholar]
- 126.Pickering LK, Baker CJ, Freed GL, Gall SA, Grogg SE, Poland GA, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:817–40. doi: 10.1086/605430. [DOI] [PubMed] [Google Scholar]
- 127.Freed GL, Cowan AE, Clark SJ. Primary care physician perspectives on reimbursement for childhood immunizations. Pediatrics. 2009;124(Suppl 5):S466–71. doi: 10.1542/peds.2009-1542F. [DOI] [PubMed] [Google Scholar]