Abstract
Pneumatosis cystoides intestinalis (PCI) is a rare condition that may be associated with a variety of diseases. The presenting clinical picture may be very heterogeneous and represent a challenge for the clinician. In the present paper we describe both a common and an uncommon clinical presentation of PCI and review the pertaining literature. Our cases confirm that, apart from asymptomatic cases, the clinical presentation of PCI may be widely different and suggest that a new onset of stipsis might be the presenting symptom. Diagnosis might be suggested by a simple X-ray of the digestive tract showing a change in the characteristics of the intestinal wall in two-thirds of these patients. However, one third of the patients do not have a suggestive X-ray and require a computed tomography (CT) scan/nuclear magnetic resonance that may reveal a thickened bowel wall containing gas to confirm the diagnosis and distinguish PCI from intraluminal air or submucosal fat. CT also allows the detection of additional findings that may suggest an underlying, potentially worrisome cause of PCI such as bowel wall thickening, altered contrast mucosal enhancement, dilated bowel, soft tissue stranding, ascites and the presence of portal air. Our results also point out that clinicians and endoscopists should be aware of the possible presentations of PCI in order to correctly manage the patients affected with this disease and avoid unnecessary surgeries. The increasing number of colonoscopies performed for colon cancer screening makes PCI more frequently casually encountered and/or provoked, therefore the possible endoscopic appearances of this disease should be well known by endoscopists.
Keywords: Pneumatosis cystoides intestinalis, Pneumoperitoneum, Treatment, Hyperbaric oxygen, Endoscopy
INTRODUCTION
Pneumatosis cystoides intestinalis (PCI) is a rare disease characterized by the presence of gaseous cysts containing nitrogen, hydrogen and carbon dioxide[1] in the intestinal wall that may be iatrogenic[2-5] or associated with a wide variety of conditions[6-9].
In particular, the cysts are located beneath the serosa and mucosa of the intestine with an increase, in recent years, of cases of colonic localization due to an increase in the number of examinations with barium and colonoscopies[10].
Jamart[11] in a study of 919 cases in 1979 found a prevalence of 42% for ileal localization, and 36% for the colon, in the remaining 22% of cases both the small and the large intestine were involved.
The exact etiology of the disease is still unknown. PCI may appear in association with ileal surgery[12], colonoscopies[5], chronic pulmonary disease[13], connective tissue disorders[14] and ingestion of sorbitol[15] or lactulose[16].
Various theories have been proposed: mechanical, bacterial and pulmonary. According to the mechanical theory, the bowel gas is pushed through a mucosal defect into lymphatic channels and is then distributed distally by peristalsis[17]. This may happen secondarily to a bowel obstruction that may be caused by trauma, surgery and colonoscopy leading to increased intraluminal pressure[18] and this could explain the association between these maneuvers and PCI. However this theory does not explain the high content of hydrogen present in the cysts[19].
The bacterial theory proposes that submucosal localization of fermenting Clostridia and Escherichia Coli leads to the production of gas which is retained by the submucosa and lymphatic channels. In fact, in animal experiments the introduction of bacteria in the gut wall by injection causes the pneumatosis and these cysts have a high content of hydrogen[13]. This theory is also supported by the resolution of pneumatosis with the use of metronidazole for bacterial overgrowth[20].
The pulmonary theory is demonstrated in patients with asthma and chronic bronchitis and argues that the gas freed by the rupture of the alveoli, travels through the mediastinum into the retroperitoneal space and then comes through the perivascular spaces in the intestinal wall[21].
Some recent reports[22] show an association between PCI and treatment with alpha-glucosidase inhibitor. The explanation would be the fermentation of carbohydrates by the intestinal bacterial flora with production of intestinal gas. The absorption of these carbohydrates is inhibited by αGI. Here we describe two cases of pneumatosis cystoides intestinalis.
CASE REPORT
Case 1
A 49 years old male (S.A.) presented to the gastroenterology outpatient clinic for abdominal pain. The pain was crampy and diffuse with no clear localization in the abdomen and had no clear relationship with meals or evacuation. Bowel habit was characterized by chronic constipation; the abdomen showed no relevant physical findings, in particular there was no palpable mass. The patient also suffered from a chronic obstructive pulmonary disease and had finger clubbing. Routine biochemical examinations were within normal values as well as inflammation indices. The physician prescribed a colonoscopy revealing melanosis coli, a sessile polyp 2 cm in diameter and two sessile formations with a large base and a reddened but regular overlying mucosa (Figure 1). The formations appeared soft when touched with a closed biopsy forceps and collapsed when biopsied suggesting the presence of air. The endoscopist performed multiple biopsies along the colon. The pathologist described the presence of optically empty spaces in the biopsies confirming the hypothesis of pneumatosis cystoides intestinalis. The patient was then referred to the pneumologist for oxygen therapy. At a subsequent colonoscopy the air cysts appeared reduced in volume and the patient referred pain reduction to the endoscopist. The patient did not present to subsequent control visits.
Figure 1.
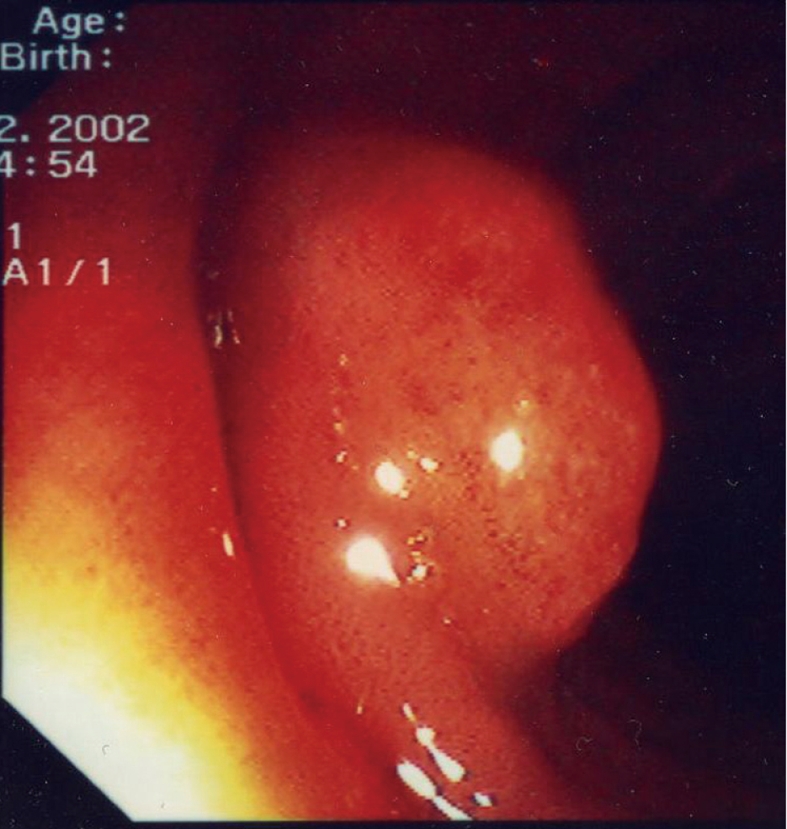
This photograph shows a typical endoscopic appearance of a larger cysts with a reddened overlying mucosa.
Case 2
N.F. 44 years old presented to the GI Unit for the re-evaluation of a known celiac disease. Despite adequate gluten free diet the patient complained of a worsening of his symptoms dominated by stipsis and abdominal distension. The patient suffered from recurrent subocclusive episodes and referred recent tetanic crisis from hypocalcemia on treatment with calcium and vitamin D. Biochemical analysis on admission revealed a megaloblastic anemia related to a Vitamin B12 deficiency and hypomagnesemia. Physical examination was consistent with abdominal distension and revealed hyperreflexia of the extremities. The patient had no fever nor any other remarkable finding.
The first postulated hypothesis, based on patient history was the complication of a celiac disease with intestinal lymphoma. A plain X ray of the abdomen revealed distension caused by dilatation of colonic loops leading to diaphragm elevation. The patient was then prescribed an upper GI endoscopy and an abdomen computed tomography (CT). The esophagogastroduodenoscopy revealed a normal endoscopic appearance of the mucosa and orientated biopsies were done in the distal duodenal mucosa. Histological examination revealed normal villi with a mild, non significant, lymphocytic infiltrate (below 25%) consistent with a celiac disease in remission responding to gluten free diet. The abdomen CT scan showed a complex picture including fecal stasis in the colon, severe distension of the large bowel with the presence of free air under the diaphragm (Figure 2A), and small air bubbles in the rectum wall (Figure 2B). Despite the radiologic picture, the patient was feeling well, with distended but tractable abdomen and no Blumberg sign. Therefore, the patient was kept fasting and received liquids and antibiotics iv. Subsequent radiologic evaluation of the small and large bowel, using gastrografin as contrast media, revealed no perforation suggesting that the free air might have come from the rupture of a subserosal air cyst. The patient then started hyperbaric therapy (5 treatments of 90 min: 2.5 atmospheres, 75 min of oxygen respiration divided in 3 cycles) with prompt amelioration of bowel movements and subjective feeling. The patient became asymptomatic and apyretic with normal bowel movements and was dismissed with antibiotic therapy.
Figure 2.
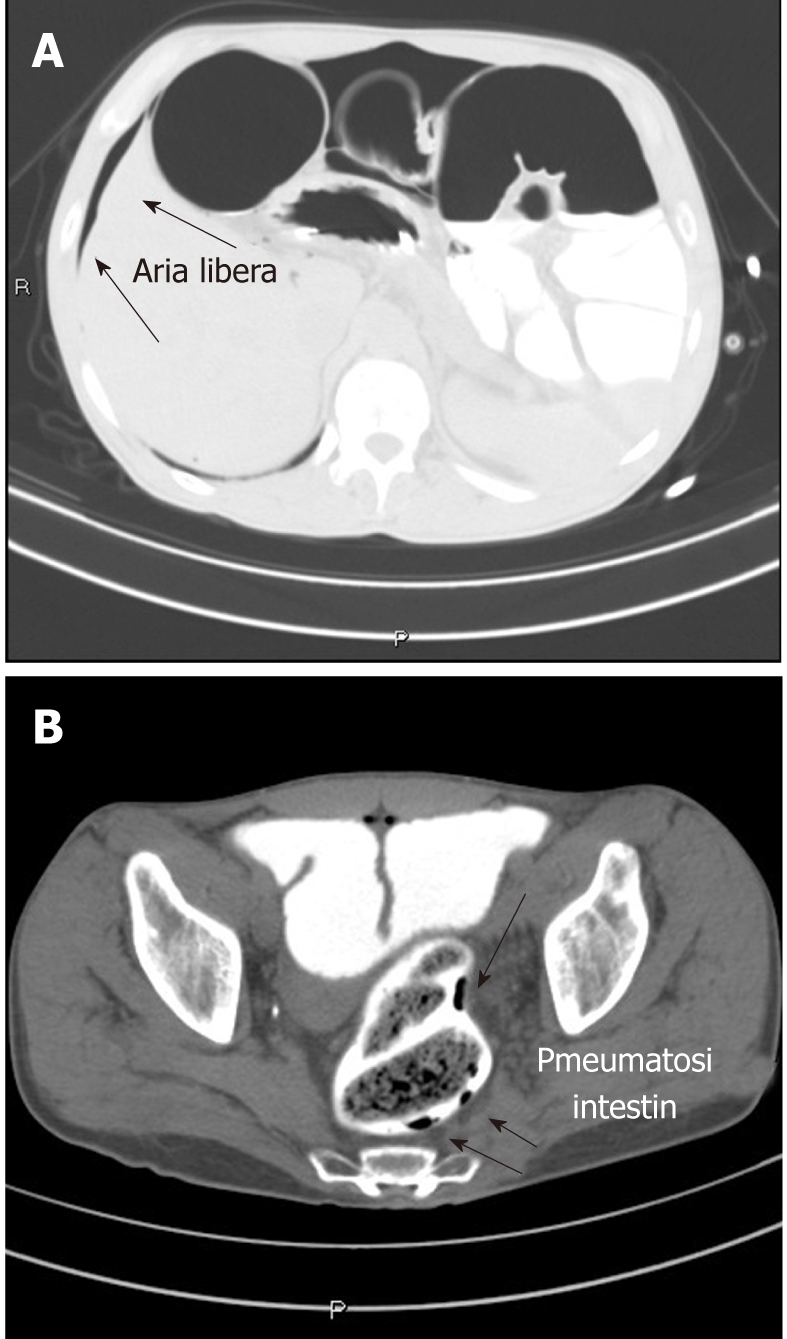
Computed tomography scan image. A: Presence of free air (arrows) in the abdomen; B: Presence of air in the bowel wall (arrows).
DISCUSSION
There is no characteristic clinical presentation of pneumatosis. Patients may be asymptomatic or complain of pain and abdominal distension, diarrhea and rectal blood loss with a mortality rate that may reach 75%[23]. Apart from the cases associated with chronic intestinal pseudo-obstruction[20,24,25], the majority of cases reported in the literature present with diarrhea; in the present manuscript we described two cases of PCI that presented with stipsis. The first was diagnosed after a colonoscopy that put the suspect of PCI, while the second required a CT scan because of the atypical presentation and the misleading anamnesis. In fact, the history of celiac disease lead the clinicians to hypothesize a complication of the pre-existent disease more than the onset of a new pathology. It is difficult to say whether the motility defects are a cause or a result of the pathologic condition and we are not aware of any longitudinal study evaluating such a question. However, on a purely hypothetical basis, it seems more reasonable to think that motility defects are secondary to PCI or to underlying pathological process that may have lead to PCI (i.e., ischemia, diverticular disease, etc.).
Colonoscopy is frequently requested to exclude colonic lesions. The endoscopic appearance of PCI is typically dual: multiple white small cysts coupled to a sub-atrophic mucosa or larger cysts (up to 3 cm) with a reddened overlying mucosa[26]. The cysts usually collapse when biopsied. Nowadays, given the increasing number of colonoscopies performed because of the colon cancer screening programs, the endoscopists should be aware of the endoscopic appearance of this rare pathology. In fact, some patients may be asymptomatic and in such cases the clinical suspect may rely on the endoscopist performing the procedure. In our case 1, the endoscopist cautiously biopsied the cyst because of his unusual appearance that was not suggestive of a typical polyp. This allowed a confirmation of the suspect and avoided an unnecessary snare polypectomy with the related costs and complications.
A simple X-ray of the digestive tract may show a change in the characteristics of the intestinal wall in two-thirds of these patients leading to further investigations. However, one third of the patients do not have a suggestive X-ray and require a CT scan/magnetic resonance imaging, showing a thickened bowel wall containing gas to confirm the diagnosis[27]. Suggestive images on plain radiography comprise different pattern of radiolucency: linear, small bubbles or collection of larger cysts[27]. CT is more sensitive than plain radiography in distinguishing PCI from intraluminal air or submucosal fat. In fact, CT more easily visualizes the presence of air in the bowel wall. Furthermore, CT allows the detection of additional findings that may suggest an underlying, potentially worrisome cause of PCI, i.e., bowel wall thickening, altered contrast mucosal enhancement, dilated bowel, soft tissue stranding, ascites, and the presence of portal air[28].
The intestinal pneumatosis may experience various complications, in particular, Goel et al[29] described the complications of pneumatosis of the small intestine which may be intestinal or extra-intestinal. Intestinal complications are obstruction caused by the cysts (i.e., fecal impaction) and perforation from stercoral ulceration. The extra-intestinal complications are adhesions or compression of adjacent structures by large masses of cysts.
For the resolution of these complications surgical treatment is often required because sometimes we have a picture of pneumoperitoneum due to rupture of cysts.
To determine the need for surgical therapy Knechtle et al[23] found a correlation between the clinical presentation, the need for surgery and the final outcome. It is necessary to evaluate six physical parameters, like pain, diarrhea, fever, tenderness, rectal blood loss and hypotension, and their severity coupled to clinical laboratory tests including white blood cell count, aspartate aminotransferases, alanine aminotransferases, alkaline phosphatase, pH, bicarbonate, lactic acid and amylase.
Surgical therapy is still a second-line therapy, chosen especially for complications, the first approach is oxygen therapy. It is also our opinion that the clinical decisions should not rely only on the radiologic picture of pneumoperitoneum, but should be coupled to the clinical symptoms (i.e., the positivity of the Blumberg sign). In fact, when the mucosa is intact surgery may be avoidable as in our case 2.
The rationale of oxygen treatment is based on increasing partial pressure of oxygen in blood and thus increasing the pressure gradient of the gas in the cysts. Cysts release gases contained within them and refills with oxygen which is then metabolized leading to resolution[26].
Oxygen therapy can be made through humidified oxygen administered by Venturi mask (6 L/min) or nasal cannula (4 L/min). However, treatment with oxygen at high doses can be toxic. The patient may experience a narcotic effect and therefore lung function should be monitored closely (during therapy) by measuring the vital capacity, daily blood gas estimations and chest radiography. A decrease in lung vital capacity can be a early parameter of oxygen toxicity[30].
To reduce the duration of oxygen administration hyperbaric oxygen can be used at a pressure of 2.5 atmosphere for up to 2 h a day [31]. To decrease the recurrence rate oxygen therapy should be continued until two days after the disappearance of cysts[32].
In conclusion, our cases confirm that the clinical presentation of PCI may be very heterogeneous and suggest that a new onset of stipsis might be the presenting symptom. Furthermore, it should be taken into account that the patients may also be totally asymptomatic. The clinicians and the endoscopist should be aware of the possible presentations of PCI in order to correctly manage the patients affected with this disease and avoid unnecessary surgeries. It is possible that with the increasing number of colonoscopies performed for colon cancer screening PCI is casually encountered and/or provoked, therefore the possible endoscopic appearances of this disease should be known.
Footnotes
Peer reviewer: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany
S- Editor Sun H L- Editor Logan S E- Editor Xiong L
References
- 1.Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25:839–845. doi: 10.1136/gut.25.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groninger E, Hulscher JB, Timmer B, Tamminga RY, Broens PM. Free air intraperitoneally during chemotherapy for acute lymphoblastic leukemia: consider pneumatosis cystoides intestinalis. J Pediatr Hematol Oncol. 2010;32:141–143. doi: 10.1097/MPH.0b013e3181ced397. [DOI] [PubMed] [Google Scholar]
- 3.Clemente G, Chiarla C, Giovannini I, De Rose AM, Astone A, Barone C, Nuzzo G. Gas in portal circulation and pneumatosis cystoides intestinalis during chemotherapy for advanced rectal cancer. Curr Med Res Opin. 2010;26:707–711. doi: 10.1185/03007990903566798. [DOI] [PubMed] [Google Scholar]
- 4.Mimatsu K, Oida T, Kawasaki A, Kano H, Kuboi Y, Aramaki O, Amano S. Pneumatosis cystoides intestinalis after fluorouracil chemotherapy for rectal cancer. World J Gastroenterol. 2008;14:3273–3275. doi: 10.3748/wjg.14.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCollister DL, Hammerman HJ. Air, air, everywhere: pneumatosis cystoides coli after colonoscopy. Gastrointest Endosc. 1990;36:75–76. doi: 10.1016/s0016-5107(90)70936-4. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JA, Adler DG. Pneumatosis coli in the setting of severe ulcerative colitis: a case report. Dig Dis Sci. 2006;51:185–191. doi: 10.1007/s10620-006-3106-2. [DOI] [PubMed] [Google Scholar]
- 7.Breitinger A, Kozarek R, Hauptman E. Pneumatosis cystoides intestinalis in Crohn’s disease. Gastrointest Endosc. 2003;57:241. doi: 10.1067/mge.2003.10. [DOI] [PubMed] [Google Scholar]
- 8.Wood BJ, Kumar PN, Cooper C, Silverman PM, Zeman RK. Pneumatosis intestinalis in adults with AIDS: clinical significance and imaging findings. AJR Am J Roentgenol. 1995;165:1387–1390. doi: 10.2214/ajr.165.6.7484571. [DOI] [PubMed] [Google Scholar]
- 9.Dovrish Z, Arnson Y, Amital H, Zissin R. Pneumatosis intestinalis presenting in autoimmune diseases: a report of three patients. Ann N Y Acad Sci. 2009;1173:199–202. doi: 10.1111/j.1749-6632.2009.04807.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim KM, Lee CH, Kim KA, Park CM. CT Colonography of pneumatosis cystoides intestinalis. Abdom Imaging. 2007;32:602–605. doi: 10.1007/s00261-007-9216-2. [DOI] [PubMed] [Google Scholar]
- 11.Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg) 1979;26:419–422. [PubMed] [Google Scholar]
- 12.Wandtke J, Skucas J, Spataro R, Bruneau RJ. Pneumatosis intestinalis as a complication of jejunoileal bypass. AJR Am J Roentgenol. 1977;129:601–604. doi: 10.2214/ajr.129.4.601. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11:111–118. doi: 10.1007/s003840050031. [DOI] [PubMed] [Google Scholar]
- 14.Sequeira W. Pneumatosis cystoides intestinalis in systemic sclerosis and other diseases. Semin Arthritis Rheum. 1990;19:269–277. doi: 10.1016/0049-0172(90)90049-l. [DOI] [PubMed] [Google Scholar]
- 15.Duncan B, Barton LL, Eicher ML, Chmielarczyk VT, Erdman SH, Hulett RL. Medication-induced pneumatosis intestinalis. Pediatrics. 1997;99:633–636. doi: 10.1542/peds.99.4.633. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman AL, Gupta JK, Ingegno AP. Pneumatosis coli following treatment with lactulose. N Y State J Med. 1979;79:1896–1899. [PubMed] [Google Scholar]
- 17.Galandiuk S, Fazio VW. Pneumatosis cystoides intestinalis. A review of the literature. Dis Colon Rectum. 1986;29:358–363. doi: 10.1007/BF02554132. [DOI] [PubMed] [Google Scholar]
- 18.Heer M, Altorfer J, Pirovino M, Schmid M. Pneumatosis cystoides coli: a rare complication of colonoscopy. Endoscopy. 1983;15:119–120. doi: 10.1055/s-2007-1021484. [DOI] [PubMed] [Google Scholar]
- 19.Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20:1008–1011. doi: 10.1136/gut.20.11.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tak PP, Van Duinen CM, Bun P, Eulderink F, Kreuning J, Gooszen HG, Lamers CB. Pneumatosis cystoides intestinalis in intestinal pseudoobstruction. Resolution after therapy with metronidazole. Dig Dis Sci. 1992;37:949–954. doi: 10.1007/BF01300397. [DOI] [PubMed] [Google Scholar]
- 21.St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68–75. doi: 10.1001/archsurg.138.1.68. [DOI] [PubMed] [Google Scholar]
- 22.Tsujimoto T, Shioyama E, Moriya K, Kawaratani H, Shirai Y, Toyohara M, Mitoro A, Yamao J, Fujii H, Fukui H. Pneumatosis cystoides intestinalis following alpha-glucosidase inhibitor treatment: a case report and review of the literature. World J Gastroenterol. 2008;14:6087–6092. doi: 10.3748/wjg.14.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212:160–165. doi: 10.1097/00000658-199008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attar A, Pocard M, Messing B. Pneumatosis cystoides intestinalis in primary intestinal pseudo-obstruction: a nonsurgical cause of pneumoperitoneum. Clin Gastroenterol Hepatol. 2005;3:A21. doi: 10.1016/s1542-3565(05)00847-5. [DOI] [PubMed] [Google Scholar]
- 25.Luks FI, Chung MA, Brandt ML, Hertecant J, Roy CC, Blanchard H, Bensoussan AL. Pneumatosis and pneumoperitoneum in chronic idiopathic intestinal pseudoobstruction. J Pediatr Surg. 1991;26:1384–1386. doi: 10.1016/0022-3468(91)91039-2. [DOI] [PubMed] [Google Scholar]
- 26.Rennenberg RJ, Koek GH, Van Hootegem P, Stockbrügger RW. Pneumatosis cystoides intestinalis, four cases of a rare disease. Neth J Med. 2002;60:22–25. [PubMed] [Google Scholar]
- 27.Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188:1604–1613. doi: 10.2214/AJR.06.1309. [DOI] [PubMed] [Google Scholar]
- 28.Olson DE, Kim YW, Ying J, Donnelly LF. CT predictors for differentiating benign and clinically worrisome pneumatosis intestinalis in children beyond the neonatal period. Radiology. 2009;253:513–519. doi: 10.1148/radiol.2532090168. [DOI] [PubMed] [Google Scholar]
- 29.Goel A, Tiwari B, Kujur S, Ganguly PK. Pneumatosis cystoides intestinalis. Surgery. 2005;137:659–660. doi: 10.1016/j.surg.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Holt S, Gilmour HM, Buist TA, Marwick K, Heading RC. High flow oxygen therapy for pneumatosis coli. Gut. 1979;20:493–498. doi: 10.1136/gut.20.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61:423–426. [PubMed] [Google Scholar]
- 32.Boerner RM, Fried DB, Warshauer DM, Isaacs K. Pneumatosis intestinalis. Two case reports and a retrospective review of the literature from 1985 to 1995. Dig Dis Sci. 1996;41:2272–2285. doi: 10.1007/BF02071412. [DOI] [PubMed] [Google Scholar]


