Abstract
Background
The growing number of cancer survivors combined with a looming shortage of oncology specialists will require greater coordination of post-treatment care responsibilities between oncologists and primary care physicians (PCPs). However, data are limited regarding these physicians’ views of cancer survivors’ care.
Objective
To compare PCPs and oncologists with regard to their knowledge, attitudes, and practices for follow-up care of breast and colon cancer survivors.
Design and Subjects
Mailed questionnaires were completed by a nationally representative sample of 1,072 PCPs and 1,130 medical oncologists in 2009 (cooperation rate = 65%). Sampling and non-response weights were used to calculate estimates to reflect practicing US PCPs and oncologists.
Main Measures
PCPs and oncologists reported their 1) preferred model for delivering cancer survivors’ care; 2) assessment of PCPs’ ability to perform follow-up care tasks; 3) confidence in their knowledge; and 4) cancer surveillance practices.
Key Results
Compared with PCPs, oncologists were less likely to believe PCPs had the skills to conduct appropriate testing for breast cancer recurrence (59% vs. 23%, P < 0.001) or to care for late effects of breast cancer (75% vs. 38%, P < 0.001). Only 40% of PCPs were very confident of their own knowledge of testing for recurrence. PCPs were more likely than oncologists to endorse routine use of non-recommended blood and imaging tests for detecting cancer recurrence, with both groups departing substantially from guideline recommendations.
Conclusion
There are significant differences in PCPs’ and oncologists’ knowledge, attitudes, and practices with respect to care of cancer survivors. Improving cancer survivors’ care may require more effective communication between these two groups to increase PCPs’ confidence in their knowledge, and must also address oncologists’ attitudes regarding PCPs’ ability to care for cancer survivors.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1808-4) contains supplementary material, which is available to authorized users.
Key Words: cancer care, cancer survivorship, physician survey, physician attitudes
INTRODUCTION
One in three persons experiences cancer during their lifetime, and nearly 12 million Americans are cancer survivors1. Optimal cancer survivor care requires surveillance for recurrence or progression and second cancers, caring for long-term and late medical effects of cancer or its treatment, providing psychosocial support, and managing comorbid conditions2. The rapidly increasing survivor population and looming shortages of both oncology specialists3,4 and primary care physicians (PCPs)5,6 present challenges to ensuring high quality follow-up care for cancer survivors7,8. Prior research has shown that appropriate follow-up surveillance testing is associated with more frequent visits to oncologists9,10. However, responsibility for follow-up care often falls to the PCP, since many survivors do not see an oncologist annually11,12, and visits to oncologists decline sharply after five years post-treatment13. These trends may contribute to the considerable variability observed in the delivery of follow-up care10,12,14.
There is growing consensus that inadequate follow-up care may be related to a fragmented health system that impedes communication and care coordination2. Greater coordination of care between PCPs and oncologists has been shown to improve both the quality of and survivors’ satisfaction with follow-up care15–20. Various care delivery models may optimize coordination, including a “shared care” model involving greater PCP involvement, or use of specialty clinics led by oncology nurses or physician assistants4,21–23. However, little is known about physician attitudes or other potential barriers to implementing these models. Prior physician surveys were either conducted outside the US24–27 or in selected samples from academic centers28. There are also limited data on potential physician barriers reflecting US medical practices, such as deficits in knowledge and unfavorable attitudes towards shared care and other alternative models of cancer survivor care.
To address this gap, we conducted a large, nationally representative survey of 2,202 practicing US physicians regarding survivorship care—the Survey of Physician Attitudes Regarding the Care of Cancer Survivors (SPARCCS)—in 2009. SPARCCS was designed to examine and compare the attitudes, knowledge, roles, and usual practices of PCPs and oncologists regarding different components of follow-up care for breast and colon cancer survivors. The ultimate goal of SPARCCS was to obtain information for improving the quality of care for survivors.
In this report, we present the first in a series of results from SPARRCS describing US physicians’ knowledge and attitudes toward improving follow-up cancer care. Our aims in this first report are to describe and compare PCPs’ and oncologists’: 1) preferred model of follow-up care; 2) perceptions of PCPs’ skills in providing follow-up care; 3) confidence in knowledge of components of follow-up care; and 4) cancer surveillance practices.
METHODS
SPARCCS was co-sponsored by the National Cancer Institute (NCI) and the American Cancer Society (ACS). Approval for the study was obtained from NCI’s IRB, and from the U.S. Office of Management and Budget.
Sample Design
We used the American Medical Association (AMA) Physician MasterFile to obtain a nationally representative sample of physicians29,30. We used a stratified sampling strategy within relevant physician specialties (hematology/oncology, family medicine, general internal medicine, and obstetrics/gynecology, including gynecology-only physicians). Within each stratum, the frame was sorted by the following AMA variables: census region, metropolitan status, age category, sex, and “mail undeliverable” status to achieve even coverage of these variables within the sample. To be eligible for the survey, physicians were required to practice in a non-federal setting, be under 76 years of age, and spend 20% or more of their professional time caring for patients. Furthermore, oncologists had to provide care for breast or colon cancer patients within the prior year, and PCPs had to work in an office-based practice. Eligibility was determined from the AMA Masterfile and responses to screener telephone calls or the questionnaire.
Recruitment
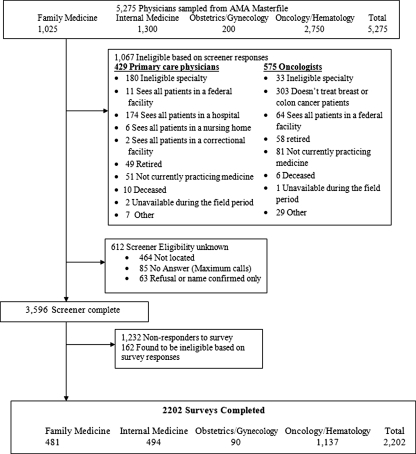
Screener telephone calls were placed to the offices of sampled physicians to verify eligibility for survey participation and contact information. Physicians whose office address and specialty could not be confirmed were classified as non-locatable. Of the 5,275 physicians in the initial sample, 20% were ineligible, 9% were non-locatable, and 1% refused to participate.
The remaining 3,596 physicians received the SPARCCS questionnaires by mail, with a $50 incentive check and telephone follow-up of non-respondents. The survey was fielded from March through December, 2009. Non-responders received up to four mailings: three to the office and one to the physician’s home address. Reminder telephone calls were placed after the 3rd and 4th mailings.
Figure 1 shows the disposition of the survey sample. The combined screener and survey response rates were calculated using the American Association of Public Opinion Research’s standard methods31. The weighted survey response rate that excludes non-locatable physicians was 65.1% (also defined as the "cooperation" rate). The survey’s absolute weighted response rate using the AAPOR RR3 formula, which incorporates unscreened physicians with unknown eligibility, was 57.6%.
Figure 1.
Sample flow diagram for the 2009 Survey of Physician Attitudes Regarding the Care of Cancer Survivors (SPARCCS).
Survey Instrument and Measures
We focused on breast and colon cancer because of the availability of evidence-based guidelines for surveillance of recurrent cancer32,33, high prevalence of survivors, and long survival periods. Separate questionnaires were developed for oncologists and for PCPs. The questionnaires contained identical as well as specialty-specific items covering several content domains and constructs to facilitate comparisons across groups. Most survey items were adapted from previously developed surveys of physicians relating to cancer-focused health care15,26,34–37. Several new items were developed by the investigators. The questionnaires were revised based on the results of cognitive testing in a convenience sample of nine PCPs and nine oncologists. The full survey instruments are available as supplementary material online at.
For the current analysis, we used three main survey questions, described below.
Models of Follow-up Care We asked physicians to identify their preferred model for providing follow-up care using a single item. The response options included 1) PCPs having primary responsibility; 2) oncologists having primary responsibility; 3) oncologists and PCPs sharing responsibility (shared care model); 4) specialized survivorship clinics led by physicians, or 5) survivorship clinics led by oncology nurses, certified nurse practitioners, or physician assistants.
Perceived Skills of PCPs, and Confidence in Knowledge Separate items asked oncologists and PCPs whether they agreed or disagreed with statements regarding PCPs’ skills relating to 1) initiating screening and diagnostic evaluations to detect recurrent cancer, 2) caring for the effects of cancer or its treatment, and 3) providing psychosocial support for survivors. Another item asked about physicians’ confidence in their own knowledge of the same three components of care. For the first item on perceived skills, a 5-point Likert response scale was used, and we grouped respondents into those who “strongly” or “somewhat” agreed or disagreed with each statement, excluding those who “neither agreed nor disagreed”. For the item on confidence, a three-point scale was used, and we grouped responses as either “very confident” versus “somewhat” or “not at all” confident since very few respondents (<10%) were in the latter group.
Surveillance Care Practices We used hypothetical clinical vignettes to assess surveillance practices because of their known validity for measuring physicians’ actual behaviors38–40. Our vignette posed the following question: “There are different beliefs about appropriate cancer surveillance testing for survivors of breast cancer. How often do you believe the following cancer surveillance tests should be performed for a breast cancer survivor with the following characteristics: 55-year old woman, status post adjuvant chemotherapy for early stage breast cancer four years ago, currently asymptomatic, no evident disease, no significant co-morbidities, not on endocrine therapy for her cancer?” A stage 3 colon cancer survivor with similar characteristics (except no mention of endocrine therapy) was a second vignette. For each vignette, respondents were asked how frequently various clinical, laboratory, or imaging tests should be performed using three pre-defined time intervals in addition to the response options “only if indicated”, “never”, “don’t know” or other. We used professional guidelines from the American Society of Clinical Oncology32,33, which are nearly identical to those of the National Comprehensive Cancer Network41,42, to categorize each test as recommended or not. Physicians who endorsed use of a “non-recommended” surveillance test at any of the other pre-defined time intervals were considered to be routine users of the test.
Data Analysis
Estimates for the entire population of practicing PCPs and oncologists in the US that met our eligibility criteria were based upon weighted analysis that adjusted for under-coverage of the sampling proportions and for survey non-response. The SAS (Version 9.2) procedure “SurveyFreq” was used to incorporate jackknife replicate weights in the estimation of the weighted frequency distributions and to calculate associated 95% confidence limits. We used 2-sided chi-square tests to compare PCPs’ and oncologists’ responses. We calculated estimates for each item excluding respondents who did not answer that particular item. Less than 1.5% of all respondents skipped the items asking about knowledge or perceptions, and 3.5% (n = 79) skipped the item on preferred models of follow-up care.
RESULTS
Table 1 provides the demographic characteristics of the final survey sample of 1072 PCPs and 1130 oncologists. The final sample was heterogeneous with respect to multiple personal and practice setting characteristics, reflecting the spectrum of physicians in these specialties in the US health care system. To assess non-response bias, we compared the 2202 survey responders with the 2006 non-responders within each specialty stratum, finding no statistically significant differences on AMA Masterfile variables including age, gender, board certification, specialty, region, or US training (data not shown).
Table 1.
Characteristics of Physicians Respondents
| Primary Care Physicians (n = 1072, weighted N =140,353) | Oncologists (n = 1130, weighted N = 7950) | |||
|---|---|---|---|---|
| Weighted Column % | Weighted Column % | |||
| Age | ||||
| < 40 years old | 22 | 30 | ||
| 40–49 years old | 33 | 29 | ||
| 50–59 years old | 31 | 25 | ||
| 60+ years old | 14 | 16 | ||
| Gender | ||||
| Female | 35 | 27 | ||
| Male | 65 | 73 | ||
| Race-Ethnicity | ||||
| Hispanic | 7 | 4 | ||
| Asian | 15 | 28 | ||
| Black or African-American | 5 | 2 | ||
| White | 70 | 63 | ||
| Other | 3 | 3 | ||
| US Trained | ||||
| Yes | 76 | 64 | ||
| No | 24 | 36 | ||
| Boarded | ||||
| Yes | 82 | 90 | ||
| No | 18 | 10 | ||
| Specialty | ||||
| Family Medicine | 43% | NA | ||
| Internal Medicine | 37% | |||
| Ob/Gyn | 20% | |||
| Breast or colon cancer patients treated per week * | Breast | Colon | ||
| 0 | 2 | 4 | ||
| 1–5 | NA | 12 | 23 | |
| 5–20 | 51 | 61 | ||
| 20+ | 34 | 12 | ||
| Breast or colon cancer patients treated in the last 12 months * | Breast | Colon | NA | |
| 0 | 2 | 5 | ||
| 1–5 | 21 | 37 | ||
| 5–20 | 40 | 38 | ||
| 20+ | 29 | 12 | ||
| In a month percent of time spent in providing patient care | ||||
| Less than 50 % | 5 | 10 | ||
| 51–90 % | 44 | 58 | ||
| More than 90 % | 51 | 32 | ||
| Percentage of patients uninsured * | ||||
| <=5% | 62 | 67 | ||
| 6-25% | 29 | 21 | ||
| 26-100% | 5 | 4 | ||
| Main practice location * | ||||
| Full- or part-owner of a physician practice or employee of practice | 66 | 56 | ||
| Employee of a large medical group or health care system or HMO | 17 | 11 | ||
| Employee of a hospital or clinic | 16 | 30 | ||
*Frequencies do not always add to 100% due to missing values
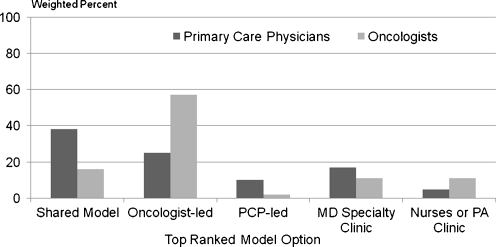
Models of Follow-up Care Figure 2 compares PCPs’ and oncologists’ preferred model for follow-up care. Among PCPs, the shared care model was preferred by 38%, while 25% believed that oncologists should hold primary responsibility for follow-up care, and 10% preferred a PCP-led model. In contrast, oncologists most often preferred an oncologist-led model (57%), while only 16% chose a shared care model, and 2% a PCP-led model. Similar proportions of PCPs and oncologists (a total of 22% when summing the two options for specialized clinics) endorsed specialized survivorship care clinics overall. Among these physicians, oncologists were evenly divided between physician-led versus nurse or PA-led clinics, while PCPs favored physician-led clinics.
Figure 2.
Preferred model For cancer follow-up care: Dark bars= primary care physicians; Lighter bars=Oncologists. Y-Axis shows percent (weighted) of physicians responding with their top ranked preference among the following 5 options for models for the delivery of cancer survivors’ care: 1. PCPs and oncologists share responsibility; 2. Medical Oncologists have primary responsibility; 3. PCPs have primary responsibility; 4. Specialized clinics led by physicians who focus on survivor care; 5. Specialized clinics led by Oncology Nurses, Certified Registered Nurse Practitioners, or Physician Assistants. Five percent (5%) of PCPs and 3% of oncologists endorsed more than 1 option as their top preferred model, and are not included in the figure. Chi-sq. test of difference in preference by physician group was P < 0.001.
Perceived Skills and Knowledge of PCPs Table 2 shows physicians’ perceptions of PCPs’ follow-up care skills. A majority (59%) of PCPs but only 23% of oncologists strongly or somewhat agreed that PCPs have the necessary skills to provide follow-up care related to the effects of breast cancer or its treatment. Similarly, 75%of PCPs, but only 38% of oncologists, agreed that PCPs have the skills necessary to initiate appropriate screening or diagnostic work-up to detect recurrent breast cancer. Only 8% of oncologists but 51% of PCPs believed that PCPs are better able than oncologists to provide psychosocial support for breast cancer survivors (all P < 0.001). Physicians’ perceptions of PCP skills in providing colon cancer survivor care were virtually identical to those for breast cancer.
Table 2.
Perceptions of PCP’s1 Skills Regarding Follow-Up Cancer Care
| Primary Care Physicians (n = 1072) | Oncologists (n = 1130) | |
|---|---|---|
| Weighted % Who Either Strongly or Somewhat Agreed With Statement (95% CL’s) | ||
| For Breast Cancer Survivors | ||
| PCPs have skills necessary to provide follow-up care related to the effects of cancer or its treatment | 59 (56–62) | 23 (21–26) |
| PCPs have skills necessary to initiate appropriate screening or diagnostic work-up to detect recurrent cancer | 75 (72–77) | 38 (35–41) |
| PCPs are better able than oncologists to provide psychosocial support | 51 (48–54) | 8 (6–10) |
| For Colon Cancer Survivors | ||
| PCPs have skills necessary to provide follow-up care related to the effects of cancer or its treatment | 58 (55–61) | 24 (21–27) |
| PCPs have skills necessary to initiate appropriate screening or diagnostic work-up to detect recurrent cancer | 74 (71–77) | 38 (35–41) |
| PCPs are better able than oncologists to provide psychosocial support | 51 (48–54) | 8 (6–10) |
Results are derived from Survey item #5 which asked: To what extent do you agree or disagree with the following statements regarding patients who have already completed active treatment for early stage breast or colon cancer? Response options were: strongly disagree, somewhat disagree, neither disagree nor agree, somewhat Agree, strongly agree.
Statistical significance of chi-square tests for differences by physician groups were all P < 0.001
1 Primary Care Physicians (PCPs)
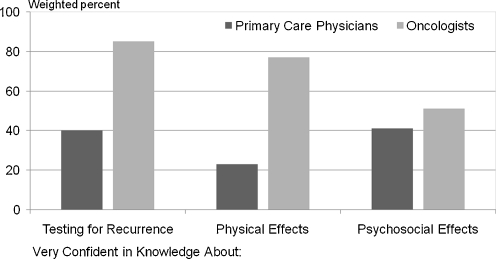
Figure 3 shows physicians’ confidence in their knowledge about various components of follow-up care for breast cancer survivors. While 85% of oncologists were “very confident” about appropriate tests for detecting recurrent disease, only 40% of PCPs expressed this level of confidence (P < 0.001). A large difference was found in reported confidence in caring for late physical effects of cancer, with 23% of PCPs and 77% of oncologists expressing high confidence in their knowledge. While still statistically significant (P < 0.001), the smallest difference in confidence between practitioner groups was observed in caring for psychosocial effects of cancer, with 41% of PCPs and 51% of oncologists reporting being “very confident” in their knowledge of caring for these effects. Results for colon cancer were comparable to those presented in Figure 2.
Figure 3.
Confidence in knowledge about breast cancer follow-up care components. Dark bars=primary care physicians; Lighter bars=Oncologists. Y-Axis shows percent (weighted) of physicians responding that they were “very confident” versus “not at all confident” or “somewhat confident” to the following question: How confident do you feel about your knowledge of the following aspects of cancer-related follow-up care for breast cancer survivors? a. Appropriate surveillance testing to detect recurrent cancer; b. long-term and late physical adverse effects of cancer and cancer treatment; c. the potential adverse psychosocial outcomes of cancer or its treatment. Fewer than 2% responded “Don’t Know” and are excluded from the figure. Chi-sq. tests of differences by physician group for each of the 3 components were all P < 0.001.
Surveillance Care Practices We used clinical vignettes describing characteristics of two hypothetical cancer survivors at 4-years post- diagnosis to elicit physicians’ recommended use of multiple tests and exams for detecting recurrent cancer. When asked about recommended intervals for performing physical exams for 4-year breast cancer survivors, 72% of oncologists recommended 6-month intervals, and 20% recommended yearly intervals. In contrast, 40% of PCPs recommended 6-month intervals, and 53% recommended annual exams (P < 0.001). Both oncologists’ and PCs’ recommended intervals for colon cancer survivors were nearly identical to these estimates.
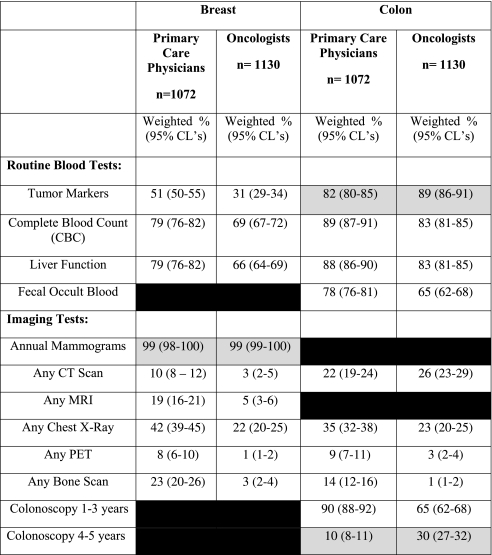
Table 3 shows the percentage of physicians recommending different blood and imaging tests for detecting recurrent cancer. The gray-shaded areas show guideline-recommended tests for surveillance testing of cancer survivors; all other tests shown in the table are not guideline-recommended. With respect to the recommended tests, nearly all physicians endorsed annual mammograms for breast cancer, and a high percentage (over 80%) endorsed serum tumor markers for colon cancer. Guidelines recommend the use of CT scans annually for up to 3 years post-treatment for colon cancer survivors, but not beyond 3 years, and thus we did not consider CT scans as “recommended” for our hypothetical 4-year survivor. Only 10% of PCPs and 30% of oncologists recommended colonoscopy exams every 4–5 years, consistent with guidelines (both P < 0.001); while 90% of PCPs and 65% of oncologists recommended more frequent colonoscopy exams (every 1–3 years) than guidelines specify (P < 0.001).
Table 3.
Percent of US Physicians Recommending Routine Use of Tests and Exams to Detect Recurrent Cancer
Hypothetical Vignettes depicting 4-year survivors for each cancer type were used to elicit physicians’ recommended use of blood and imaging tests to detect recurrent cancer. Routine blood tests are defined as testing every 3–4 months, 6-months or annually. Gray-shaded areas indicate those tests or exams which were recommended by professional guidelines for follow-up care. Blacked-out areas represent tests that were not asked on the survey.
Using chi-square tests, differences by physician group for annual mammograms were not statistically significant. All other tests of differences by physician groups were all P < 0.001, except for liver function tests for colon cancer (P = 0.003) and CT scan for colon cancer (P = 0.03).
More than two-thirds of all physicians departed substantially from guidelines in recommending routine blood tests for cancer survivors; the proportion of PCPs was only slightly higher than oncologists for most of these tests. Non-recommended imaging tests were endorsed by both physician groups much less frequently than were blood tests. Both physician groups demonstrated substantial overuse of chest X-rays and CT scans. PCPs were much more likely than oncologists to endorse non-guideline imaging tests such as chest X-rays, bone scans and MRI.
DISCUSSION
SPARCCS provides current nationally representative data comparing U.S. PCPs’ and oncologists’ knowledge, attitudes, and practices regarding the care of cancer survivors. The findings reported in this paper have several important implications for health policy and research aimed at improving the care of cancer survivors. First, we found disagreement among PCPs and oncologists regarding the ideal model of care for cancer survivors (Fig. 1). While most oncologists favored an oncologist-led model, nearly half of PCPs favored a shared care model or one led by PCPs.
These results suggest an unfavorable view among oncologists regarding a central role for PCPs in caring for cancer survivors—an interpretation reinforced by our results showing oncologists’ generally negative perceptions of PCPs’ skills in caring for survivors. The results also reflect mixed views among PCPs about assuming increased responsibility for survivorship care. These findings are significant, because lack of receptiveness to PCP involvement in cancer survivors’ care, especially among oncologists, could compromise efforts to promote shared care or PCP-led delivery models, which may be a key strategy to meet the care needs of the many survivors who see only their PCP annually11–13.
However, 22% of physicians in both groups endorsed the alternative model of specialty clinics either led by physicians, oncology nurses, or physician assistants, such as those already established by some cancer centers43. With PCP shortages projected5,6 and likely to accelerate due to efforts to expand access to primary care under health care reform, this model may offer a more feasible and cost-efficient alternative to physician-led programs4,21–23. Given the anticipated shortage of oncologists and growth in the cancer survivor population, there is a clear need for closer examination of the costs and effectiveness of such alternative delivery models for cancer survivor care. Our findings suggest that a sizable segment of physicians may be receptive to such models.
Consistent with their attitudes towards models of care, many PCPs reported uncertainty about their own skill levels and lack of confidence in their knowledge of cancer survivor care. For example, less than 60% agreed that PCPs had the necessary skills to care for treatment effects in survivors of breast or colon cancer. Furthermore, less than half of PCPs felt very confident in their knowledge of testing for recurrence or caring for psychosocial effects of cancer, and only 23% felt very confident in their knowledge of caring for the late physical effects of cancer or its treatments (Fig. 3). Oncologists’ opinions about PCP skills in these domains were more negative; far less than half of oncologists agreed that PCPs had the skills necessary to provide care for the late effects of cancer or initiate appropriate testing to detect recurrences.
Taken as a whole, these findings suggest significant attitudinal barriers among both PCPs and oncologists that could impede implementation of new delivery models in which PCPs assume greater responsibility for cancer survivor care. This approach is consonant with movement toward establishing patient-centered medical homes for all patients44–46. Successful implementation of this type of model, however, may first require directly addressing the unfavorable attitudes of many oncologists and some PCPs regarding PCPs’ ability to care for cancer survivors.
Yet the problem is not merely one of overcoming unfavorable attitudes; our study also suggests that many PCPs—and even some oncologists—may lack critical knowledge or training to care for cancer survivors. The responses to the clinical vignettes reflect that both PCPs and oncologists deviate from guidelines by endorsing more testing, and at more frequent intervals, than the guidelines suggest (Table 3). Although both groups departed substantially from guidelines, PCPs diverge more substantially than do oncologists, consistent with our findings of deficits in confidence regarding their knowledge of follow-up care (Fig. 3). The observed systematic bias towards excessive use of non-recommended surveillance tests in both physician groups may contribute to increasing health care costs or iatrogenic harms among cancer survivors47–49. The reasons for physicians’ overuse of follow-up blood and imaging tests remain to be elucidated, but may be due in part to the practice of defensive medicine, reimbursement incentives for office-based lab testing, or uncertainty regarding best care practices given the limited evidence base informing the development of clinical practice guidelines and areas of disagreement between the guidelines of different professional groups. Despite uncertainty regarding the validity of guidelines, our results suggest a need for broader training about appropriate post-treatment surveillance testing.
Interestingly, specialty differences in recommendations for clinic follow-up and physical examinations showed a different pattern than those observed for surveillance testing. Professional guidelines suggest performing physical exams every 6 months for colon cancer survivors and annually for breast cancer survivors. However, our results showed that physicians’ recommended intervals for physical exams were nearly identical for the two survivor groups, with PCPs favoring annual and oncologists opting for 6-month intervals. Responses may reflect the care patterns and experiences most familiar to each of these practitioner groups, rather than lack of awareness of guidelines; for example, annual health examinations are a common practice among PCPs.
The current study has several strengths and limitations. SPARCCS is a large, nationally representative survey designed to obtain the perspectives of US-based PCPs and oncologists practicing in the full spectrum of health care delivery settings, and it builds upon prior physician surveys conducted in other countries or in smaller, less representative physician groups15,24,26,28,50. Our survey had a good response rate and no measurable response bias, and results are generalizable to US PCPs and oncologists. However, our limited focus on two common cancers may underestimate knowledge gaps, given that PCPs likely have lower awareness for rarer cancers, their treatments and long-term adverse effects. Finally, our analyses in this initial overview paper were descriptive, and did not assess the association between multiple physician characteristics or practice setting variables and our main outcomes; these more detailed analyses for each outcome will be addressed in future separate papers.
Our study suggests several key insights regarding implementation of the Institute of Medicine (IOM) recommendations to improve cancer survivor care45. First, PCPs require more training and education to enhance their knowledge and confidence in providing quality follow-up cancer care44. Succinct, explicit, patient-specific, actionable information in the form of ‘survivorship care plans’, as recommended by the IOM, might potentially address much of this concern and also help address any underuse of evidence-based surveillance testing45. Such efforts should also ensure that PCPs are informed of follow-up care guidelines. A randomized controlled trial demonstrated that when PCPs are made aware of such guidelines, patient outcomes including complications, recurrence, and quality of life are similar to those of patients followed by oncologists51. Second, efforts to improve awareness and adherence to guidelines may reduce routine use of non-guideline surveillance testing among both PCPs and oncologists. However, it is unlikely that guideline adherence will occur without changes in coverage policies or reimbursement, informed by systematic research on the benefits and harms of such testing. Third, oncologists' willingness to share survivorship care responsibilities with PCPs or other allied health professionals must be further explored and negotiated. Finally, our results should be interpreted in light of prior research showing that survivors have a keen interest in their own follow-up care, and that greater communication about their expectations would likely enhance the quality of survivor care15,28,45,52. Integrating survivors’ perspectives and preferences will help providers, payers, policymakers, and researchers to develop and disseminate communication strategies such as survivorship care plans, and improved models of care that ensure survivors are no longer “lost in transition” between specialists and PCPs.
ELECTRONIC SUPPLEMENTARY MATERIALS
(PDF 292 kb)
(PDF 289 kb)
Acknowledgements
The authors gratefully acknowledge those who have contributed significantly to this work: The staff of Westat Inc., (Bethesda, MD) who helped in the design and planning of the SPARCCS survey, and who collected all of the survey data; and Ms. Tania Lobo, M.S., at Georgetown University, who provided assistance with data analysis.
Funding for the Survey of Physicians Attitudes Regarding the Care of Cancer Survivors (SPARCCS) was provided by National Cancer Institute (Contract numberHSN261200700068C) and the American Cancer Society Intramural Research funds. The views expressed in this article do not necessarily represent the views of either the federal government or the American Cancer Society.
Selected findings in this article were presented at the 5th Biennial Cancer Survivorship Research Conference: Recovery and Beyond, June 2010, Washington, DC.
Conflict of Interest None Disclosed.
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: Lost in transition. Washington: Institute of Medicine, National Academies Press; 2005. [Google Scholar]
- 3.Warren JL, Mariotto AB, Meekins A, Topor M, Brown ML. Current and future utilization of services from medical oncologists. J Clin Oncol. 2008;26:3242–47. doi: 10.1200/JCO.2007.14.6357. [DOI] [PubMed] [Google Scholar]
- 4.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists : challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salsberg E, Grover A. Physician workforce shortages: implications and issues for academic health centers and policymakers. Acad Med. 2006;81:782–87. doi: 10.1097/00001888-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Starfield B, Fryer GE., Jr The primary care physician workforce: ethical and policy implications. Ann Fam Med. 2007;5:486–91. doi: 10.1370/afm.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulman LN, Jacobs LA, Greenfield S, Jones B, McCabe MS, Syrjala K, et al. Cancer Care and Cancer Survivorship Care in the United States: Will We Be Able to Care for These Patients in the Future? J Oncol Pract. 2009;5:119–23. doi: 10.1200/JOP.0932001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunnell CA. Will We Be Able to Care For Cancer Patients In The Future? Oncology. 2011;24:1–9. [PubMed] [Google Scholar]
- 9.Field TS, Doubeni C, Fox MP, Buist DS, Wei F, Geiger AM, et al. Under utilization of surveillance mammography among older breast cancer survivors. J Gen Intern Med. 2008;23:158–63. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 11.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25:1074–81. doi: 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 12.Snyder CF, Earle CC, Herbert RJ, Neville BA, Blackford AL, Frick KD. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073–79. doi: 10.1200/JCO.2007.11.9859. [DOI] [PubMed] [Google Scholar]
- 13.Pollack LA, Adamache W, Ryerson AB, Eheman CR, Richardson LC. Care of long-term cancer survivors: physicians seen by Medicare enrollees surviving longer than 5 years. Cancer. 2009;115:5284–95. doi: 10.1002/cncr.24624. [DOI] [PubMed] [Google Scholar]
- 14.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors : a population-based analysis. Cancer. 2008;113:2029–37. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 15.Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27:2489–95. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 16.Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, et al. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–55. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 17.Grunfeld E. Primary care physicians and oncologists are players on the same team. J Clin Oncol. 2008;26:2246–47. doi: 10.1200/JCO.2007.15.7081. [DOI] [PubMed] [Google Scholar]
- 18.Haggstrom DA, Arora NK, Helft P, Clayman ML, Oakley-Girvan I. Follow-up care delivery among colorectal cancer survivors most often seen by primary and subspecialty care physicians. J Gen Intern Med. 2009;24(Suppl 2):S472–S479. doi: 10.1007/s11606-009-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nekhlyudov L, Latosinsky S. The interface of primary and oncology specialty care: from symptoms to diagnosis. J Natl Cancer Inst Monogr. 2010;2010:11–17. doi: 10.1093/jncimonographs/lgq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debono D. Coping with the oncology workforce shortage: transitioning oncology follow-up care to primary care providers. J Oncol Pract. 2010;6:203–5. doi: 10.1200/JOP.777005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–24. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 22.Ferrell BR, Winn R. Medical and nursing education and training opportunities to improve survivorship care. J Clin Oncol. 2006;24:5142–48. doi: 10.1200/JCO.2006.06.0970. [DOI] [PubMed] [Google Scholar]
- 23.Grant M, Economou D, Ferrell BR. Oncology nurse participation in survivorship care. Clin J Oncol Nurs. 2010;14:709–15. doi: 10.1188/10.CJON.709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earle CC, Grunfeld E, Coyle D, Cripps MC, Stern HS. Cancer physicians' attitudes toward colorectal cancer follow-up. Ann Oncol. 2003;14:400–405. doi: 10.1093/annonc/mdg101. [DOI] [PubMed] [Google Scholar]
- 25.Nissen MJ, Beran MS, Lee MW, Mehta SR, Pine DA, Swenson KK. Views of primary care providers on follow-up care of cancer patients. Fam Med. 2007;39:477–82. [PubMed] [Google Scholar]
- 26.Giudice ME, Grunfeld E, Harvey BJ, Piliotis E, Verma S. Primary care physicians' views of routine follow-up care of cancer survivors. J Clin Oncol. 2009;27:3338–45. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- 27.Brazil K, Sussman J, Bainbridge D, Whelan T. Who is responsible? The role of family physicians in the provision of supportive cancer care. J Oncol Pract. 2010;6:19–24. doi: 10.1200/JOP.091060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung WY, Neville BA, Earle CC. Associations among cancer survivorship discussions, patient and physician expectations, and receipt of follow-up care. J Clin Oncol. 2010;28:2577–83. doi: 10.1200/JCO.2009.26.4549. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin LM, Adamache W, Klabunde CN, Kenward K, Dahlman C, Warren L. Linking physician characteristics and Medicare claims data: issues in data availability, quality, and measurement. Med Care. 2002;40:IV–95. doi: 10.1097/00005650-200208001-00012. [DOI] [PubMed] [Google Scholar]
- 30.Cherkin D, Lawrence D. An evaluation of the American Medical Association's Physician masterfile as a data source–one state's experience. Med Care. 1977;15:767–69. doi: 10.1097/00005650-197709000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Standard definitions: final dispositions of case codes and outcome rates for surveys. 6th edition. 2009. American Association for Public Opinion Research.
- 32.Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–97. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 33.Desch CE, Benson AB, III, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–19. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 34.Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians' colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352–62. doi: 10.1016/S0091-7435(02)00066-X. [DOI] [PubMed] [Google Scholar]
- 35.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keating NL, Landrum MB, Rogers SO, Jr, Baum SK, Virnig BA, Huskamp HA, et al. Physician factors associated with discussions about end-of-life care. Cancer. 2010;116:998–1006. doi: 10.1002/cncr.24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klabunde CN, Ambs A, Keating NL, He Y, Doucette WR, Tisnado D, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029–36. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandelblatt JS, Berg CD, Meropol NJ, Edge SB, Gold K, Hwang YT, et al. Measuring and predicting surgeons' practice styles for breast cancer treatment in older women. Med Care. 2001;39:228–42. doi: 10.1097/00005650-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Peabody JW, Luck J, Glassman P, Jain S, Hansen J, Spell M, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141:771–80. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]
- 40.Yeazel MW, Lindstrom Bremer KM, Center BA. A validated tool for gaining insight into clinicians' preventive medicine behaviors and beliefs: the preventive medicine attitudes and activities questionnaire (PMAAQ) Prev Med. 2006;43:86–91. doi: 10.1016/j.ypmed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. Breast Cancer Guidelines, Version 2.2008. 2008.
- 42.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™. Colon Cancer Guidelines, Version 2.2011. 1-1-2010.
- 43.Shapiro CL, McCabe MS, Syrjala KL, Friedman D, Jacobs LA, Ganz PA, et al. The LIVESTRONG Survivorship Center of Excellence Network. J Cancer Surviv. 2009;3:4–11. doi: 10.1007/s11764-008-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hewitt M, Ganz PA. Implementing Cancer Survivorship Care Planning: Workshop Summary. Washington: Institute of Medicine, National Academies Press; 2007. [Google Scholar]
- 46.Grunfeld E. Cancer survivorship: a challenge for primary care physicians. Br J Gen Pract. 2005;55:741–42. [PMC free article] [PubMed] [Google Scholar]
- 47.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 48.Smith-Bindman R. Is computed tomography safe? N Engl J Med. 2010;363:1–4. doi: 10.1056/NEJMp1002530. [DOI] [PubMed] [Google Scholar]
- 49.Lauer MS. Elements of danger–the case of medical imaging. N Engl J Med. 2009;361:841–43. doi: 10.1056/NEJMp0904735. [DOI] [PubMed] [Google Scholar]
- 50.Watson EK, Sugden EM, Rose PW. Views of primary care physicians and oncologists on cancer follow-up initiatives in primary care: an online survey. J Cancer Surviv. 2010;4:159–66. doi: 10.1007/s11764-010-0117-y. [DOI] [PubMed] [Google Scholar]
- 51.Grunfeld E, Mant D, Yudkin P, Adewuyi-Dalton R, Cole D, Stewart J, et al. Routine follow up of breast cancer in primary care: randomised trial. BMJ. 1996;313:665–69. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi-Dalton R, Stewart J, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705–10. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 292 kb)
(PDF 289 kb)