Abstract
AIM: To evaluate the therapeutic effects of abdominal decompression plus continuous regional arterial infusion (CRAI) via a drug delivery system (DDS) in severe acute pancreatitis (SAP) patients with abdominal compartment syndrome (ACS).
METHODS: We presented our recent experience in 8 patients with SAP. The patients developed clinical ACS, which required abdominal decompression. During the operation, a DDS was inserted into the peripancreatic artery (the catheter was inserted from the right gastroepiploic artery until it reached the junction between the pancreaticoduodenal and gastroduodenal artery). Through this DDS, a protease inhibitor, antibiotics and octreotide were infused continuously. The duration of the regional artery infusion ranged from 8 to 41 d. The outcomes and the changes in the APACHE II score, computed tomography (CT) severity index and intra-abdominal pressure (IAP) of the patients were retrospectively evaluated.
RESULTS: Eight patients with an initial APACHE IIscore of 18.9 (range, 13-27) and a Balthazar CT severity index of 9.1 (range, 7-10) developed severe local and systemic complications. These patients underwent subsequent surgical decompression and CRAI therapy because of intra-abdominal hypertension (IAH). After a mean interval of 131.9 ± 72.3 d hospitalization, 7 patients recovered with decreased APACHE II scores, CT severity indexes and IAP. The mean APACHE II score was 5.4 (range, 4-8), the CT severity index was 2.3 (range, 1-3), and IAP decreased to 7.7 mmHg (range, 6-11 mmHg) 60 d after operation. One patient died of multiple organ failure 1 wk after surgery.
CONCLUSION: CRAI and laparotomic decompression might be a therapeutic option for SAP patients with ACS.
Keywords: Severe acute pancreatitis, Arterial infusion, Laparotomy, Abdominal compartment syndrome
INTRODUCTION
Acute pancreatitis is an inflammatory disease that is self-limited in the majority of patients and resolves within 48-72 h. However, approximately 20% of the patients develop a more severe form of the disease with evidence of organ dysfunction and complications such as pancreatic necrosis, abscess or pseudocyst. This acute form is classified as severe acute pancreatitis (SAP) according to the Atlanta classification and has a mortality approaching 30%[1]. Previous studies have shown that 60% of the patients developed organ failure on admission, and those with persistent organ failure had the worst outcomes[2].
The current treatment paradigm calls for non-operative management of SAP as long as there is no evidence of infection. However, there is a subset of patients with acute pancreatitis who may need an urgent laparotomy in the absence of an infection to decompress the clinically significant abdominal compartment syndrome (ACS)[3].
SAP with ACS, which is defined as a sustained intra-abdominal pressure (IAP) greater than 20 mmHg that is associated with the development of organ dysfunction or failure[4], is the most severe form of acute pancreatitis and has a high morbidity and mortality. SAP with ACS injures not only the pancreas itself but also the surrounding organs. Despite various treatment protocols, including intensive care therapy and blood filtration, the mortality rate of SAP with ACS is still reported to be 30%-60%[2].
In a number of recent studies, continuous regional arterial infusion therapy (CRAI) for SAP using protease inhibitors and antibiotics has been shown to control the inflammation of the pancreas and to prevent the extension of the inflammatory process, which would decrease the rate of infection and mortality[5-11].
Accordingly, in this study, we used a new system during our decompressing surgical procedure in 8 patients suffering from SAP with ACS, and the therapeutic effects of continuous regional intra-arterial infusion were retrospectively evaluated.
MATERIALS AND METHODS
Eight patients, 6 men and 2 women, with a mean age of 51.5 year (35-66 year) were diagnosed with SAP at Xiamen University Zhongshan Hospital from April 2009 to July 2010. SAP was diagnosed based on clinical manifestation, biological findings and contrast-enhanced abdominal computed tomography (CT) within 3 d after admission. These patients presented with multiple organ dysfunction (MOD) or multiple organ failure (MOF) within 3 d of admission, and they continued to deteriorate under intensive medical support (Table 1).
Table 1.
Multiple organ dysfunction or multiple organ failure within 3 d after admission
| Dysfunction (failure) | n (%) |
| Pulmonary insufficiency | 8 (100.0) |
| Requiring mechanical ventilation | 8 (100.0) |
| Renal insufficiency | 7 (87.5) |
| Requiring dialysis | 6 (75.0) |
| Shock | 7 (87.5) |
| Requiring catecholamines | 8 (100.0) |
| Sepsis (or SIRS) | 8 (100.0) |
| Coagulopathy | 7 (87.5) |
| Hepatic dysfunction (failure) | 7 (87.5) |
| Cardiovascular disable | 7 (87.5) |
| Gastrointestinal bleeding | 7 (87.5) |
| Center nerve system problem | 5 (62.5) |
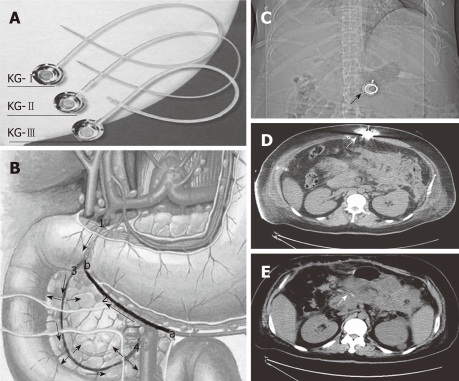
Laparotomy was performed 3-9 d after the onset of SAP in these patients because of clinical deterioration despite intensive medical care and persistent abdominal hypertension. The abdominal pressure was measured by the bladder technique[4]. In contrast to the traditional surgical procedure for SAP, necronectomy (debridement) was not performed. For abdominal decompression and placement of wide-bore drains for continuous postoperative irrigation, the catheter of a drug delivery system (DDS) was inserted into the peripancreatic artery. Because the pancreas has a multiple-sourced blood supply, an appropriate drug distribution in the pancreas can not be achieved unless the catheter is properly placed. The proper position of the catheter tip was decided based on the methylthionine chloride injection. By injecting methylthionine chloride from the head of DDS, we could find the dyeing area, and adjust the position of catheter tip. In cases which it was difficult to find the right gastroepiploic artery, an intraoperative ultrasound was used for guiding. The injection head of the DDS was subcutaneously placed for postoperative drug delivery (Figure 1), and the patients subsequently received an arterial infusion with protease inhibitor, antibiotics and octreotide. In addition, patients with biliary stones had emergency surgery such as endoscopic sphincterotomy, endoscopic nasobiliary drainage, cholecystectomy or common bile duct lithotomy. If we had difficulties with the closure after laparotomy, we left the abdomen open and applied a temporary closure device until suturing again.
Figure 1.
Procedure of placing drug delivery system and imaging after drug delivery system was inserted. A: Drug delivery system; B: Anatomy and how it is inserted; 1: Gastroduodenal artery; 2: Right gastroepiploic artery; 3: Superior pancreatico-duodenal artery; and 4: Inferior pancreatico-duodenal artery (arrows indicate the direction of drug delivery and blood flow); a: The inserting point; and b: The end in which the catheter should be inserted; C: Abdominal X-ray film showed the subcutaneous drug delivery system (DDS) head (arrow); D: Computed tomography (CT) scan showed the subcutaneous DDS and the drug delivery needle inside the DDS (arrow); E: DDS catheter around the pancreas (arrow) in the CT scan.
Ulinastatin (l00 000 U), antibiotics (imipenem/cilastatin 0.5 g) and octreotide (0.3 mg) were dissolved into the saline (48 mL) and continually infused through the DDS twice a day. To ensure the therapeutic effects, the infusion was continued even after the patients’ conditions had improved and until the Balthazar CT severity index had decreased to 3 or less.
All patients were treated in the intensive care unit (ICU). Fluid, electrolytes, albumin, and insulin were replaced dependent on central venous pressure (6-10 mm H2O), hematocrit (30%-35%), urinary excretion, and blood glucose measurement. Assisted ventilation was begun if the partial pressure of oxygen could not be maintained at a level of > 60 mmHg with an oxygen mask. In patients with progressive renal failure (serum creatinine > 3.0 mg/dL), hemodialysis or continuous venovenous hemodialysis (CVVHD) was performed.
The clinical data of patients, transvesical measurement of IAP, CT severity index[12], APACHE II score[13], presence of MOD, local-regional complications and outcome were examined and reported.
RESULTS
The severity of the pancreatitis was judged according to the APACHE II score[1,14,15] , with a score of > 8 points considered indicative of severe disease[16]. In our patients, the APACHE II score ranged from 13 to 27 points, with a mean of 18.9 points. The Balthazar CT severity index was evaluated at each CT study[12]. The CT severity index of the 8 patients ranged from 7 to 10 (average 9.1) before arterial infusion. The abdominal pressure of the patients was measured before operation, and the results suggested that every patient had high abdominal pressure and was diagnosed as having ACS. The average abdominal pressure was 29.1 mmHg (range, 23-38 mmHg). The cause of the pancreatitis was cholelithiasis in 4 cases, alcoholism in 1, hyperlipidemia in 2, and unknown in 1. The arterial infusion was started 3-9 d after the onset of SAP in these patients, and lasted 8-41 d (mean, 26.3 d).
The clinical conditions of the patients improved within 3 d after decompression and the initiation of the arterial infusion. The APACHE II score was decreased from 13-27 (mean, 18.1) to 4-8 (mean, 5.4), and the CT severity index from 7-10 (mean, 9.0) to 1-3 (mean, 2.3), respectively, 60 d after the operation. IAP decreased to 7.7 mmHg (range, 6-11 mmHg). One patient died of MOF, and all other patients were discharged from the hospital within 68-259 d (mean, 131.9 d). The patients are all in good health now. The catheter was removed percutaneously 1 year after discharge, and there were no complications related to the procedure or drug delivery after long-term catheter placement. The clinical data are shown in Table 2 and Figure 2.
Table 2.
Clinical data of 8 patients with severe acute pancreatitis treated by postoperative peripancreatic artery infusion
| Patients | Sex/age (yr) | Area of pancreatitis | Operation | AI (d) |
IAP |
ApacheII |
CT-SI (d) |
ICU (d) | Hosp. (d) | Outcome | |||
| Pre | Post | Pre | Post | Pre | Post | ||||||||
| 1 | M/35 | Entire | 3 | 31 | 35 | 11 | 27 | 6 | 10 | 3 | 45 | 259 | Recovered |
| 2 | F/42 | Entire | 4 | 8 | 38 | 18 | 24 | 18 | 10 | 8 | 11 | 11 | Dead |
| 3 | M/64 | Entire | 5 | 25 | 26 | 8 | 13 | 4 | 7 | 1 | 34 | 68 | Recovered |
| 4 | M/53 | Entire | 8 | 18 | 30 | 7 | 16 | 5 | 9 | 3 | 64 | 101 | Recovered |
| 5 | M/49 | Entire | 6 | 41 | 31 | 9 | 21 | 8 | 10 | 3 | 58 | 159 | Recovered |
| 6 | F/66 | Entire | 3 | 35 | 24 | 6 | 19 | 4 | 10 | 3 | 44 | 186 | Recovered |
| 7 | M/57 | Entire | 4 | 19 | 26 | 6 | 16 | 5 | 9 | 1 | 33 | 74 | Recovered |
| 8 | M/46 | Entire | 5 | 33 | 23 | 7 | 15 | 6 | 8 | 2 | 40 | 76 | Recovered |
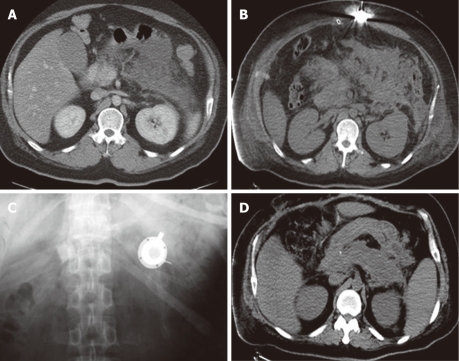
Figure 2.
Imaging comparison between preoperation and postoperation. A: Abdominal computed tomography (CT) scan before the operation; B: Abdominal CT scan after the operation with the drug delivery system (DDS) inserted; C: Abdominal X-ray film showing the subcutaneous DDS head (white); D: CT scan showing the recovered pancreas. No more fluid could be seen around the pancreas (CT scan before discharge).
DISCUSSION
SAP injures not only the pancreas itself but also the surrounding organs, culminating in ACS and MOF in many cases. Despite the various treatment protocols, including intensive care therapy and blood filtration, SAP is still has a high mortality. The basic principles of the initial management of acute pancreatitis are adequate monitoring of vital signs, fluid replacement, correction of any electrolyte imbalance, nutritional support, and prevention of local and systemic complications.
Infected necrosis is generally accepted as an indication for surgery, but there is a subset of acute pancreatitis which may need an urgent laparotomy in the absence of infection to decompress abdominal pressure, which is unique as a compartment syndrome and virtually affects all organ systems within the body. Pathophysiologically, it deranges cardiovascular hemodynamics, respiratory and renal functions and may eventually lead to multi-organ failure. In addition, the gold standard for the treatment of established ACS is surgical decompression of the abdomen[17].
Our main purpose was to decompress the intra-abdominal hypertension (IAH). A double drainage tube was placed in peritoneal cavity around the region of pancreas, through which the patient’s cavity was persistently douched using a large amount of saline solution. This can help drain the intra-abdominal hemorrhagic ascites, alleviate IAH, dilute the inflammatory mediators and activated amylase, and reduce toxin absorption through the peritoneum. In addition, we placed a DDS for postoperative treatment based on the positive results of regional infusion reported by other authors[5-11]. In contrast to their methods of vascular intervention using an angiocatheter, we inserted the DDS catheter into the pancreaticoduodenal artery from the right gastroepiploic artery to the gastroduodenal artery during the procedure. The DDS was subcutaneously fixed and did not result in any discomfort after drug delivery. In the ICU, the DDS could be easily used for the continuous injection of protease inhibitors, antibiotics and octreotide. This device was simple, safe and did not hinder the patient’s movement. No complications related to the procedure or the device setting were observed, even after long-term placement.
Regarding the drug infusion, because acute pancreatitis is an autodigestive disease, protease inhibition has been the focus of experimental and clinical research. However, in clinical settings, the effect of protease inhibitors in the treatment of acute pancreatitis is still controversial. Some randomized, controlled trials failed to demonstrate any significant benefits[18,19]. For this reason, in Europe and the United States, protease inhibitors are not usually applied in the treatment of acute pancreatitis. In Japan, however, protease inhibitors are often applied, and in particular, it has been demonstrated that the CRAI of protease inhibitors and antibiotics are beneficial for severe acute necrotizing pancreatitis[5-11]. They believed that there were many reasons why the protease inhibitors were not as effective as expected in the experimental studies, such as the timing of administration, the concentration of the protease inhibitor in the pancreatic tissue, the diminution of the vasculature of the pancreas and so on. With intravenous administration, the concentration of the agent reaching the pancreas eventually becomes insufficient for controlling the inflammation. As a result of drug dilution as well as serum and hepatic metabolism, most of the aprotinin administered intravenously tended to go into the liver and thereafter accumulate in the kidneys, with only a small amount of aprotinin distributed in the pancreas. In contrast, with intra-arterial administration in experimental studies, the local concentration in the pancreas was high enough to improve the biochemical indices of inflammation and survival[20,21].
Because pancreatic and extrapancreatic infections are determining factors leading to death in patients with SAP, much attention has been paid to the potential role for antibacterial prophylaxis, especially in those patients with pancreatic necrosis. Many studies on the prophylactic effect of antibiotics have demonstrated that broad-spectrum antibiotics with good pancreatic tissue penetration decreased the incidence of infectious complications and mortality[22,23]. The antibacterial agent of first choice is likely to be imipenem because it reaches a higher distribution in the pancreatic tissue and provides higher bactericidal activity against most of the bacteria present in pancreatic infection compared with other types of antibiotics. An alternative antibiotic regimen is either ciprofloxacin or ofloxacin in combination with metronidazole[24]. It also has been suggested that the effect was induced markedly by intra-arterial administration.
Octreotide reduces exocrine pancreatic secretion in acute pancreatitis, which would decrease pancreatic autodigestion, and it may also significantly prevent the bacterial translocation by preventing mucosal damage[25]. As a treatment guideline for acute pancreatitis, octreotide has been widely used in clinical practice, although the results of clinical investigations using somatostatin or its analogue are controversial. In a multicenter randomized controlled study with a large number of patients (n = 302) with an adequate level of disease severity, no benefit of octreotide on progression or outcome was found[26], but other research suggests that octreotide may have a beneficial effect in the treatment of SAP[27]. One study suggested that octreotide seemed to have a dose- and time-dependent effect on histopathology and lipid peroxidation: an early bolus application of octreotide reduced the severity of histopathological changes in acute pancreatitis and decreased lipid peroxidation in the pancreatic tissue samples. However, a late bolus application and continuous intravenous infusion did not influence pancreatitis or lipid peroxidation[28].
According to the above-mentioned clinical and experimental results, in this study, we used a combination of these three kinds of drugs via DDS as regional therapy in the 8 patients. Their clinical conditions improved after the treatment and the overall mortality was 12.5%, which is much lower than that reported in the literature. These results suggest that CRAI plus laparotomic decompression might be a therapeutic choice for SAP with ACS.
COMMENTS
Background
Severe acute pancreatitis (SAP), characterized by intricate mechanism, variant symptoms, poor prognosis and multiple complications, seriously threatens the life of patients. About 11% of SAP patients suffer from abdominal compartment syndrome (ACS). SAP complicated by ACS has a mortality rate of that is 30%-60%.
Research frontiers
ACS has been recognized as a contributing factor for the multiple organ failure commonly seen in SAP. Surgical decompression is the preferred method of treatment for ACS. Although decompression has a significant effect in lowering IAP, the mortality still remains high in SAP patients with ACS. Some recent studies showed that continuous regional arterial infusion of protease inhibitors and antibiotics may reduce the mortality rate and incidence of infectious complications in SAP.
Innovations and breakthroughs
SAP with ACS is the most severe form of acute pancreatitis and has a high morbidity and mortality which need an urgent laparotomy. In this study, a new system was applied during the decompressing surgical procedure in 8 patients. In addition to abdominal decompression and the placement of wide-bore drains for continuous postoperative irrigation, the catheter of a drug delivery system (DDS) was inserted into the peripancreatic artery for postoperative continuous regional arterial infusion, which increased the tissue concentration of the drugs in the inflamed pancreas and improved the biochemical indices of inflammation and survival.
Applications
The DDS applied for continuous regional arterial infusion (CRAI) in this study is simple, safe and easy to implement. These positive results suggest that CRAI plus laparotomic decompression might be a therapeutic choice for SAP with ACS.
Peer review
Even if it is not accepted by all, the presence of an ACS in severe pancreatitis is a very difficult complication to deal with. The paper reports a small group of patients with such a complication treated with a multidisciplinary approach (surgery, antibiotics, anti protease, etc.). It is difficult to understand the real value of each therapy, but the utility of the paper lies in reporting the complexity of such a disease.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30872484
Peer reviewer: Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Roma-Sapienza, Viale del Policlinico 155, Roma 00161, Italy
S- Editor Wu X L- Editor Ma JY E- Editor Xiong L
References
- 1.Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gecelter G, Fahoum B, Gardezi S, Schein M. Abdominal compartment syndrome in severe acute pancreatitis: an indication for a decompressing laparotomy? Dig Surg. 2002;19:402–404; discussion 402-404. doi: 10.1159/000065820. [DOI] [PubMed] [Google Scholar]
- 4.Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–962. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Matsuno S, Ogawa M, Watanabe S, Atomi Y. Continuous regional arterial infusion (CRAI) therapy reduces the mortality rate of acute necrotizing pancreatitis: results of a cooperative survey in Japan. J Hepatobiliary Pancreat Surg. 2001;8:216–220. doi: 10.1007/s005340170019. [DOI] [PubMed] [Google Scholar]
- 6.Ino Y, Arita Y, Akashi T, Kimura T, Igarashi H, Oono T, Furukawa M, Kawabe K, Ogoshi K, Ouchi J, et al. Continuous regional arterial infusion therapy with gabexate mesilate for severe acute pancreatitis. World J Gastroenterol. 2008;14:6382–6387. doi: 10.3748/wjg.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa M, Ogino H, Shimohira M, Hara M, Shibamoto Y. Continuous regional arterial infusion therapy for acute necrotizing pancreatitis due to Mycoplasma pneumoniae infection in a child. Cardiovasc Intervent Radiol. 2009;32:581–584. doi: 10.1007/s00270-008-9457-7. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K. Antiproteases in the treatment of acute necrotizing pancreatitis: continuous regional arterial infusion. JOP. 2007;8:526–532. [PubMed] [Google Scholar]
- 9.Mikami Y, Takeda K, Omura N, Abe H, Fukuyama S, Motoi F, Egawa S, Sunamura M, Matsuno S. New strategy for acute necrotizing pancreatitis: Continuous Regional Arterial Infusion (CRAI) therapy. Rocz Akad Med Bialymst. 2005;50:101–105. [PubMed] [Google Scholar]
- 10.Imaizumi H, Kida M, Nishimaki H, Okuno J, Kataoka Y, Kida Y, Soma K, Saigenji K. Efficacy of continuous regional arterial infusion of a protease inhibitor and antibiotic for severe acute pancreatitis in patients admitted to an intensive care unit. Pancreas. 2004;28:369–373. doi: 10.1097/00006676-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Sunamura M, Shibuya K, Kobari M, Matsuno S. Role of early continuous regional arterial infusion of protease inhibitor and antibiotic in nonsurgical treatment of acute necrotizing pancreatitis. Digestion. 1999;60 Suppl 1:9–13. doi: 10.1159/000051446. [DOI] [PubMed] [Google Scholar]
- 12.Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 13.Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–205. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 14.Malfertheiner P, Domínguez-Muñoz JE. Prognostic factors in acute pancreatitis. Int J Pancreatol. 1993;14:1–8. doi: 10.1007/BF02795224. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 16.Wilson C, Imrie CW. Current concepts in the management of pancreatitis. Drugs. 1991;41:358–366. doi: 10.2165/00003495-199141030-00004. [DOI] [PubMed] [Google Scholar]
- 17.Scheppach W. Abdominal compartment syndrome. Best Pract Res Clin Gastroenterol. 2009;23:25–33. doi: 10.1016/j.bpg.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Valderrama R, Pérez-Mateo M, Navarro S, Vázquez N, Sanjosé L, Adrián MJ, Estruch J. Multicenter double-blind trial of gabexate mesylate (FOY) in unselected patients with acute pancreatitis. Digestion. 1992;51:65–70. doi: 10.1159/000200877. [DOI] [PubMed] [Google Scholar]
- 19.Dervenis C, Johnson CD, Bassi C, Bradley E, Imrie CW, McMahon MJ, Modlin I. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini consensus conference. Int J Pancreatol. 1999;25:195–210. doi: 10.1007/BF02925968. [DOI] [PubMed] [Google Scholar]
- 20.Satoh H, Harada M, Tashiro S, Shiroya T, Imawaka H, Machii K. The effect of continuous arterial infusion of gabexate mesilate (FOY-007) on experimental acute pancreatitis. J Med Invest. 2004;51:186–193. doi: 10.2152/jmi.51.186. [DOI] [PubMed] [Google Scholar]
- 21.Mikami Y, Takeda K, Matsuda K, Qiu-Feng H, Fukuyama S, Egawa S, Sunamura M, Matsuno S. Rat experimental model of continuous regional arterial infusion of protease inhibitor and its effects on severe acute pancreatitis. Pancreas. 2005;30:248–253. doi: 10.1097/01.mpa.0000153328.54569.28. [DOI] [PubMed] [Google Scholar]
- 22.Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480–483. [PubMed] [Google Scholar]
- 23.Sharma VK, Howden CW. Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta-analysis. Pancreas. 2001;22:28–31. doi: 10.1097/00006676-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Hartwig W, Werner J, Uhl W, Büchler MW. Management of infection in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:423–428. doi: 10.1007/s005340200052. [DOI] [PubMed] [Google Scholar]
- 25.Güler O, Akturan S, Kisli E, Dolapçi I, Caydere M, Akova A. Acute pancreatitis, bacterial translocation, and different octreotide regimens: an experimental study. Surg Today. 2009;39:876–883. doi: 10.1007/s00595-009-3972-2. [DOI] [PubMed] [Google Scholar]
- 26.Uhl W, Büchler MW, Malfertheiner P, Beger HG, Adler G, Gaus W. A randomised, double blind, multicentre trial of octreotide in moderate to severe acute pancreatitis. Gut. 1999;45:97–104. doi: 10.1136/gut.45.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paran H, Mayo A, Paran D, Neufeld D, Shwartz I, Zissin R, Singer P, Kaplan O, Skornik Y, Freund U. Octreotide treatment in patients with severe acute pancreatitis. Dig Dis Sci. 2000;45:2247–2251. doi: 10.1023/a:1026679106463. [DOI] [PubMed] [Google Scholar]
- 28.Wenger FA, Kilian M, Heukamp I, Foitzik T, Jacobi CA, Guski H, Schimke I, Müller JM. Effects of octreotide in acute hemorrhagic necrotizing pancreatitis in rats. J Gastroenterol Hepatol. 2007;22:1872–1876. doi: 10.1111/j.1440-1746.2006.04627.x. [DOI] [PubMed] [Google Scholar]