Abstract
AIM: To evaluate the clinical significance of oxidative stress markers in patients with hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC).
METHODS: Sixty-four consecutive patients who were admitted to Kagoshima University Medical and Dental Hospital were enrolled in this retrospective study. All patients had chronic liver disease (CLD) due to infection with HCV. Thirty patients with HCV-related HCC, 34 with HCV-related CLD without HCC (non-HCC), and 20 healthy volunteers (HVs) were enrolled. Possible associations between serum manganese superoxide dismutase (MnSOD) and thioredoxin (TRX) levels and clinical parameters or patient prognosis were analyzed over a mean follow-up period of 31.7 mo.
RESULTS: The serum MnSOD levels were significantly higher in patients with HCV-related HCC than in patients without HCC (P = 0.03) or HVs (P < 0.001). Similarly, serum TRX levels were also significantly higher in patients with HCV-related HCC than in patients without HCC (P = 0.04) or HVs (P < 0.01). However, serum levels of MnSOD and TRX were not correlated in patients with HCC. Among patients with HCC, the overall survival rate (OSR) was lower in patients with MnSOD levels ≥ 110 ng/mL than in patients with levels < 110 ng/mL (P = 0.01), and the OSR tended to be lower in patients with TRX levels < 80 ng/mL (P = 0.05). In addition, patient prognosis with HCC was poorest with serum MnSOD levels ≥ 110 ng/mL and serum TRX levels < 80 ng/mL. Furthermore, a multivariate analysis using a Cox proportional hazard model and serum levels of five factors (MnSOD, prothrombin time, serum albumin, serum α-fetoprotein (AFP), and serum des-γ-carboxy prothrombin) revealed that MnSOD levels ≥ 110 ng/mL (risk ratio: 4.12, 95% confidential interval: 1.22-13.88, P = 0.02) and AFP levels ≥ 40 ng/mL (risk ratio: 6.75; 95% confidential interval: 1.70-26.85, P < 0.01) were independent risk factors associated with a poor patient prognosis.
CONCLUSION: Serum MnSOD and TRX levels are potential clinical biomarkers that predict patient prognosis in HCV-related HCC.
Keywords: Oxidative stress, Manganese superoxide dismutase, Thioredoxin, Hepatitis C virus, Hepatocellular carcinoma
INTRODUCTION
As a significant cause of global cancer morbidity and mortality, hepatocellular carcinoma (HCC) is the fifth- and seventh-most frequently diagnosed cancer worldwide in men and women, respectively, and is the second- and sixth-most frequent cause of cancer deaths in men and women, respectively[1]. HCC is most frequently caused by persistent infection with hepatitis C or B virus. Early HCC diagnosis and better treatments have helped to improve the prognosis for patients with HCC. Also, interferon (IFN)-based treatments not only eliminate hepatitis C virus (HCV) infection, but also prevent HCC in patients with chronic hepatitis C (CHC)[2]. However, IFN-based therapies do not always effectively eliminate HCV infection or prevent HCC. Thus, biomarkers that are indicative of HCC pathological condition would have many clinical benefits, including aiding in the selection of the most appropriate treatment for a patient’s disease.
Oxidative stress results from an imbalance in the production of reactive oxygen species (ROS) and the antioxidative defenses that maintain a cellular redox state. ROS include superoxide anions, hydrogen peroxide, hydroxyl radicals and nitric oxide, all of which are indispensable elements in many biochemical processes[3]. ROS are mainly derived from Kupffer and inflammatory cells in the liver[4], and upon exposure to other cells are thought to induce apoptosis, necrosis, inflammation, immune responses, fibrosis and tissue regeneration[5]. In liver disease, there is an overproduction of ROS from endogenous sources such as the mitochondria, peroxisomes, and activated inflammatory cells. In particular, ROS of mitochondrial origin were recently reported to be elevated in patients with alcoholic liver disease, non-alcoholic steatohepatitis (NASH)[6,7] and HCV-related chronic liver disease (CLD)[8]. Conversely, cells are protected from oxidative stress by intracellular antioxidants such as glutathione (GSH) and thioredoxin (TRX) and by various antioxidant enzymes such as superoxide dismutase (SOD), GSH peroxidase, catalase, and heme oxygenase-1[9-11]. Collectively, the relative expression levels of these molecules may serve as biomarkers for various liver diseases, including HCV-related HCC.
Manganese SOD (MnSOD) is an antioxidant enzyme that catalyzes the dismutation of the highly reactive superoxide anion to O2 and to the less reactive species H2O2. We have previously demonstrated that MnSOD expression was induced in primary cultured hepatocytes that were loaded with hydrogen peroxide in vitro and that serum MnSOD levels can be used to distinguish between NASH and simple steatosis in patients with nonalcoholic fatty liver disease[7]. However, the clinical significance of serum MnSOD levels in HCV-related CLD has not been fully investigated.
TRX was originally discovered in Escherichia coli as a proton donor for ribonucleotide reductase[12]. Subsequently, the human TRX gene was cloned as an adult T-cell leukemia-derived factor and was originally described as an interleukin-2 receptor inducer present in the cell culture supernatant of human T-lymphotropic virus type-1 -transformed cells[13]. TRX expression is induced by various oxidative stressors in patients with acquired immunodeficiency syndrome[14], Sjögren’s syndrome[15], rheumatoid arthritis[16], and malignant neoplasms[17,18]. Previous studies have reported that serum TRX is an oxidative stress marker and that serum TRX levels increase in patients with HCV-related CLD during liver fibrosis progression[19]. In addition, serum TRX levels are reported to be elevated in patients with NASH compared to patients with simple steatosis[20]. However, the clinical significance of elevated TRX levels among patients infected with HCV in relation to HCC diagnosis and prognosis has not been elucidated.
In this study, we aimed to clarify the clinical significance of serum levels of MnSOD and TRX in patients with HCV-related CLD, and in particular among patients with HCC.
MATERIALS AND METHODS
Patients
Sixty-four consecutive patients who were admitted to Kagoshima University Medical and Dental Hospital between December 2006 and November 2008 were enrolled in this retrospective study. All patients had CLD due to an HCV infection and were diagnosed with HCC (30 patients; HCC group) or without HCC (34 patients; non-HCC group). Twenty healthy volunteers (HVs) were also enrolled in this study.
In this study, HCC was diagnosed based on findings from abdominal ultrasound, abdominal computed tomography, and serum levels of α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP, also known as PIVKA-II). Patients were excluded from this study if they were positive for hepatitis B surface antigen; other types of hepatitis, including autoimmune hepatitis and alcoholic liver disease; or other malignancies.
The study endpoint was patient death, the available follow-up date, or December 31, 2010. Patient follow-up periods ranged from 5.1 to 44.6 mo, with a mean observation time of 31.7 mo. Informed consent was obtained from all study patients and healthy controls. This study was approved by the ethical committees of Kagoshima University Graduate School of Medical and Dental Sciences and Kagoshima University Medical and Dental Hospital.
Laboratory markers
The clinical laboratory parameters assessed included platelet count (Plt), prothrombin time (PT), albumin (Alb), total bilirubin (T-Bil), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), AFP and DCP. The serologically defined HCV genotype (HCV serotype) was determined using a serological genotyping assay kit (Immunocheck F-HCV Grouping; International Reagents Co., Tokyo, Japan). If the HCV serotype could not be determined, the HCV genotype was evaluated using the HCV Core Genotype assay (SRL, Tokyo, Japan). HCV genotype 1b was included with serotype I, while genotypes 2a and 2b were included with serotype II. No other HCV genotype was detected in this study population. HCV RNA titers were quantified using either quantitative RT-PCR (Amplicor monitor version 2, Roche, Tokyo, Japan) or the Cobas TaqMan PCR assay (Roche, Tokyo, Japan). Patients were categorized as having a high viral load if their values were 100 KIU/mL or greater based on quantitative RT-PCR analysis, or 5 log IU/mL or more based on the Cobas TaqMan PCR assay.
Evaluation of clinical stage
Hepatic function was assessed in the HCC group using Child-Pugh staging based on both clinical (ascites and encephalopathy) and laboratory (Alb, T-Bil, and PT) parameters. HCC clinical stage was assessed based on a patient’s Cancer of the Liver Italian Program (CLIP) score, which was calculated by adding points for the following four variables: Child-Pugh stage, tumor morphology, AFP value, and portal venous invasion[21,22]. The Japan Integrated Staging (JIS) system[23,24], developed by the Liver Cancer Study Group of Japan and based on a combination of Child-Pugh stage and HCC TNM classification, was used to clinically stage HCC.
Serum MnSOD and TRX levels
Serum was obtained from peripheral blood samples by centrifugation at 4000 g for 5 min at room temperature. Serum samples were frozen at -80 °C until further use. Serum MnSOD or TRX levels were measured using the Human Superoxide Dismutase 2 (AbFRONTIER, Seoul, Korea) and human thioredoxin (Redox Bio Science, Kyoto, Japan) ELISAs, respectively.
Statistical analysis
Results are expressed as the mean and standard deviation. P values less than 0.05 were regarded as statistically significant. Statistical analyses were performed using the Fischer’s exact test or the Mann-Whitney U test, as appropriate. The area under the curve (AUC) was calculated for the receiver operating characteristic (ROC) curve in order to measure the overall accuracy of the test. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of diagnostic test were additionally determined according to the protocol described previously[25]. Differences among the three groups were evaluated using the Kruskal-Wallis test followed by Dunn’s multiple comparison tests. Correlation coefficients were calculated using Spearman’s rank correlation analysis. The Kaplan-Meier method was used to estimate death for each parameter that had been identified at enrollment, and the death distribution curves were compared using the log-rank test. Univariate and multivariate analyses of patient outcome risk ratios were performed using Cox’s proportional hazards regression analyses. All statistical analyses were conducted using PASW Statistics v. 18 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics and classification according to the presence of hepatocellular carcinoma
Table 1 summarizes the baseline clinical characteristics of the 64 patients who were classified based on the presence or absence of HCC. Age, sex, and clinical laboratory parameters, including Plt, PT, Alb, T-Bil, γ-GTP, AFP and DCP, were significantly different between these two groups.
Table 1.
Patient clinical characteristics
| Characteristics | Non-HCC group(n = 34) | HCC group(n = 30) | P value1 |
| Age (yr) | 62.3 ± 11.0 | 72.2 ± 7.5 | < 0.001 |
| Sex (male/female) | 10/24 | 21/9 | < 0.01 |
| Plt (× 104/μL) | 17.0 ± 5.5 | 10.3 ± 5.2 | < 0.001 |
| PT (%) | 99.7 ± 13.3 | 77.6 ± 11.8 | < 0.001 |
| Alb (g/dL) | 4.3 ± 0.4 | 3.6 ± 0.6 | < 0.001 |
| T-Bil (mg/dL) | 0.8 ± 0.3 | 1.5 ± 0.8 | < 0.001 |
| ALT (IU/L) | 44.8 ± 30.2 | 52.0 ± 28.2 | 0.12 |
| γ-GTP (IU/L) | 31.3 ± 16.1 | 56.2 ± 44.3 | < 0.01 |
| AFP (ng/mL) | 7.2 ± 22.8 | 85.9 ± 197.6 | < 0.001 |
| DCP (mAU/mL) | 22.8 ± 14.7 | 485.5 ± 1982.6 | 0.001 |
| HCV serotype group (1/2) | 18/10 (n = 28) | 21/3 (n = 24) | 0.06 |
| HCV RNA level (high/low) | 28/5 (n = 33) | 21/4 (n = 25) | 0.99 |
Data are shown as the mean ± SD. n: Number of patients or the number of samples analyzed.
Differences between mean values were evaluated using either the Fischer’s exact test or the Mann-Whitney U test, as appropriate. Plt: Platelet count; PT: Prothrombin time; Alb: Albumin; T-Bil: Total bilirubin; ALT: Alanine aminotransferase; γ-GTP: γ-glutamyl transpeptidase; AFP: alpha-fetoprotein; DCP: des-γ-carboxy prothrombin; HCV: Hepatitis C virus; RNA: Ribonucleic acid.
Serum MnSOD and TRX levels in hepatocellular carcinoma patients
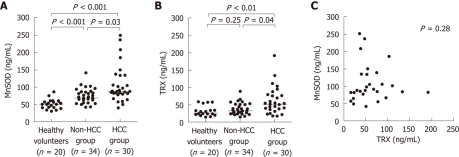
Serum MnSOD levels were significantly higher in patients with HCC compared to patients without HCC (P = 0.03) and HVs (P < 0.001) (Figure 1A). The serum TRX levels were also significantly higher in the HCC group compared to the non-HCC group (P = 0.04) and HV group (P < 0.01) (Figure 1B). However, there was no correlation between these two markers in the HCC group (P = 0.28, r = 0.20) (Figure 1C).
Figure 1.
Serum levels of manganese superoxide dismutase and thioredoxin in the hepatocellular carcinoma, non-hepatocellular carcinoma and healthy volunteer groups. A: Serum manganese superoxide dismutase (MnSOD) levels were significantly higher in the hepatocellular carcinoma (HCC) group than in either the non-HCC group (P = 0.03) or the healthy volunteers (HV) group (P < 0.001); B: Serum thioredoxin (TRX) levels were also significantly higher in the HCC group than in either the non-HCC group (P = 0.04) or the HV group (P < 0.01); C: No significant correlation was detected between serum MnSOD and TRX levels in the HCC group.
Diagnostic value of serum MnSOD and TRX levels for patients with hepatocellular carcinoma and hepatitis C virus infection
Serum AFP and DCP concentrations are established diagnostic markers for HCC. To evaluate the utility of MnSOD and TRX for the diagnosis of HCC, we measured AFP and DCP expression in addition to MnSOD and TRX expression. In an AUC-ROC analysis, AFP was the strongest diagnostic marker for HCC (AUC-ROC, 0.90). AUC-ROCs for MnSOD, TRX and DCP were 0.73, 0.77 and 0.77, respectively. Additional analyses showed that the accuracy of AFP (≥ 40 ng/mL) for diagnosis of HCC was higher than that of MnSOD (≥ 110 ng/mL) (Table 2), while the combination of AFP and MnSOD was a more accurate marker of HCC than either marker alone.
Table 2.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy of manganese superoxide dismutase and α-fetoprotein serum levels for diagnosis of hepatocellular carcinoma in all patients (%)
| Factors | Sensitivity | Specificity | PPV | NPV | Accuracy |
| MnSOD (≥ 110 ng/mL) | 26.7 | 97.1 | 88.9 | 60.0 | 64.1 |
| AFP (≥ 40 ng/mL) | 33.3 | 97.1 | 90.9 | 62.3 | 67.2 |
| Combination1 | 46.7 | 94.1 | 87.5 | 66.7 | 71.9 |
MnSOD ≥ 110 ng/mL and/or AFP ≥ 40 ng/mL. PPV: Positive predictive value; NPV: Negative predictive value; MnSOD: Manganese superoxide dismutase; AFP: α-fetoprotein.
Association of serum MnSOD or TRX levels with laboratory data in the HCC group
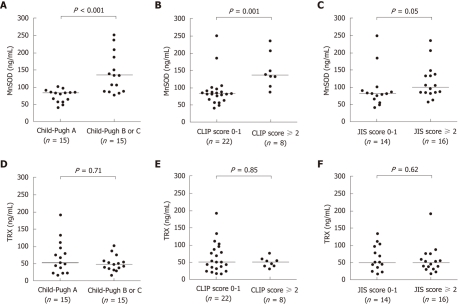
Serum MnSOD levels for the 30 patients in the HCC group were positively correlated with serum AFP and DCP levels and were negatively correlated with serum Alb levels (Table 3). Serum MnSOD levels were also significantly higher in patients with two or more HCC tumors than in patients with a single HCC tumor [average ± SD (ng/mL), 125.4 ± 50.9 vs 87.4 ± 48.8, P = 0.008], although HCC tumor size was not associated with serum MnSOD levels. In addition, HCC patient serum MnSOD levels increased in parallel with the Child-Pugh stage, CLIP score and JIS score (Figure 2A-C). In contrast, serum TRX levels were only associated with platelet counts (Table 3). Serum TRX levels were not associated with HCC tumor number or size. Furthermore, there were no significant correlations between serum TRX levels for various scores (Figure 2D-F).
Table 3.
Correlation between serum manganese superoxide dismutase or thioredoxin levels and laboratory data in the hepatocellular carcinoma group
| Factors |
HCC group (n = 30) |
|||
|
Serum MnSOD levels |
Serum TRX levels |
|||
| Correlation coefficient | P value | Correlation coefficient | P value | |
| Age (yr) | -0.97 | 0.61 | 0.11 | 0.55 |
| Plt (× 104/μL) | 0.03 | 0.89 | 0.66 | < 0.001 |
| PT (%) | -0.36 | 0.05 | 0.12 | 0.53 |
| Alb (g/dL) | -0.63 | < 0.001 | 0.19 | 0.33 |
| T-Bil (mg/dL) | 0.25 | 0.18 | 0.05 | 0.79 |
| ALT (IU/L) | 0.12 | 0.52 | 0.15 | 0.42 |
| γ-GTP (IU/L) | 0.30 | 0.11 | 0.28 | 0.13 |
| AFP (ng/mL) | 0.38 | 0.04 | 0.11 | 0.57 |
| DCP (mAU/mL) | 0.57 | 0.001 | 0.12 | 0.52 |
P values were assessed by Spearman’s rank correlation analysis. MnSOD: Manganese superoxide dismutase; TRX: Thioredoxin; HCC: Hepatocellular carcinoma; Plt: Platelet count; PT: Prothrombin time; Alb: Albumin; T-Bil: Total bilirubin; ALT: Alanine aminotransferase; γ-GTP: γ-glutamyl transpeptidase; AFP: α-fetoprotein; DCP: des-γ-carboxy prothrombin.
Figure 2.
Clinical significance of serum manganese superoxide dismutase and thioredoxin levels in hepatocellular carcinoma. In the hepatocellular carcinoma (HCC) group, differences in serum manganese superoxide dismutase (MnSOD) and thioredoxin (TRX) levels were evaluated based on Child-Pugh stage, cancer of the liver italian program (CLIP) score and Japan integrated staging (JIS) score. A: Serum MnSOD levels were significantly higher in patients with Child-Pugh B or C compared to those with Child-Pugh A (P < 0.001); B: Serum MnSOD levels in patients with a CLIP score of 2 or greater were significantly higher compared to levels in patients with a CLIP score of 0 or 1 (P = 0.001); C: In addition, serum MnSOD levels tended to be higher in patients with a JIS score of 2 or greater compared to patients with a JIS score of 0 or 1 (P = 0.05); D-F: In contrast, serum TRX levels were not significantly different based on Child-Pugh stage, CLIP score or JIS score.
Overall survival rate based on serum MnSOD or TRX levels in the HCC group
In the HCC group, the overall patient survival rate was significantly lower (P = 0.01) in patients with MnSOD levels ≥ 110 ng/mL compared to patients with levels < 110 ng/mL (Figure 3A). In addition, the overall survival rate tended to be lower (P = 0.05) in patients with TRX levels < 80 ng/mL compared to those with levels ≥ 80 ng/mL (Figure 3B). Furthermore, among all HCC groups, patients who had both serum MnSOD levels ≥ 110 ng/mL and TRX levels < 80 ng/mL had a significantly poorer prognosis. Conversely, patients with a serum TRX level ≥ 80 ng/mL had a favorable prognosis, regardless of their serum MnSOD level (Figure 3C).
Figure 3.
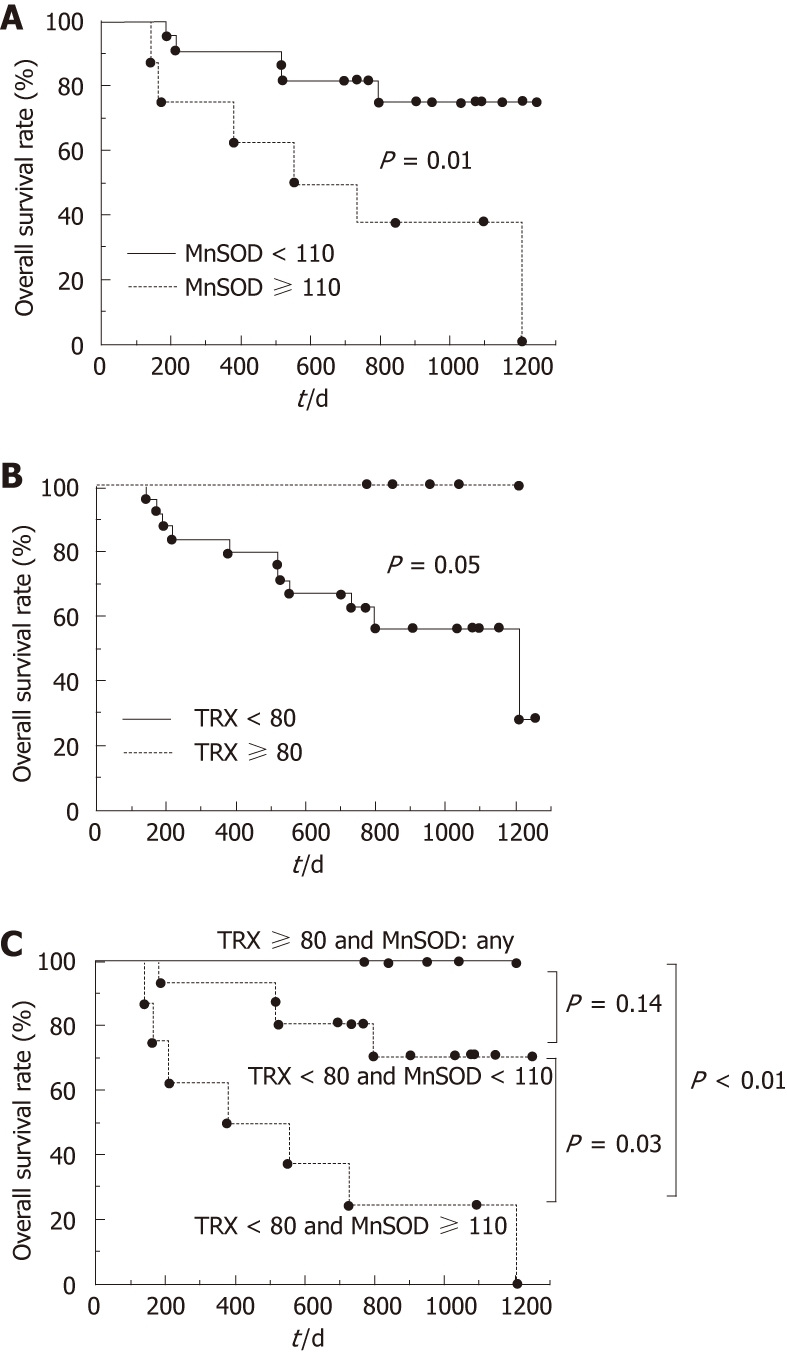
Overall hepatocellular carcinoma patient survival based on serum levels of manganese superoxide dismutase or thioredoxin. Overall survival was plotted using the Kaplan-Meier method after separation into two or three groups defined as follows: A: Manganese superoxide dismutase (MnSOD) < 110 ng/mL or ≥ 110 ng/mL; B: Thioredoxin (TRX) < 80 ng/mL or ≥ 80 ng/mL; TRX ≥ 80 ng/mL, TRX < 80 ng/mL; C: MnSOD < 110 ng/mL, or TRX < 80 ng/mL and MnSOD ≥ 110 ng/mL. The overall survival rate was lower in patients with MnSOD levels ≥ 110 ng/mL (P = 0.01) (A). Also, cumulative patient survival rate tended to be lower in patients with TRX levels < 80 ng/mL (P = 0.05) (B). Among these groups, patients with serum TRX levels < 80 ng/mL and serum MnSOD levels ≥ 110 ng/mL had the poorest prognosis (C).
In addition to serum MnSOD and TRX levels, other possible prognostic factors were also investigated in the HCC group. A univariate analysis (log-rank test) revealed that the survival rate was significantly different between patients with high and low levels of MnSOD, PT, Alb, AFP and DCP, but not other factors such as TRX (Table 4). A multivariate analysis using a Cox proportional hazard model and five markers (MnSOD, PT, Alb, AFP and DCP) selected based on the results of the univariate analysis revealed that MnSOD levels ≥ 110 ng/mL and AFP levels ≥ 40 ng/mL were independent risk factors that were associated with a poor patient prognosis (Table 5). In addition, similar results were obtained from a similar multivariate analysis using the same five factors and TRX, supporting the finding that TRX is not an independent risk factor associated with HCC prognosis. Furthermore, patient Child-Pugh stage, CLIP score and JIS score, which were calculated based on several factors including clinical symptoms and laboratory data, were also prognostic factors for patients with HCC (Table 4). A multivariate analysis using the three markers of MnSOD, Child-Pugh stage and CLIP score indicated that Child-Pugh stage was also a significant prognostic factor (risk ratio: 6.19, 95% confidential interval: 1.33-28.95, P = 0.02).
Table 4.
Univariate analysis of prognostic factors in the hepatocellular carcinoma group
| Factors | Category | Number | P value1 |
| Single marker | |||
| MnSOD (ng/mL) | < 110/≥ 110 | 22/8 | 0.01 |
| TRX (ng/mL) | < 80/≥ 80 | 24/6 | 0.05 |
| Age (yr) | < 70/≥ 70 | 12/18 | 0.23 |
| Plt (× 104/μL) | < 10/≥ 10 | 19/11 | 0.38 |
| PT (%) | < 80/≥ 80 | 15/15 | 0.02 |
| Alb (g/dL) | < 3.5/≥ 3.5 | 15/15 | 0.02 |
| T-Bil (mg/dL) | < 1.5/≥ 1.5 | 18/12 | 0.34 |
| ALT (IU/L) | < 40/≥ 40 | 11/19 | 0.58 |
| γ-GTP (IU/L) | < 50/≥ 50 | 17/13 | 0.98 |
| AFP (ng/mL) | < 40/≥ 40 | 20/10 | <0.01 |
| DCP (mAU/mL) | < 40/≥ 40 | 16/14 | 0.02 |
| Staging system | |||
| Child-Pugh stage | A/≥ B | 16/14 | < 0.01 |
| CLIP score | 0-1/≥ 2 | 22/8 | 0.01 |
| JIS score | 0-1/≥ 2 | 14/16 | 0.41 |
P values were assessed using the log-rank test. MnSOD: Manganese superoxide dismutase; TRX: Thioredoxin; Plt: Platelet count; PT: Prothrombin time; Alb: Albumin; T-Bil: Total bilirubin; ALT: Alanine aminotransferase; γ-GTP: γ-glutamyl transpeptidase; AFP: Alpha-fetoprotein; DCP: Serum des-γ-carboxy prothrombin; CLIP: Cancer of the Liver Italian Program; JIS: Japan Integrated Staging.
Table 5.
Multivariate analysis of prognostic factors in the hepatocellular carcinoma group
| Factors | Risk ratio | 95% CI | P value |
| MnSOD (≥ 110 ng/mL) | 4.12 | 1.22-13.88 | 0.02 |
| AFP(≥ 40 ng/mL) | 6.75 | 1.70-26.85 | < 0.01 |
95% CI: 95% confidence interval; MnSOD: Manganese superoxide dismutase; AFP: α-fetoprotein.
DISCUSSION
HCV infection is the most important known contributor to the etiology of HCC. An increasing incidence of HCC has been largely attributed to a rise in HCV infections in the general population during the last 50 to 60 years[26]. During HCV infection, ROS production increases and persists throughout the infection. In addition, ROS are thought to play a major role in the pathogenesis of chronic inflammatory changes in the liver, leading to increased hepatic fibrosis and decreased hepatic function. In this study, we have shown that both serum MnSOD and TRX levels are elevated in patients with HCV-related HCC, with no correlation between these two markers. In addition, serum MnSOD and TRX levels were a useful predictor of overall patient survival. Serum MnSOD and TRX levels are reported to be biomarkers of oxidative stress in several diseases, including liver disease[7,14,17,19,27-29]. There were a small number of enrolled patients in this study and other contributors to liver diseases such as chronic hepatitis B infection should be further evaluated. However, our study has clearly demonstrated the clinical significance of these markers in patients with HCV-related HCC.
Serum MnSOD and TRX levels should both reflect hepatic oxidative stress. The results of the current study showed that both of these markers were increased in the HCC group relative to levels in the non-HCC group and the HV group (Figure 1A and B). However, there was no correlation between these two markers in the HCC group (Figure 1C). MnSOD is primarily localized to the mitochondrial matrix[3] and abnormal mitochondrial morphologies are frequently observed in CHC[8]. Therefore, MnSOD may be an indicator of mitochondrial disorders that are induced by oxidative stress. On the other hand, there are two TRX proteins, cytoplasmic TRX1 and mitochondrial TRX2[30]. TRX1 negatively regulates the apoptosis signal-regulating kinase 1 (ASK1)-c-Jun N-terminal kinase/P38 apoptotic pathway by binding to and inhibiting the kinase activity of ASK1, which plays an important role in ROS-induced cellular responses[31]. TRX2 is an essential regulator of mitochondrial ROS levels that has been associated with mitochondrial outer membrane permeability[32]. In the present study, we examined the serum levels of TRX1, but not TRX2, using a sandwich ELISA. Thus, the MnSOD and TRX proteins that were examined in this study have different origins in the mitochondria and cytoplasm, respectively, which could contribute to the lack of correlation between these two markers.
Several studies have shown that the HCV core protein directly inhibits the electron transport system and modulates apoptosis, transcription, and cell signaling[33]. Abdalla et al[34] reported that expression of not only the HCV core protein but also the HCV NS proteins increases ROS and further showed that the presence of these proteins can increase endogenous expression levels of antioxidant enzymes and prooxidants such as MnSOD. Several reports have shown that serum MnSOD levels in patients with HCV-related CLD[35-37] are associated with various clinical findings, such as fibrosis and hepatic oxidative stress. However, the significance of serum MnSOD levels has not been fully examined in patients with HCC. We previously reported that serum MnSOD levels may be correlated with fibrosis in patients with NAFLD[7]. In addition, serum MnSOD levels decreased in patients with CHC after administration of an interferon-based treatment (data not shown). These results indicate that serum MnSOD levels are likely associated with hepatic fibrosis or oxidative stress in patients with CHC. In the present study, however, MnSOD levels were not associated with platelet counts, which is a simple predictor of hepatic fibrosis in this patient population[38]. Thus, advanced hepatic fibrosis or oxidative stress may be one reason why serum MnSOD levels have diagnostic and prognostic utility with HCC, but other mechanisms should also be considered.
The present study revealed that serum MnSOD levels were significantly higher in the HCC group than in the non-HCC group (Figure 1A). In the HCC group, serum MnSOD levels were negatively correlated with serum Alb and tended to negatively correlate with PT (Table 3); these results showed an association between MnSOD and Child-Pugh stage (Figure 2A). It is known that in humans, MnSOD activity is comparatively higher in the liver compared to other tissues[39]. In addition, although a previous immunohistochemical study showed that MnSOD expression was higher in both cancerous and non-cancerous liver tissues from patients with HCC, this positive immunoreactivity was strongly observed in non-cancerous liver tissues, especially in normal hepatocytes surrounding HCC, regenerative small hepatocytes in the tumor boundary, and mononuclear inflammatory cells in necroinflammatory lesions[40]. Furthermore, ROS are overproduced by Kupffer cells and inflammatory cells in liver disease[5,41]. In the present study, serum MnSOD levels were also positively correlated with the serum tumor markers AFP and DCP (Table 3) and with Child-Pugh stage and CLIP score (Figure 2). These results indicate that increased MnSOD expression reflects hepatocyte oxidative stress and correlates with decreased hepatic function, increased hepatic fibrosis and ROS production by inflammatory cells in liver cirrhosis. These features comprise the main background characteristics leading to HCC and may be associated with the indirect effects of liver cancer progression. These associations may also explain why serum MnSOD levels predicted the overall survival of patients with HCC.
It was previously reported that serum levels of TRX, which is a stress-induced protein, increase relative to the degree of hepatic fibrosis, and that high serum concentrations of TRX may indicate advanced hepatic fibrosis[19,20]. In contrast, it has also been reported that a higher degree of hepatic fibrosis is associated with lower platelet counts[38]. Therefore, the present study may present a conflict, since results indicated that serum TRX level was positively correlated with platelet count. A previous report showed that the survival rate following LPS plus GalN-induced hepatitis was much higher in transgenic mice overexpressing TRX than in wild-type mice, and that thioacetamide-induced hepatic fibrosis was suppressed in TRX transgenic mice compared to wild-type mice[42]. Although it is still unclear why TRX and platelet counts are positively correlated, we speculate that elevated serum TRX in patients with HCC and advanced hepatic fibrosis potentially improves overall survival by suppressing oxidative stress[43]. In addition, patients with HCC, low levels of TRX, and high levels of MnSOD, which may be indicative of excessive oxidative stress without TRX attenuation, have the poorest prognosis. This result supports the hypotheses presented above. In order to better assess these findings, future studies are needed that incorporate sequential observations of serum TRX and MnSOD levels over time in patients with chronic hepatitis, cirrhosis and HCC.
Serum MnSOD and TRX may be useful biomarkers for HCC diagnosis (Figure 1). AFP is also a diagnostic marker for HCC, and the present results indicate that AFP can be used to distinguish between patients with and without HCC (Table 2). However, AFP is not a sufficiently sensitive marker for identification of the majority of patients with small HCCs[44,45], and AFP testing is not currently included in the recommendations for HCC surveillance in the updated HCC guidelines published by the American Association for the Study of Liver Disease[46]. Therefore, clinicians and clinical researchers should consider using MnSOD and TRX as diagnostic biomarkers for early HCC or as additional markers in a HCC surveillance program using ultrasonography or AFP. In addition, it is highly important to know whether these markers decrease in response to HCC therapy and reductions in tumor burden. These markers also may have utility in patients on a transplant waiting list who are treated with neo-adjuvant therapy for tumor downstaging.
Our study demonstrated that elevated serum AFP level is indicative of a poor prognosis for patients with HCC (Table 4), as was previously reported[47]. The CLIP score, which is calculated based on four factors such as the AFP value, was also useful to predict the prognosis of HCC patients in this study as well as in a previous report[48]. Other markers such as the protein survivin have been reported as poor prognostic factors for HCC[49]. Similarly, MnSOD was an independent predictive factor for overall survival in the HCC group (Figure 3A, Table 5). Although TRX was not an independent predictor of overall survival in patients with HCC (Table 4), we speculate that a combination assay using both MnSOD and TRX could be used to predict overall patient survival. It will be important to conduct further prospective evaluations of each individual marker as well as a combination of these markers using a large number of patients.
In conclusion, serum MnSOD and TRX levels increased as HCV-related chronic liver disease progressed, especially among patients with HCC. Although there was no correlation between serum levels of MnSOD and TRX, higher serum MnSOD levels and lower TRX levels in patients with HCC trended towards an indication of poor patient prognosis. These results suggest that serum MnSOD and TRX levels are not only a potential biomarker for HCV-related progressed liver disease, but may also serve as prognostic markers in HCC.
ACKNOWLEDGMENTS
We thank Ms. Yuko Nakamura for technical assistance.
COMMENTS
Background
During hepatitis C virus (HCV) infection, production of reactive oxygen species (ROS) is persistently increased throughout HCV infection. ROS are thought to play an important role in the pathogenesis of chronic inflammatory changes in the liver, which may lead to the development of hepatic fibrosis, decreased hepatic function or hepatocellular carcinoma (HCC). However, there is little information currently available regarding serum oxidative stress markers in patients with HCV-related HCC.
Research frontiers
Cells are protected from oxidative stress by antioxidant enzymes such as superoxide dismutase (SOD) and by intracellular antioxidants such as thioredoxin (TRX). Serum manganese SOD (MnSOD) and TRX are thought to be biomarkers for various liver diseases, including HCV-related liver disease, but these possibilities have not been fully investigated. In this study, the authors demonstrated the clinical significance of serum levels of MnSOD and TRX in patients with HCV-related HCC.
Innovations and breakthroughs
Although there was no correlation between serum levels of MnSOD and TRX, serum levels of both markers increased as HCV-related chronic liver disease progressed, and in particular among patients with HCC. In addition, higher serum MnSOD levels and lower TRX levels tended to indicate a poor prognosis among patients with HCC.
Applications
Serum MnSOD and TRX levels are not only potential biomarkers for progression of HCV-related liver disease, but they may also serve as prognostic markers for patients with HCC. Therefore, clinicians should consider using serum levels of MnSOD and TRX as diagnostic biomarkers for early HCC or as additional markers in HCC surveillance programs. In addition, it will be important to know whether these markers change after therapy for liver disease, including HCC.
Peer review
Oxidative stress is closely associated with carcinogenesis. If oxidative stress markers could be useful in predicting clinical outcome in chronic hepatitis C and HCV-related HCC, they would provide us with a practical and informative tool. However, there are some limitations of this investigation, including a relatively small number of patients studied. Thus, the overall assessment is “good”.
Footnotes
Supported by (in part) Grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labour and Welfare of Japan
Peer reviewers: Assy Nimer, MD, Assistant Professor, Liver Unit, Ziv Medical Centre, BOX 1008, Safed 13100, Israel; Andre Castro Lyra, MD, Associate Professor, Federal University of Bahia, Head, Gastro-Hepatology Unit, Hospital Sao Rafael, Monte Tabor Foundation, Salvador, Bahia 40296 720, Brazil
S- Editor Tian L L- Editor Logan S E- Editor Xiong L
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2001;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zhang CH, Xu GL, Jia WD, Li JS, Ma JL, Ge YS. Effects of interferon treatment on development and progression of hepatocellular carcinoma in patients with chronic virus infection: A meta-analysis of randomized controlled trials. Int J Cancer. 2010:Epub ahead of print. doi: 10.1002/ijc.25767. [DOI] [PubMed] [Google Scholar]
- 3.Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 5.Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 6.Niemelä O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 7.Takami Y, Uto H, Tamai T, Sato Y, Ishida Y, Morinaga H, Sakakibara Y, Moriuchi A, Oketani M, Ido A, et al. Identification of a novel biomarker for oxidative stress induced by hydrogen peroxide in primary human hepatocytes using the 2-nitrobenzenesulfenyl chloride isotope labeling method. Hepatol Res. 2010;40:438–445. doi: 10.1111/j.1872-034X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 8.Mottola G, Cardinali G, Ceccacci A, Trozzi C, Bartholomew L, Torrisi MR, Pedrazzini E, Bonatti S, Migliaccio G. Hepatitis C virus nonstructural proteins are localized in a modified endoplasmic reticulum of cells expressing viral subgenomic replicons. Virology. 2002;293:31–43. doi: 10.1006/viro.2001.1229. [DOI] [PubMed] [Google Scholar]
- 9.Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity: lessons from transgenic and knockout mice (Review) Int J Mol Med. 2001;7:13–19. doi: 10.3892/ijmm.7.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69:3113–3119. doi: 10.1016/s0024-3205(01)01417-5. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 13.Teshigawara K, Maeda M, Nishino K, Nikaido T, Uchiyama T, Tsudo M, Wano Y, Yodoi J. Adult T leukemia cells produce a lymphokine that augments interleukin 2 receptor expression. J Mol Cell Immunol. 1985;2:17–26. [PubMed] [Google Scholar]
- 14.Nakamura H, De Rosa SC, Yodoi J, Holmgren A, Ghezzi P, Herzenberg LA, Herzenberg LA. Chronic elevation of plasma thioredoxin: inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc Natl Acad Sci USA. 2001;98:2688–2693. doi: 10.1073/pnas.041624998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurimoto C, Kawano S, Tsuji G, Hatachi S, Jikimoto T, Sugiyama D, Kasagi S, Komori T, Nakamura H, Yodoi J, et al. Thioredoxin may exert a protective effect against tissue damage caused by oxidative stress in salivary glands of patients with Sjögren’s syndrome. J Rheumatol. 2007;34:2035–2043. [PubMed] [Google Scholar]
- 16.Lemarechal H, Allanore Y, Chenevier-Gobeaux C, Ekindjian OG, Kahan A, Borderie D. High redox thioredoxin but low thioredoxin reductase activities in the serum of patients with rheumatoid arthritis. Clin Chim Acta. 2006;367:156–161. doi: 10.1016/j.cca.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Deng ZH, Cao HQ, Hu YB, Wen JF, Zhou JH. TRX is up-regulated by fibroblast growth factor-2 in lung carcinoma. APMIS. 2011;119:57–65. doi: 10.1111/j.1600-0463.2010.02692.x. [DOI] [PubMed] [Google Scholar]
- 18.Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumida Y, Nakashima T, Yoh T, Nakajima Y, Ishikawa H, Mitsuyoshi H, Sakamoto Y, Okanoue T, Kashima K, Nakamura H, et al. Serum thioredoxin levels as an indicator of oxidative stress in patients with hepatitis C virus infection. J Hepatol. 2000;33:616–622. doi: 10.1034/j.1600-0641.2000.033004616.x. [DOI] [PubMed] [Google Scholar]
- 20.Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, Nakajima Y, Ishikawa H, Mitsuyoshi H, Okanoue T, et al. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32–38. doi: 10.1016/s0168-8278(02)00331-8. [DOI] [PubMed] [Google Scholar]
- 21.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 22.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 23.The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98–129. doi: 10.1007/BF02471576. [DOI] [PubMed] [Google Scholar]
- 24.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 25.Greenhalgh T. How to read a paper. Papers that report diagnostic or screening tests. Br Med J. 1997;315:540–543. doi: 10.1136/bmj.315.7107.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–S26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Ono M, Sekiya C, Ohhira M, Ohhira M, Namiki M, Endo Y, Suzuki K, Matsuda Y, Taniguchi N. Elevated level of serum Mn-superoxide dismutase in patients with primary biliary cirrhosis: possible involvement of free radicals in the pathogenesis in primary biliary cirrhosis. J Lab Clin Med. 1991;118:476–483. [PubMed] [Google Scholar]
- 28.Kawaguchi T, Suzuki K, Matsuda Y, Nishiura T, Uda T, Ono M, Sekiya C, Ishikawa M, Iino S, Endo Y. Serum-manganese-superoxide dismutase: normal values and increased levels in patients with acute myocardial infarction and several malignant diseases determined by an enzyme-linked immunosorbent assay using a monoclonal antibody. J Immunol Methods. 1990;127:249–254. doi: 10.1016/0022-1759(90)90075-7. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto H, Kobayashi H, Ogasawara K, Yamakado M, Ohno M. Association of the manganese superoxide dismutase polymorphism with vasospastic angina pectoris. J Cardiol. 2010;55:205–210. doi: 10.1016/j.jjcc.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Masutani H, Ueda S, Yodoi J. The thioredoxin system in retroviral infection and apoptosis. Cell Death Differ. 2005;12 Suppl 1:991–998. doi: 10.1038/sj.cdd.4401625. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Masutani H, Oka S, Tanaka T, Yamaguchi-Iwai Y, Nakamura H, Yodoi J. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J Biol Chem. 2006;281:7384–7391. doi: 10.1074/jbc.M509876200. [DOI] [PubMed] [Google Scholar]
- 33.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 34.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 35.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahon P, Sutton A, Pessayre D, Rufat P, Ziol M, Ganne-Carrie N, Charnaux N, Trinchet JC, Gattegno L, Beaugrand M. Manganese superoxide dismutase dimorphism and iron overload, hepatocellular carcinoma, and death in hepatitis C virus-infected patients. Clin Gastroenterol Hepatol. 2007;5:630–635. doi: 10.1016/j.cgh.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Clemente C, Elba S, Buongiorno G, Guerra V, D’Attoma B, Orlando A, Russo F. Manganese superoxide dismutase activity and incidence of hepatocellular carcinoma in patients with Child-Pugh class A liver cirrhosis: a 7-year follow-up study. Liver Int. 2007;27:791–797. doi: 10.1111/j.1478-3231.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Y, Hoshida Y, Kato N, Moriyama M, Otsuka M, Taniguchi H, Kawabe T, Omata M. A simple combination of serum type IV collagen and prothrombin time to diagnose cirrhosis in patients with chronic active hepatitis C. Hepatol Res. 2004;30:214–220. doi: 10.1016/j.hepres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Westman NG, Marklund SL. Copper- and zinc-containing superoxide dismutase and manganese-containing superoxide dismutase in human tissues and human malignant tumors. Cancer Res. 1981;41:2962–2966. [PubMed] [Google Scholar]
- 40.Aida Y, Maeyama S, Takakuwa T, Uchikoshi T, Endo Y, Suzuki K, Taniguchi N. Immunohistochemical expression of manganese superoxide dismutase in hepatocellular carcinoma, using a specific monoclonal antibody. J Gastroenterol. 1994;29:443–449. doi: 10.1007/BF02361241. [DOI] [PubMed] [Google Scholar]
- 41.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 42.Okuyama H, Nakamura H, Shimahara Y, Uyama N, Kwon YW, Kawada N, Yamaoka Y, Yodoi J. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol. 2005;42:117–123. doi: 10.1016/j.jhep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Okuyama H, Son A, Ahsan MK, Masutani H, Nakamura H, Yodoi J. Thioredoxin and thioredoxin binding protein 2 in the liver. IUBMB Life. 2008;60:656–660. doi: 10.1002/iub.102. [DOI] [PubMed] [Google Scholar]
- 44.Kanmura S, Uto H, Sato Y, Kumagai K, Sasaki F, Moriuchi A, Oketani M, Ido A, Nagata K, Hayashi K, et al. The complement component C3a fragment is a potential biomarker for hepatitis C virus-related hepatocellular carcinoma. J Gastroenterol. 2010;45:459–467. doi: 10.1007/s00535-009-0160-5. [DOI] [PubMed] [Google Scholar]
- 45.Kanmura S, Uto H, Kusumoto K, Ishida Y, Hasuike S, Nagata K, Hayashi K, Ido A, Stuver SO, Tsubouchi H. Early diagnostic potential for hepatocellular carcinoma using the SELDI ProteinChip system. Hepatology. 2007;45:948–956. doi: 10.1002/hep.21598. [DOI] [PubMed] [Google Scholar]
- 46.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson PJ, Melia WM, Palmer MK, Portmann B, Williams R. Relationship between serum alpha-foetoprotein, cirrhosis and survival in hepatocellular carcinoma. Br J Cancer. 1981;44:502–505. doi: 10.1038/bjc.1981.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266–2273. [PubMed] [Google Scholar]
- 49.Ye CP, Qiu CZ, Huang ZX, Su QC, Zhuang W, Wu RL, Li XF. Relationship between survivin expression and recurrence, and prognosis in hepatocellular carcinoma. World J Gastroenterol. 2007;13:6264–6268. doi: 10.3748/wjg.v13.i46.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]