Abstract
BACKGROUND
Despite reductions in morbidity and mortality and changes in guidelines, little is known regarding changes in asthma treatment patterns.
OBJECTIVE
To examine national trends in the office-based treatment of asthma between 1997 and 2009.
PARTICIPANTS AND DESIGN
We used the National Ambulatory Care Survey (NAMCS) and the National Disease and Therapeutic Index™ (NDTI), nationally representative audits of office-based physicians, to examine patients diagnosed with asthma less than 50 years of age.
MEASUREMENTS
Visits where asthma was diagnosed and use of six therapeutic classes (short-acting β2 agonists [SABA], long-acting β2 -agonists [LABA], inhaled steroids, antileukotrienes, anticholinergics, and xanthines).
RESULTS
Estimates from NAMCS indicated modest increases in the number of annual asthma visits from 9.9 million [M] in 1997 to 10.3M during 2008; estimates from the NDTI suggested more gradual continuous increases from 8.7M in 1997 to 12.6M during 2009. NAMCS estimates indicated declines in use of SABAs (from 80% of treatment visits in 1997 to 71% in 2008), increased inhaled steroid use (24% in 1997 to 33% in 2008), increased use of fixed dose LABA/steroid combinations (0% in 1997 to 19% in 2008), and increased leukotriene use (9% in 1997 to 24% in 2008). The ratio of controller to total asthma medication use increased from 0.5 (1997) to a peak of 0.7 (2004). In 2008, anticholinergics, xanthines, and LABA use without concomitant steroids accounted for fewer than 4% of all treatment visits. Estimates from NDTI corroborated these trends.
CONCLUSIONS
Changes in office-based treatment, including increased inhaled steroid use and increased combined steroid/long-acting β2-agonist use coincide with reductions in asthma morbidity and mortality that have been demonstrated over the same period. Xanthines, anticholinergics, and increasingly, LABA without concomitant steroid use, account for a very small fraction of all asthma treatments.
KEY WORDS: primary care, respiratory disease, pharmacotherapy
INTRODUCTION
Despite substantial decreases in morbidity and mortality in the past decade,1,2 asthma continues to pose a considerable burden in the United States. Characterized by chronic inflammation and reversible narrowing of the small airways, it is estimated that asthma affected more than 10% of the population in 2006.3–5 The direct and indirect costs attributable to asthma are expected to reach $20.7 billion by 2010.6 A quarter of these costs are attributable to pharmacologic treatments.2
In 1997, the National Heart, Lung, and Blood Institute (NHLBI) published its second Expert Panel Report (EPR-2) that suggested a role for long-acting beta-agonists for long-term prevention of symptoms when added to anti-inflammatory agents. However, subsequent studies raised increasing concern regarding potential increases in life-threatening asthma exacerbations and asthma-related deaths among such users.7 In response to these concerns, the FDA held an advisory panel on the safety of long-acting β2-agonists in 2005,8 ultimately issuing a black box warning regarding their use in March 2006.9 Following release of the Guidelines for the Diagnosis and Management of Asthma (EPR-3) by the NHLBI, treatment with long acting β2-agonists was recommended only in combination with inhaled steroids when steroids alone were insufficient.10 These treatment recommendations were reiterated by the FDA in early 2010.
Recent trends in asthma treatment in response to these and other changes in care, such as the increasing availability of fixed dose inhaled corticosteroid/long-acting beta agonist combinations, have not been well established. We sought to characterize changes in asthma treatment patterns using two nationally representative audits of office-based prescribing with a focus on recent trends in the use of inhaled steroids, long-acting beta-agonists, and the combination of these two agents among individuals diagnosed with asthma less than 50 years of age.
METHODS
Audits of Office-based Care
We used data from the National Ambulatory Care Survey (NAMCS) and the National Disease and Therapeutic Index™ (NDTI), nationally representative audits of office-based physicians conducted by the National Center for Health Statistics and IMS Health, respectively. These data are routinely used to evaluate changes in diagnosis and treatment patterns in ambulatory based practice. Data from both the NAMCS and the NDTI are reported from 1997 through 2008 and 2009, respectively. Although the universe of physicians from which samples are drawn varies modestly between NAMCS and the NDTI, both include most physician specialties delivering office-based care and exclude non-patient care disciplines such as pathology, radiology, and critical care medicine.
The National Ambulatory Medical Care Survey (NAMCS) is a publicly accessible, national survey that provides comprehensive information about the provision and use of office-based medical care services in the United States. The survey is collected from a representative sample of visits to non-federal employed office-based physicians who are primarily engaged in direct patient care. NAMCS utilizes a multistage probability sample design involving samples of 112 geographic primary sampling units (PSUs), physician practices within PSUs, and patient visits within physician practices. Physicians are randomly assigned to a one-week reporting period during which time data from a systematic random sample of visits are recorded on a patient record form. Physicians provide data on patients’ symptoms, physicians’ diagnoses, and medications ordered or provided. Additionally, physicians provide data on patient demographics and details on services provided such as diagnostic procedures, patient management, and planned future treatment.
The National Disease and Therapeutic Index (NDTI) is an audit of office-based prescribing conducted by IMS Health. The collection of NDTI data involves 4,800 physicians randomly selected within strata defined by specialty and geographic area. Participants record information on all clinical contacts during two consecutive workdays per quarter. These encounters are mostly office-visits (85-90%) but also include phone calls with patients and physician visits to patients in hospitals and nursing homes; we excluded encounters that were not office-based from the present analysis. The NDTI generates approximately 350,000 annual contact records. For each record, physicians record all applicable diagnoses and then for each diagnosis record the specific medications ordered or mentioned to treat that condition. Each record of a drug therapy within the NDTI is linked to a specific six-digit taxonomic code capturing diagnostic information similar to the International Classification of Diseases 9th Revision (ICD-9). Drug reporting reflects the physician’s best knowledge of new or continuing prescription and non-prescription medications. Based on the sample records, national estimates are constructed.
Eligibility Criteria and Therapies Analyzed
For both NAMCS and NDTI, all data is restricted to office visits. Our primary unit of analysis was treatment visits; visits in which asthma was diagnosed and one or more treatment was mentioned by the physician. Using NAMCS, we selected ICD-9 codes inclusive of all asthma subtypes, including allergy induced and asthmatic bronchitis. We used a similar process to select eligible patient records from the NDTI. We focused our analyses on four classes of inhaled therapy (long-acting β2-agonists, short-acting β2-agonists, anticholinergics, and inhaled corticosteroids) and two classes of oral systemic therapies (leukotriene modifiers and xanthines) most commonly used for the treatment of asthma. We excluded omalizumab from our analysis, since it is an injectable therapy that may be less well captured by the office-based encounter data available.
We assigned each agent to a therapeutic class using IMS Health’s Uniform System of Classification codes. As NAMCS provides generic drug information and much of the NDTI classification was based on brand name, we then used the Cerner Multum (Denver, Colorado) generic to brand crosswalk provided by NAMCS to apply the same classification system to NAMCS data in order to generate matching classes. We examined the ratio of controller therapies to all therapies used as a measure of quality of care.11,12 To do so, we categorized agents as controller medicines used for long-term asthma control (i.e., inhaled corticosteroids, cromones, long-acting β2-agonists, and leukotriene modifiers), and reliever or rescue medicines used for short-term symptomatic relief (i.e., short-acting inhaled β2-agonists, anti-cholinergics). In these cases, fixed dose combinations containing two controller agents (e.g., long-acting β2-agonist/steroid) were counted as contributing to both classes.
Analyses
We used descriptive statistics to conduct our analyses, and accounted for a change in the data collection that occurred in NAMCS during 2006.13 Analyses of data from NAMCS were conducted applying the sample weights that account for the multi-step design of the office-based survey. Since the misclassification of asthma and other pulmonary diseases, especially chronic obstructive pulmonary disease (COPD), increases as subjects age, we also compared our primary analyses (limited to those less than 50 years of age), with sensitivity analyses that did not apply an age restriction.
RESULTS
Sample Characteristics
Table 1 compares the demographic characteristics of patients less than 50 years of age diagnosed with asthma in 2008 between the NAMCS and the NDTI. In general, patient records included in NAMCS were more likely to be those of males (51% vs. 45%), less than 19 years of age (56% vs. 40%), seen by pediatricians (41% vs. 30%). The proportion of individuals with prior visits during the previous 12 months was similar among visits within the NAMCS and the NDTI.
Table 1.
Characteristics of Patients Less than 50 Years of Age Diagnosed with Asthma in 2008
| NDTI N = 11.9 Million Visits | NAMCS N = 16.7 Million Visits | |
|---|---|---|
| Gender, % | ||
| Male | 50.9 | 45.1 |
| Female | 47.2 | 52.8 |
| Unspecified | 1.9 | 2.1 |
| Age, % | ||
| <18 | 56.0 | 64.1 |
| 18–29 | 14.0 | 16.0 |
| 30–39 | 14.1 | 16.1 |
| 40-49 | 15.9 | 18.2 |
| Physician specialty, % | ||
| Pediatricians | 40.6 | 30.2 |
| Other Primary Care Physicians* | 33.6 | 39.9 |
| All Other Physicians** | 25.8 | 29.9 |
| Past Visits in last 12 months, % | ||
| None | 27.8 | 25.6 |
| 1–2 | 43.9 | 43.2 |
| 3–5 | 13.2 | 14.7 |
| 6 or more | 7.4 | 8.7 |
| Unknown or unspecified | 7.7 | 7.9 |
* Includes internal medicine, family practice, and general practice
**Includes physicians such as pulmonologists and allergy/immunologists
NAMCS=National Ambulatory Medical Care Survey; NDTI=National Disease and Therapeutic Index; Visits represent projected estimates
Asthma Treatments by Treatment Class
Table 2 provides examples of the therapeutic classes examined, their availability as generics, their date of FDA approval, and the total volume of use among individuals less than 50 years of age in 2008 based on NAMCS. For example, in 2008 there were an estimated 6.9 million [M] treatment visits in which short acting β2 agonists were mentioned, 3.2M mentions of inhaled steroids, and 1.9M mentions of fixed dose combinations including long acting β2 agonists and inhaled corticosteroids.
Table 2.
Examples of Asthma Medications by Treatment Class, 2008
| Therapeutic class | Total Drug Uses, 2008 (Millions) | Example of member of class | Available as Generic | Date of FDA Approval |
|---|---|---|---|---|
| Inhaled single agent | ||||
| Short acting β2-agonists | 6.9 | Albuterol (e.g., Proventil HFA) | Yes | Mar-82 |
| Inhaled steroids | 3.2 | Fluticasone Propionate (Flovent HFA) | No | Sep-00 |
| Long acting β2-agonists | 0.045 | Salmeterol Xinafoate (Serevent Diskus) | No | May-01 |
| Anticholinergics | 0.167 | Titropium Bromide (Spiriva Handihaler) | No | May-04 |
| Inhaled combination agent | ||||
| Long acting β2-agonists/steroids | 1.9 | Fluticasone-Salmeterol (Advair Diskus) | No | Mar-01 |
| Anticholinergics/β2 Stimulants | 0.076 | Albuterol Sulfate/Ipratropium (Combivent) | No | Feb-97 |
| Systemic treatments | ||||
| Leukotrienes | 2.3 | Montelukast (Singulair) | No | Feb-98 |
| Xanthines | 0.054 | Theophylline (e.g., Theo-Dur) | Yes | May-78 |
Combination therapies (e.g., combined steroid/long-acting β2-agonist fixed dose combinations) are not counted as either steroid or long-acting β2-agonist; data on drug uses derived from the National Ambulatory Medical Care Survey (2008) and limited to individuals less than 50 years of age
Trends in Asthma Treatment Visits
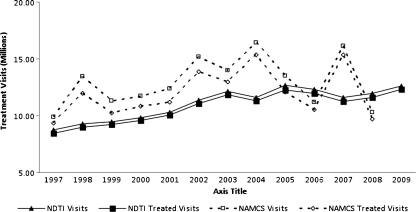
Figure 1 depicts trends in the number of NAMCS and NDTI office-based visits for asthma, as well as the fraction of these where one or more asthma treatment was mentioned. During most of the years examined, trends were similar between the two data sources, although NAMCS estimates exhibited much greater yearly variation. Estimates from NAMCS indicated modest increases in the number of annual asthma visits from 9.9 million [M] in 1997 to 10.3M during 2008; estimates from the NDTI suggested more gradual continuous increases from 8.7M in 1997 to 12.6M during 2009. The fraction of annual visits where at least one asthma treatment was mentioned (treatment visits) ranged between 85%–95% (NAMCS) and 96%–98% (NDTI).
Figure 1.
Total treatment visits for asthma among individuals less than 50 years of age, United States, 1997–2009 (NDTI and NAMCS). Treated visits represent office based physician visits in which one or more treatments were newly prescribed, renewed, or mentioned as therapy. Sources: IMS Health’s National Disease and Therapeutic Index, 1997–2009; National Ambulatory Medical Care Survey, 1997–2008.
Use of Individual Therapies
There were marked changes in the rates of use of most asthma therapies between 1997 and 2009 (Table 3). For example, data from NAMCS suggested decreases in the number of visits where asthma was diagnosed and a treatment was mentioned (treatment visits) with a short-acting β2-agonist, from 80% of treatment visits in 1997 to 71% of treatment visits in 2008. Table 3 also indicates increased inhaled steroid use (24% of treatment visits in 1997 to 33% of treatment visits in 2008), increased use of fixed dose LABA/steroid combinations (0% in 1997 to 19% in 2008), and increased leukotriene use (9% in 1997 to 24% in 2008). In 2008, anticholinergics, xanthines, and LABAs without concomitant steroid accounted for fewer than 4% of all treatment visits.
Table 3.
Percent of Asthma Treatment by Category, 1997–2009*
| National Ambulatory Medical Care Survey | |||||||||||||
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| Short acting β2-agonists | 80 | 82 | 76 | 80 | 63 | 61 | 65 | 67 | 61 | 73 | 67 | 71 | --- |
| Inhaled steroids | 24 | 42 | 37 | 32 | 46 | 31 | 28 | 20 | 32 | 24 | 31 | 33 | --- |
| Anticholinergics | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 4 | 1 | 1 | 2 | 2 | --- |
| Leukotrienes | 9 | 6 | 17 | 13 | 14 | 18 | 27 | 31 | 28 | 22 | 33 | 24 | --- |
| Xanthines | 8 | 5 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | --- |
| Long acting β2-agonists | 7 | 9 | 16 | 9 | 15 | 4 | 4 | 1 | 1 | 1 | 0 | 0 | --- |
| Long acting β2-agonists/Steroida | 0 | 0 | 0 | 0 | 9 | 24 | 19 | 24 | 21 | 19 | 19 | 19 | --- |
| Total, N (millions) | 9.4 | 12.0 | 10.2 | 10.8 | 11.2 | 13.9 | 12.9 | 15.3 | 12.1 | 10.6 | 15.4 | 9.7 | --- |
| National Disease and Therapeutic Index | |||||||||||||
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| Short acting β2-agonists | 75 | 73 | 71 | 67 | 63 | 61 | 62 | 60 | 64 | 68 | 67 | 66 | 70 |
| Inhaled steroids | 39 | 38 | 39 | 40 | 37 | 27 | 25 | 25 | 22 | 28 | 26 | 26 | 29 |
| Anticholinergics | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Leukotrienes | 7 | 12 | 19 | 23 | 23 | 25 | 27 | 25 | 25 | 26 | 26 | 25 | 22 |
| Xanthines | 6 | 5 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Long acting β2-agonists | 12 | 14 | 13 | 16 | 13 | 7 | 4 | 3 | 1 | 2 | 1 | 0 | 0 |
| Long acting β2-agonists/Steroida | 0 | 0 | 0 | 0 | 13 | 23 | 27 | 31 | 33 | 27 | 28 | 30 | 26 |
| TOTAL, N (millions) | 8.4 | 9.0 | 9.2 | 9.6 | 10.1 | 11.1 | 11.8 | 11.3 | 12.3 | 11.9 | 11.2 | 11.6 | 12.3 |
*Column percents may exceed 100% because individual visits may include more than one treatment; NAMCS data from 2009 is not currently available
aReflects fixed dose combinations; excludes visits where patients received extemporaneous long-acting β2-agonists and steroids separately
Comparisons of Estimates from NAMCS with NDTI
Table 3 also reflects similar data from the NDTI. Overall, the patterns were quite similar, with reductions in short-acting β2-agonist use, increases in leukotriene use and the use of fixed-dose long-acting β2-agonist/steroid combinations, and nearly no use of long-acting β2-agonists without concomitant steroids. The two data sources revealed modestly different trends for inhaled steroid mentions, with NAMCS suggesting an increase from 24% of treatment visits (1997) to 33% of treatment visits (2008) and the NDTI suggesting a decline from 39% (1997) to 26% (2008). Overall, the mean absolute difference in the estimated proportion of treatment visits accounted for by each therapy was between 2%–6%, with 5 of 84 annual estimates exceeding 10% between the two data sources.
Trends in Use of Fixed Dose Combinations and LABA Use Without Steroids
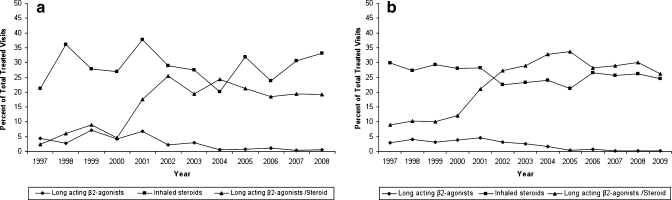
Figure 2a depicts changes in the use of inhaled steroids, long-acting β2-agonists, and LABA/steroid combinations based on data from NAMCS. In contrast to the data in Table 3, the figure includes both fixed dose combination and extemporaneously combined therapies when depicting treatment visits where combined LABA and steroid therapies were mentioned. There were low levels of LABA mentioned without concomitant steroids even in 1997 (4%), which declined to fewer than 1% of visits where a treatment was used in 2008. By contrast, the number of treatment visits in which LABA/steroid combinations were mentioned ranged between 2% and 8% of treatment visits between 1997 and 2000, then increased markedly between 2000 and 2002 before again reaching a plateau and remaining between 19% and 24% of treatment visits between 2002 and 2008. By 2008, of individuals taking both a LABA and inhaled steroid, 99% of these mentions were through the use of fixed dose combination therapies rather than extemporaneously combined therapies (data not shown). Over the time period, the use of inhaled steroids without concomitant LABA use has remained fairly steady, ranging from 21% (1997) to a peak of 38% (2001) before declining modestly to 33% (2008). Figure 2b reflects generally similar results using the NDTI.
Figure 2.
a. Trends in use of inhaled steroids, long-acting β2-agonists, and steroid/ β2-agonist combination therapy (NAMCS). “Long-acting β2-agonist/steroids" depicts both fixed dose combinations and concurrent use of separate therapies. b. Trends in use of inhaled steroids, long-acting β2-agonists, and steroid/β2-agonist combination therapy (NDTI). “Long-acting β2-agonist/steroids" depicts both fixed dose combinations and concurrent use of separate therapies.
Use of Controller and Reliever Therapies
The number of mentions of controller therapies in NAMCS increased from 4.5M treatment visits (1997) to a peak of 15.5M uses (2004) and was an estimated 9.4M uses in 2008. NDTI reflected more gradual changes with increasing controller treatment visits from 5.4M visits in 1997 to 14.0M in 2005 and then a modest decrease to 12.7M visits in 2009. Similar to NAMCS, reliever therapies remained relatively stable in NDTI, fluctuating between 6.5M and 8.5M treatment visits between 1997 and 2009. Overall, between 1997 and 2008 the ratio of controller to total therapies increased from 0.4 to 0.6 (NAMCS) and from 0.5 to 0.6 (NDTI).
Analyses of All Individuals Rather than Those Less than 50 Years of Age
Between 1997 and 2007, patients under the age of 50 represented 63%–77% of all visits where asthma was diagnosed in NAMCS. A similar fraction of all asthma patient visits (62%–73%) was accounted for by these individuals in NDTI. Overall, the observed patterns of usage did not differ markedly when analyses were conducted with this less restricted patient cohort.
DISCUSSION
We use two complementary physician audits to describe the ambulatory care of asthma among individuals less than 50 years of age from 1997 through 2009. Increases in asthma visits were evident, and most were associated with the use of one or more pharmacotherapy. Declines in short-acting β2-agonists accompanied increased steroid, long-acting β2-agonist/steroid, and leukotriene inhibitor use. By 2009 there was very infrequent use of long-acting beta-agonist use without concomitant steroids for the treatment of asthma among these individuals. For most comparisons, the National Ambulatory Medical Care Survey and the National Disease and Therapeutic Index™ yielded similar estimates of the prevalence of asthma diagnosis and treatment.
Our finding of increased use of controller therapies and reductions in short-acting β2-agonist use suggests a continuation of prior trends14 consistent with clinical guidelines emphasizing the important role of controller therapies in the treatment of asthma.15 Our results also highlight the virtual abandonment of the use of long-acting β2-agonists (LABAs) without the combined use of an inhaled steroid.10 These changes occurred fairly rapidly from 2001, when 15% of treatment visits in NAMCS included a LABA that was not a fixed-dose therapy (whether or not a steroid was used extemporaneously), to 2004, when just 1% of visits included such treatment. It is noteworthy that substantial reductions in LABA use without concomitant steroids occurred prior to FDA and NHLBI advisories, which may reflect educational campaigns undertaken after the NHLBI’s 1997 consensus guidelines as well as the emerging evidence that informed these regulatory communications, including longer-standing cautions about LABAs in Europe.
Our results also illustrate that the use of combined long-acting β2-agonists with steroids has increased considerably since 1997; during the most recent years, nearly all such use occurs via fixed dose combination therapies rather than therapies that are extemporaneously combined. While this trend may be associated with greater patient convenience, and in turn better adherence, it also increases costs and may be associated with unnecessary use of the LABA component of these fixed dose combination therapies. Although our analysis was not designed to describe the appropriateness of LABA/steroid combinations, other investigations suggest widespread overuse of these therapies, which may be encouraged by their aggressive marketing and promotion. For example, Blanchette and colleagues conducted a retrospective cohort of a commercially insured cohort and found that just 40% of individuals met criterion for appropriate use.16
In addition to examining changes in asthma diagnosis and treatment, we also compare estimates derived from the National Ambulatory Medical Care Survey and the IMS Health National Disease and Therapeutic Index™ (NDTI). Although both are nationally representative audits of office-based physicians, there are several differences between the audits, as well. The NAMCS uses a three-stage rather than a two-stage sampling procedure, includes a modestly different universe of patient-care physicians, conducts a new sample each calendar year, and generates fewer than one-tenth the number of visit records at the NDTI. The larger sample size of the NDTI, as well as the persistence of sampled physicians over multiple years, may account for NDTI’s more stable estimates of diagnosis and treatment visits as depicted in Figure 1. In addition, the NDTI generated modestly higher estimates of the fraction of asthma diagnosis visits with a treatment reported. This may be because the NDTI links each therapeutic use with a specific clinical indication, and also because the NDTI is a proprietary audit used to generate market knowledge of prescription utilization. At least two studies have compared the NAMCS with the NDTI and suggest that they yield similar though not identical estimates of office-based medication use.17,18
Our study has limitations and leaves several questions unanswered. Although these data provide a unique opportunity to examine national patterns of office-based care, the data do not include any outcomes sufficient to examine the precise role of these treatment patterns in reducing morbidity and mortality. Furthermore, our data include limited information regarding asthma severity, and thus our study was not designed to examine quality measures of ambulatory asthma care. Finally, our study did not seek to characterize the precise role of specific factors, such as regulatory communications or professional guidelines, in accounting for the changes we describe.
CONCLUSION
Changes in office-based treatment, including increased inhaled steroid use and increased combined steroid/long-acting β2-agonist use coincide with reductions in asthma morbidity and mortality that have been demonstrated over the same period. Xanthines, anticholinergics, and increasingly, long-acting β2-agonists without concomitant steroid use, account for a very small fraction of all asthma treatments.
Acknowledgements
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): National Disease and Therapeutic Index™ (1997–2009), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Support Dr. Alexander is supported by the Agency for Healthcare Research and Quality (K08 HS15699-01A1; RO1 HS0189960) and the Robert Wood Johnson Physician Faculty Scholars Program. The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data; and preparation of final approval of the manuscript prior to publication.
Contributions Conception and design: GCA and RSS; Analysis and interpretation: ASH, SZ, GCA, and RSS; Manuscript drafting: GCA; Substantive manuscript revision: ASH, SZ, GCA, and RSS; Approval of final manuscript: ASH, SZ, GCA, and RSS; Study supervision: GCA
Conflicts of Interest Dr. Alexander is a consultant for IMS Health.
References
- 1.Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. J Asthma. 2005;42:373–8. doi: 10.1081/JAS-200062995. [DOI] [PubMed] [Google Scholar]
- 2.American Lung Association Epidemiology and Statistics Unit. Trends in Asthma Morbidity and Mortality, February 2009.
- 3.Evans R, 3rd, Mullally DI, Wilson RW, et al. National trends in the morbidity and mortality of asthma in the US. Prevalence, hospitalization. and death from asthma over two decades: 1965--1984. Chest. 1987;91:65–74. doi: 10.1378/chest.91.6.65Sb. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma---United States, 1980--1999. In: Surveillance Summaries, March 29, 2002.MMWR 2002;51(No. SS-1):1--13. [PubMed]
- 5.Center for Disease Control and Prevention, National Health Interview Survey, National Center for Health Statistics, CDC. Compiled 3/18/2008. Accessed 2/9/2010
- 6.National Heart, Lung and Blood Institute. Chartbook on Cardiovascular, Lung and Blood Diseases, U.S. Department of Health and Human Services. National Institute of Health 2009.
- 7.Saltpeter S, Buckley N, Ormiston T, Salpeter E. Meta-analysis: effect of long-acting β2-agonists on severe asthma exacerbations and asthma-related deaths. Ann Int Med. 2006;144:904–12. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 8.Donohue J, Fromer, L. Long-acting beta-agonists: role in asthma management. J Fam Pract. 2006 Apr;Suppl:1–6. [PubMed]
- 9.US Food and Drug Administration Website. FDA Public Health Advisory. Serevent Diskus (salmeterol xinafoate inhalation powder), Advair Diskus (fluticasone propionate & salmeterol xinafoate powder), Foradil Aerolizer (formoterol fumarate inhalation powder). Available at: http://www.fda.gov/cder/drug/advisory/laba.htm. Accessed February 9, 2010.
- 10.National Heart Lung and Blood Institute. Expert Panel Report 3: guidelines for the Diagnosis and Management of Asthma. Bethesda (MD): 2007. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed February 9 2010.
- 11.Schatz M, Nakahiro R, Crawford W, Mendoza G, Mosen D, Stibolt TB. Asthma quality-of-care markers using administrative data. Chest. 2005;128:1968–73. doi: 10.1378/chest.128.4.1968. [DOI] [PubMed] [Google Scholar]
- 12.Schatz M, Zeiger RS, Vollmer WM, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130:43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 13.National Ambulatory Care Medical Survey. Available at: http://www.cdc.gov/nchs/ahcd/trend_analysis.htm (Accessed June 20, 2011).
- 14.Global Strategy for Asthma Management and Prevention. Bethesda: National Heart, Lung, and Blood Institute; 2002. [Google Scholar]
- 15.National Asthma Education and Prevention Program: Expert Panel Report 3. Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. National Heart, Lung, and Blood Institute. NIH Publication Number #08-5846. October 2007.
- 16.Blanchette CM, Culler SD, Ershoff D, Gutierrez B. Association between previous health care use and initiation of inhaled corticosteroid and long acting b2 adrenergic agonis combination therapy among US patients with asthma. Clin Ther. 2009;31:2574–83. doi: 10.1016/j.clinthera.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Zell ER, McCaig LF, Kupronis BA, Besser RE, Schuchat A. A comparison of the National Disease and Therapeutic Index and the National Ambulatory Medical Care Survey to evaluate antibiotic usage. In: Proceedings of the survey research methods section, American Statistical Association (2000). Alexandria (Virginia): American Statistical Association. Available: http://www.amstat.org/sections/srms/Proceedings/papers/2000_143.pdf. (Accessed June 20, 2011).
- 18.Stafford RS, Radley DC. The underutilization of cardiacmedications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41:56–61. doi: 10.1016/S0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]