ABSTRACT
BACKGROUND
Archetypal symptoms and signs are commonly absent in frail older people who are acutely unwell. This challenges both recognition of illness and monitoring of disease progression in people at high risk of prolonged hospital stays, institutionalization and death.
OBJECTIVE
To determine whether bedside assessment of balance and mobility could track acute changes in the health status of older people admitted to hospital.
DESIGN
Prospective cohort study.
PARTICIPANTS
Four hundred nine patients, with a mean age of 81.8 years, admitted to general medical and rehabilitation wards at a tertiary care teaching hospital in Halifax, Nova Scotia. No patient refused assessment, and the only exclusion criterion was age.
INTERVENTIONS
The Hierarchical Assessment of Balance and Mobility (HABAM) was completed daily during the first 2 weeks of admission. For each patient, frailty status was measured on admission by a Frailty Index based on a Comprehensive Geriatric Assessment (FI-CGA).
MAIN MEASURES
Death and discharge destination.
KEY RESULTS
Poor performance in balance, transfers and mobility was associated with adverse outcomes. Forty-eight percent of patients with the lowest scores in all three domains died, compared with none with the highest scores. The relative risk of death for people who deteriorated during the first 48 h of admission was 17.1 (95% confidence interval: 4.9–60.3). Changes in HABAM scores were related to the discharge destination: patients discharged home showed the greatest rate of improvement, whereas those discharged to institutions stabilised at a lower level of performance. Fitter patients tended to have better performance on admission and faster recovery.
CONCLUSIONS
Daily bedside observation of mobility and balance allows assessment of acute changes in the health of older people. Frailty slows recovery of mobility and balance, and reduces recovery potential. By identifying patients most vulnerable to adverse outcomes, the HABAM and FI-CGA may facilitate risk stratification in older people admitted to hospital.
KEY WORDS: postural balance, frail elderly, hospitals, geriatric assessment
INTRODUCTION
Caring for acutely ill, frail patients can be especially challenging when the archetypal symptoms and signs that help guide care in younger, fitter people are absent. A frail patient with pneumonia, for example, may have no cough or pleuritic pain,1 fever, increased white cell count2,3 or confirmatory radiographic findings.4 Working without these symptoms and signs complicates both the recognition of illness and the monitoring of its course.
Although disease might not present typically in older adults, it is rarely silent. Delirium, functional decline, immobility and falls are common in older people,5 particularly in those who are frail.6,7 Paradoxically, these are often referred to as “atypical” disease presentations.
Impaired mobility and balance are of particular interest, as they correlate strongly with an individual’s function and overall state of health8 and occur more commonly with increasing frailty.9 Episodes of mobility disability are linked with mortality in older people.10 To track overall progression and recovery in frail older adults who become ill, the Hierarchical Assessment of Balance and Mobility (HABAM) was developed.11 By graphically displaying changes in static and dynamic balance and in mobility, the HABAM provides standard descriptions of levels of mobility and balance in three domains: mobility, transfers and balance. The HABAM has been shown to be a valid, reliable, sensible and responsive measure, and unlike most other mobility assessments can be used even in patients who are bedfast or chairfast.11–13 Even so, its prognostic ability when used routinely in the clinical setting has not been evaluated.
This study is underpinned by the hypotheses that changes in balance and mobility track the disease course in older people, and that the impact of frailty on acute illness is reflected in balance and mobility performance. Our objectives were to investigate patterns of changes in mobility and balance among older inpatients in relation to frailty, and to evaluate how mobility and balance impairment and recovery are related to the risk of adverse outcomes.
METHODS
Patients and Setting
Older patients (≥65 years) admitted to general medicine and geriatric rehabilitation at the Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, were eligible. Participants were seen between February 2008 and April 2009, under the care of either EMPE or KR, working as general internists or geriatricians. No patient refused assessment, which was part of routine care. The only exclusion criterion was age.
Measures
1. Mobility and Balance
The HABAM quantifies clinical assessments of balance, transfers and ambulation (Fig. 1). Higher scores indicate greater ability. The HABAM is simple, quick and acceptable to patients.11 Standard operating definitions for the tasks in the scale have been described previously.11 It requires no specialist skills or equipment and, in our experience, usually takes at most 10 min to complete. Its construct validity and responsiveness to change are well established.11 An early version of the HABAM11 included levels that, though clinically discernable, did not identify clinically meaningful differences in outcomes (e.g. the one person pivot assist). Likewise, despite discernable differences in a two-person maximal, medium or minimal level of assistance, clinically, the requirement for a second person makes them of approximately equal value, as identified in a subsequent Rasch analysis.12 By contrast, there are both discernable and meaningful differences in the patient who requires a one person assist, versus a one person hands-on (or minimal) assistance and a one person standby assist, as these grades identify differing physical capacities and need for expertise in the attendant.
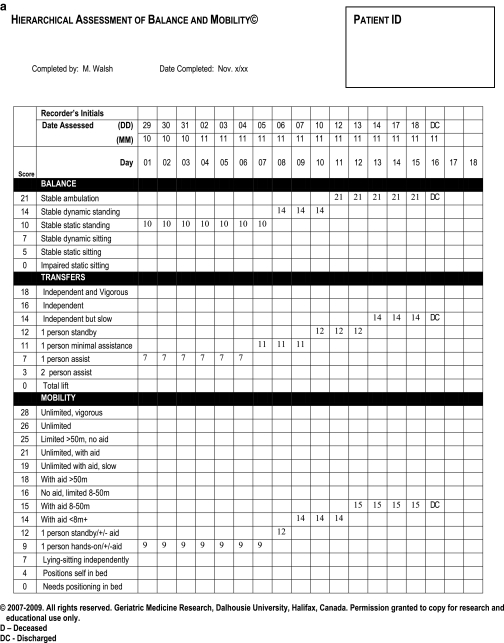
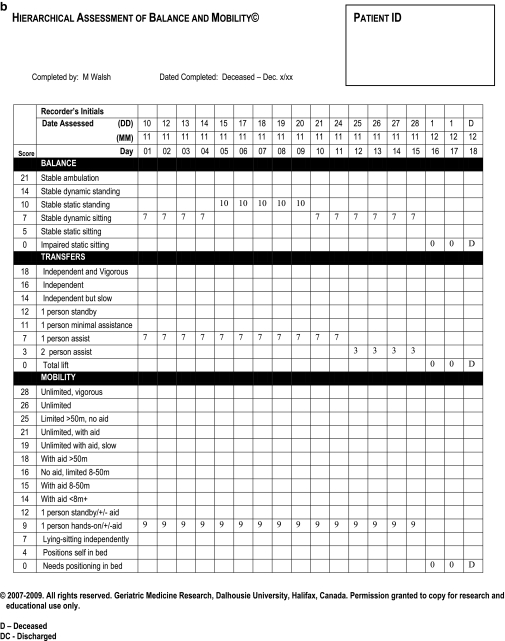
Fig. 1.
Examples of completed HABAM forms. Panel A tracks a patient who recovered and was discharged home. Panel B represents a patient who deteriorated and died.
Investigation of a range of assessors demonstrated an inter-rater reliability of the HABAM in the inpatient setting of 0.96 (95% CI 0.93–0.97).13 It was noted in the inter-rater reliability study,13 which did not formally follow all patients to discharge, that patients whose performance declined between the first assessment (day 1) and the second assessment (day 2) often died. For this reason, in this study we evaluated whether decline over the first 2 days of hospital admission was a risk for a short-term (within 30 days) adverse health outcome.
Patients were scored at their highest level of safe function, using their usual aid. Their performance was observed and recorded daily. As it tracks mobility and balance in relation to acute illness, our usual practice is for attending physicians, housestaff and, on the rehabilitation services, the ward physiotherapists to collect HABAM scores daily for only the first 14 hospital days, after which it is recorded less often. For recording purposes, the day of admission is recorded as the first hospital day, and performance is measured as observed, most often in the Emergency Department.
2. Frailty
On admission, a standardized Comprehensive Geriatric Assessment (CGA) was completed by one of the medical team as part of routine care. The CGA evaluates cognition, mood, motivation, health attitude, communication, strength, mobility, continence, nutrition, instrumental and basic activities of daily living, sleep, medical problems and medications. The CGA can also be used to quantify an individual’s degree of frailty in a Frailty Index (FI).13–15 CGA data are first coded as deficits that are either binary (e.g. 1 point = dementia is present, 0 = absent) or graded (e.g. 1 point = dependence with feeding, 0.5 = needs assistance, 0 = independent). An individual’s FI-CGA score is the sum of deficits, divided by the total number of deficits counted (here, a maximum of 52). The theoretical range is 0 to 1, with higher FI-CGA values indicating more problems and hence greater frailty.
3. Outcomes
Outcomes, including length of stay and discharge destinations, were documented contemporaneously and verified using computerised hospital records. Length of stay is reported as the median and also as the inter-quartile range, being the difference between the third and first quartiles.
Sample Size
To test that people whose scores declined were more likely to die, serial calculations, using a standard formula,16 showed that with an exposure between 5 and 10% and a relative risk of as low as 2 to 3, with 90% power and alpha = 0.05, we required a sample size of between 236–378. We opted for 378, and for a 10% rate of non-response or loss to follow-up.
Analysis
Data processing and analyses were carried out using SPSS (SYSTAT Software Inc.) and Matlab (version R2007a, MathWorks) with significance level set to p = 0.05. To investigate how frailty impacts balance and mobility, patients were divided into three groups based on the FI-CGA score on admission: low (<0.25), medium (0.25–0.5) and high (>0.5). These cutoffs were not arbitrary. A Frailty Index score of 0.25 has been proposed as the demarcation between “fitness” and “frailty” in community-dwelling older people.17 Scores of 0.5 and above, on the other hand, describe older people who are completely dependent on others for activities of daily living and have a significantly higher risk of death and institutionalisation over 5 years of follow-up.18 Mean trajectories of balance, transfers and mobility were plotted for each of these three groups and compared using a repeated measures analysis of variance, with the Student-Newman-Keuls test applied for post hoc comparisons. For presentation of the patterns of change in HABAM scores for the figures, points were smoothed using a least squares polynomial fitting procedure.
Ethics
The Research Ethics Committee of the Capital District Health Authority approved the protocol. Since all information was collected as part of routine care, additional consent was not sought beyond that specified in the patient’s admitting form, which allows use of routinely collected data. To maintain confidentiality, estimates are not disaggregated to <5 patients, and in the case descriptions below, identifying details have been altered.
RESULTS
The mean age of the 409 participants was 81.8 years (standard deviation 7.9) with more women than men (Table 1). Most patients (93.9%) lived in their own homes prior to admission, most of whom (82.9%) returned there at discharge. The Frailty Index (FI) was normally distributed around a mean value of 0.42 (SD 0.11), indicating a high burden of frailty. The median length of stay was 26 days (interquartile range 25). By 30 days, 23 patients (5.6%) had died.
Table 1.
Characteristics of Participants
| Men N = 146 | Women N = 263 | ||
|---|---|---|---|
| Age, years mean (standard deviation) | 81 (7.1) | 82 (9.8) | |
| Frailty Index mean (standard deviation) | 0.43 (0.10) | 0.41 (0.11) | |
| Pre-admission residence | Home | 381 (93.9%) | |
| Assisted-living facility | 16 (3.9%) | ||
| Nursing home | 12 (2.2%) | ||
| Number (%) died | 8 (5.5%) | 15 (5.7%) | |
| Length of stay, days median (interquartile range) | 26 (25) | ||
| Discharge destinations | Home | 320 (82.9%) | |
| Assisted-living facility | 25 (6.5%) | ||
| Nursing home | 30 (7.8%) | ||
| Transferred to another hospital or transitional care | 11 (2.8%) | ||
Examples of HABAM forms are shown in Figure 1. The patient in panel A recovered and was discharged home after 18 days, whereas panel B tracks a patient who deteriorated and died. Note that on admission, both patients needed one person’s help to transfer (transfer score = 7) and one-person hands-on assistance to walk (mobility score = 9). At baseline, they chiefly differed in balance, but their hospital courses were distinct: whereas patient A stabilized at admission level and began to improve at 6–8 days, patient B improved only slightly in balance scores before deteriorating. Scores of 0 in each of balance, transfers and mobility for 2 consecutive days preceded this patient’s death.
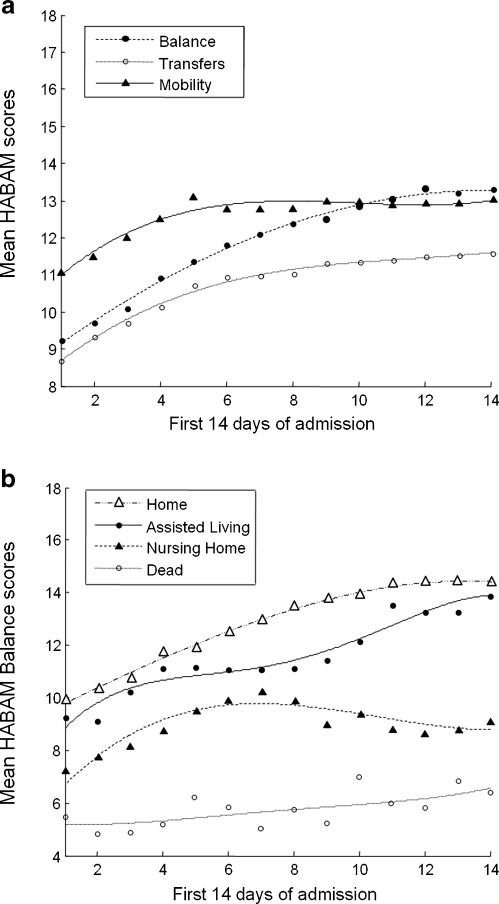
In general, the mean HABAM scores increased during the first 14 days (Fig. 2, Panel A). In each domain of mobility, transfers and balance, 2–3 point changes were observed. These changes in HABAM scores were related to discharge destinations, with similar trends in each domain. (Balance is illustrated in Fig. 2, panel B.) Patients who were discharged home had the greatest rate of improvement, whereas those discharged to nursing homes tended to plateau at a lower level. Patients who died had the lowest levels of performance on admission and of note they demonstrated no significant improvements. Deterioration in HABAM performance from the admission performance (usually observed in the Emergency Department) to the second hospital day was particularly informative. Patients with lower scores on day 2 compared to day 1 had significantly higher 30-day mortality rates (Table 2). The relative risk of death was highest in those patients who deteriorated in all three domains.
Fig. 2.
Panel A: Mean HABAM scores (y axis) during first 14 days of hospital admission (x axis) with balance scores (blue), transfers (red) and mobility (black). Panel B: Mean HABAM Balance Score (y axis) during first 14 days of hospital admission (x axis) related to discharge destinations.
Table 2.
Relative Risk of Death in Those with Worsening HABAM Scores Compared to Those with Stable or Improving Scores on Day 2 of Hospitalization
| Score stable or improved on day 2 | Score worsened on day 2 compared to day 1 | Relative risk of death (95% confidence interval) | |||
|---|---|---|---|---|---|
| Number of patients | Died within 1 month (%) | Number of patients | Died within 1 month (%) | ||
| Balance | 383 | 15 (4%) | 14 | 6 (43%) | 10.9 (3.7–32.4) |
| Transfers | 384 | 15 (4%) | 13 | 6 (46%) | 11.8 (4.0–35.4) |
| Mobility | 372 | 15 (4%) | 12 | 6 (50%) | 12.4 (4.2–37.5) |
| All three domains | 360 | 15 (4%) | 7 | 5 (71%) | 17.1 (4.9–60.3) |
More patients reached the maximum score for balance (n = 229) than for transfers (n = 56) or mobility (n = 14) with 11 achieving the highest scores for all three domains. All 11 of these patients were discharged to their pre-admission residence. Conversely, reaching the nadir of performance was associated with poor outcomes. Similar numbers of patients recorded the lowest scores for balance (n = 59), transfers (n = 43) and mobility (n = 50). Thirty-one patients scored 0 in each domain, of whom 15 died, 8 were discharged home, and 8 to a nursing home or an assisted living facility.
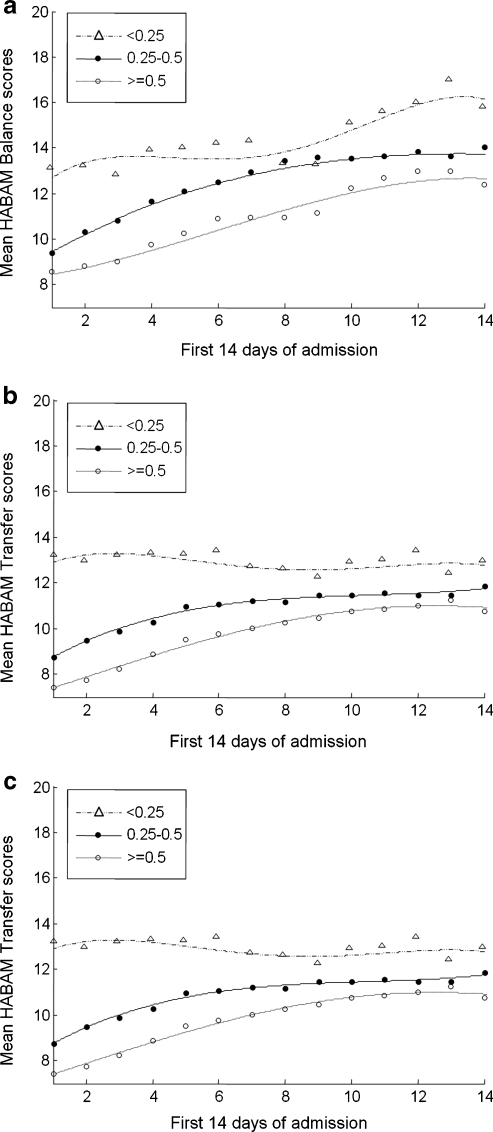
Frailty status impacted both the value of HABAM scores and their rate of change, with different effects on balance, on transfers and on mobility (Fig. 3). On admission to hospital, scores for each of balance, mobility and transfers were highest in fitter older people (FI-CGA score <0.25), intermediate for those with moderate to severe frailty (FI-CGA score 0.25–0.5) and lowest for the most frail (FI-CGA score ≥0.5). Balance scores continued to improve in each group, although differences in performance in relation to frailty status tended to persist during the first 14 days of hospital admission. Transfer scores, on the other hand, remained stable in fitter older people while continuing to improve in the most frail. Frailty status also affected mobility performance. After initial improvements in each patient group, mobility scores tended to plateau during the second week in hospital. The differences in mobility performance after 14 days remained significant: the mean HABAM score of 17 for fitter older people (FI-CGA <0.25) means that these patients were able to walk more than 50 m with an aid, whereas the most frail (FI-CGA >0.5) had a mean HABAM score = 11, which translates to needing at least one-person standby assistance, with or without an aid.
Fig. 3.
HABAM scores (y axis) during the first 14 days of admission (x axis) by frailty status. Panel A = balance, panel B = transfers, panel C = mobility. Fittest patients (FI-CGA <0.25) = ∆; moderate to severe frailty (FI-CGA 0.25–0.5) = ●; most frail (FI-CGA ≥0.5) = ο.
DISCUSSION
The Hierarchical Assessment of Balance and Mobility can track acute changes in the health status of older people and in this way offers a clinically sensible, readily communicated and quantifiable means of addressing whether a given frail, elderly, hospitalized patient is getting better or worse. Patterns are easily discerned, usually reflecting the clinical meaningfulness of the 2–3 point increments in the HABAM’s domains. Even so, not all meaningful differences translate into as few as 2–3 points: for example, the difference between impaired and stable static sitting balance is 5 points. At the individual level, not all discernable differences will have the same clinical meaning, depending on patient preferences. At the group level, interpretation of the importance of a given difference might well be done by reference to observed values in specific domains. For example, here, mean mobility scores increased from 11 to 13. This corresponds to a patient improving from needing one-person standby assistance on admission, to by day 14, being able to walk up to 8 m, independently with an aid.
Overall, the clinical meaningfulness of tracking mobility and balance is also demonstrated by poor performance in balance, transfers and mobility being associated with adverse outcomes. On any hospital day, a score of 0 in all three domains was associated with a high risk of death, as was deterioration in HABAM scores during the first 2 hospital days. Similarly, HABAM change was related to the discharge destination: patients who were discharged home showed the highest mobility, and the greatest rate of improvement, whereas those discharged to institutions stabilised at a lower level of performance.
Although other performance measures of mobility and balance have been validated in older people,19 these tests lack well-described norms, including for change.20 The de Morton Mobility Index, for example, has recently been proposed as a unidimensional measure of mobility in subacute older inpatients.21 Yet this instrument does not display results graphically, and its ability to track day-to-day changes has not been investigated. Other widely used measures, such as the Guralnik Physical Performance Test22 and the Timed Up and Go,23 have been well validated against adverse outcomes in community-dwelling older people. However, and crucial for patient care, if a patient is unable to stand or walk their abilities cannot be rated by these and most other instruments.24,25 Here, the HABAM instrument had a good span of sensitivity with no floor or ceiling effects. Fewer than 2% of the cohort reached maximum scores for balance, transfers and mobility and poor prognosis, which attends scores of 0 in all three domains, suggests this was a valid marker of poor health status rather than a floor effect of the measurement tool.
Despite its significance in relation to adverse outcomes, there is no universally accepted definition of frailty, and it continues to be operationalised in different ways. A variety of tools identify frailty as a clinical syndrome, notably Fried et al.’s frailty phenotype of ≥3 of 5 criteria (weight loss, exhaustion, weak grip strength, slow walking speed, low physical activity).26 This phenotype has been validated as a predictor of adverse outcomes in large epidemiological studies27 and was recently used to define frailty as the most common condition leading to death in community-dwelling older people.28 Slow gait speed per se is strongly linked to functional decline and disability, and has been used in isolation as a frailty measure.29 However, the omission of disorders of cognition and mood from these models is controversial: frailty in the clinical setting consists of more than weakness, slowness and wasting.30 The conceptualization of frailty as a multidimensional risk state facilitates its measurement by the number rather than by the nature of health problems.31 The Frailty Index (FI) model employs a well-defined methodology to create an index as a proportion of deficits.32 Frailty indices can be constructed from different numbers and types of variables: they are feasible and valid means of health status quantification, correlating highly with institutionalisation, worsening disability and death.33 Indeed, the risk of adverse outcomes is defined more precisely by deficit indices than by phenotypic definitions of frailty.34
The FI-CGA has been validated as a predictor of adverse outcomes for older people.15 This cohort of hospitalised older adults was very frail, evidenced by the mean value of the FI-CGA being 0.42. How this number translates clinically can be approximated from the second clinical examination cohort in the Canadian Study of Health and Aging,18 where older people were each assessed by a physician using a Clinical Frailty Scale; later an FI score was also determined. A mean FI of 0.43 (SD 0.08) there described older adults who were “severely frail, completely dependent on others for the activities of daily living”. Here, frailty status affected both time to recovery and recovery potential. This is consistent with other observations of the impact of frailty on both the presentation6 and outcomes35 of acute illness in older people. Compared to illness severity scores, frailty is more strongly associated with death36 and functional decline37 after acute illness. Although frailty is measured in different ways,30 people who are frail have a diminished capacity to effectively compensate for external stressors and to prevent further declines associated with aging and mortality.35,38 The HABAM scores in these patients showed different trajectories according to FI-CGA score: frailer patients tended to have slower rates of recovery and lower levels of performance both on admission and after 2 weeks in hospital. In this way, the FI-CGA may be a clinically feasible means to measure patients’ capacity to respond to acute illness. The HABAM, on the other hand, tracks day-to-day changes that reflect whether each patient is improving or deteriorating. The FI-CGA and HABAM thereby provide distinct but complimentary information on the health status and clinical progress of older inpatients.
Why mobility and balance relate to health status in older people can be considered by thinking about aging as the failure of a complex system.39 As deficits accumulate, redundancy is lost, resulting in a reduced ability to withstand stresses.35 As any complex system fails, the first processes to be compromised are those that require coordinated, integrated and precise interactions between many components. In humans, such high-order functions notably include upright bipedal ambulation.40 In a frail person, as a complex system close to failure, any acute illness can manifest as worsened balance and mobility.35,40
Our data must be interpreted with caution. Patients were recruited from a single hospital, the sample size is modest and small numbers of patients died. Hence, when determining the relative risk of death in those with worsening HABAM scores compared to those with stable or improving scores on day 2 of hospitalisation, our confidence intervals are wide. The length of stay of patients (median 26 days) is longer than in many other settings, as local service availability meant that many patients awaited home care or nursing home admission in their acute care beds. Reflecting earlier experience that this is where most change is seen, HABAM scores were recorded during only the first 2 weeks. Here, HABAM trajectories over the first 14 days suggested that improvement in balance was still occurring; similarly improvement in transfers was seen in those who were more frail. Tracking patients over a longer time course would provide clearer estimates of effective rehabilitation periods according to frailty status. Likewise, retrospective information about mobility and balance (from the patients themselves or, more likely, from informants) would also add value in providing an exact comparison. In addition, the HABAM requires additional validation in patients with specific mobility problems. For example, in the fully mobile but chair-bound quadriplegic or other patients with pre-existing major catastrophic disability (e.g. stroke, amputation), even though the principle of decline might pertain, the precise quantification might well require modification.
Our results suggest implications for clinical practice. As many frail patients lack traditional symptoms and signs of disease, routine use of the HABAM might improve the recognition of illness in older people and the monitoring of disease progression. Particularly for junior doctors, the divergence between the symptoms and signs that they might expect to see (given the traditional textbook emphasis on single illness presentation) and the reality of caring for frail patients can be discouraging. Showing them a non-arbitrary means of understanding how ill their patients are, and whether they are improving or getting worse, can, in our experience, greatly lessen their discouragement, and even be empowering. Additionally, presenting the results on a graph seems to be informative not just for those caring for older people, but for family members and patients. The early identification of patients likely to do well with rehabilitation, and recognition of those at risk of adverse outcomes, could help target resources more effectively. In addition, the HABAM and FI-CGA instruments may provide meaningful information on the value of different interventions for patients according to their frailty status. This latter hypothesis is the subject of current investigations by our group.
Acknowledgments
Funders The study was supported by a grant from the Fountain Innovation Fund of the QEII Health Sciences Foundation. KR receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research. The sponsor had no role in the acquisition or interpretation of data, or the decision to publish. NF is supported by a postdoctoral fellowship of the Alzheimer’s Society of Canada.
Prior Presentations This work was presented at the British Geriatrics Society Conference, November 2010.
Conflict of Interest A commercial version of the FI-CGA, known as Videx, is being developed for semi-automated use on a handheld device. Otherwise, none of the authors has any conflicts to declare.
REFERENCES
- 1.Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–24. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 2.Matsuno O, Kataoka H, Takenaka R, et al. Influence of age on symptoms and laboratory findings at presentation in patients with influenza-associated pneumonia. Arch Gerontol Geriatr. 2009;49:322–5. doi: 10.1016/j.archger.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141–5. doi: 10.1097/SMJ.0b013e318181d5b5. [DOI] [PubMed] [Google Scholar]
- 4.Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305–11. doi: 10.1016/j.amjmed.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarrett PG, Rockwood K, Carver D, Stolee P, Cosway S. Illness presentation in elderly patients. Arch Intern Med. 1995;155:1060–4. doi: 10.1001/archinte.155.10.1060. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs B. Some characteristics of geriatric patients. Scott Med J. 1969;14:243–51. doi: 10.1177/003693306901400705. [DOI] [PubMed] [Google Scholar]
- 8.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the health and retirement study. Ann Intern Med. 2007;147:156–64. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 9.Davis DHJ, Rockwood MRH, Mitnitski AB, Rockwood K. Impairments in mobility and balance in relation to frailty. Arch Gerontol Geriatr 2010; [In press]. PMID: 20678816. [DOI] [PubMed]
- 10.Gill TM, Allore HG, Hardy SE, Guo Z. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006;54:248–54. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 11.MacKnight C, Rockwood K. A hierarchical assessment of balance and mobility. Age Ageing. 1995;24:126–30. doi: 10.1093/ageing/24.2.126. [DOI] [PubMed] [Google Scholar]
- 12.MacKnight C, Rockwood K. Rasch analysis of the hierarchical assessment of balance and mobility (HABAM) J Clin Epidemiol. 2000;53:1242–7. doi: 10.1016/S0895-4356(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood K, Rockwood MRH, Andrew MK, Mitnitski A. Reliability of the hierarchical assessment of balance and mobility in frail older adults. J Am Geriatr Soc. 2008;56:1213–7. doi: 10.1111/j.1532-5415.2008.01773.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–33. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones D, Song X, Mitnitski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–71. doi: 10.1007/BF03327413. [DOI] [PubMed] [Google Scholar]
- 16.Kelsey JL, Whittemore AS, Evans AS, Thomson WD. Methods in Observational Epidemiology. 2. Oxford: Oxford University Press; 1996. [Google Scholar]
- 17.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–43. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton NA, Berlowitz DJ, Keating JL. A systematic review of mobility instruments and their measurement properties for older acute medical patients. Health Qual Life Outcomes. 2008;6:44. doi: 10.1186/1477-7525-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérennou D, Decavel P, Manckoundia P, et al. Evaluation of balance in neurologic and geriatric disorders. Ann Readapt Med Phys. 2005;48:317–35. doi: 10.1016/j.annrmp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Morton NA, Davidson M, Keating JL. The de Morton Mobility Index (DEMMI): an essential health index for an ageing world. Health Qual Life Outcomes. 2008;6:63. doi: 10.1186/1477-7525-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehab. 1986;67:387–389. [PubMed] [Google Scholar]
- 24.Berg KO, Wood-Dauphinee SL, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–11. doi: 10.3138/ptc.41.6.304. [DOI] [Google Scholar]
- 25.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–26. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 26.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Biol Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 27.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 28.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–80. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–28. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer JM, Sieber CC. Sarcopenia and frailty: a clinician’s controversial point of view. Exp Gerontol. 2008;43:674–8. doi: 10.1016/j.exger.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 32.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–9. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 34.Kulminski AM, Ukraintseva SV, Kulminskaya IV, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the cardiovascular health study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockwood K, Rockwood MRH, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58:318–323. doi: 10.1111/j.1532-5415.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- 36.Torres OH, Muñoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603–9. doi: 10.1111/j.1532-5415.2004.52492.x. [DOI] [PubMed] [Google Scholar]
- 37.Volpato S, Onder G, Cavalieri M, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med. 2007;22:668–74. doi: 10.1007/s11606-007-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Yi Z. Frailty and mortality among Chinese at advanced ages. J Gerontol B Psychol Sci Soc Sci. 2009;64:279–89. doi: 10.1093/geronb/gbn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavrilov LA, Gavrilova NS. Reliability theory of aging and longevity. In Handbook of the Biology of Aging. 6th Edition: Academic Press. [DOI] [PubMed]
- 40.Nowak A, Hubbard RE. Falls and frailty: lessons from complex systems. J R Soc Med. 2009;102:98–102. doi: 10.1258/jrsm.2009.080274. [DOI] [PMC free article] [PubMed] [Google Scholar]