Abstract
The IKK-related kinases TBK1 and IKKΣ play essential roles as regulators of innate immunity by modulating interferon and NF-κB signaling. Recent work has also implicated these non-canonical IKKs in malignant transformation. IKKΣ is amplified in approximately 30% of breast cancers and transforms cells through the activation of NF-κB signaling. TBK1 participates in RalB-mediated inflammatory responses and cell survival and is essential for the survival of non-small cell lung cancers driven by oncogenic KRAS. The delineation of target substrates and downstream activities for TBK1 and IKKΣ has begun to define their role(s) in promoting tumorigenesis. In this review, we will highlight the mechanisms by which IKKΣ and TBK1 orchestrate pathways involved in inflammation and cancer.
Introduction
The NF-κB pathway is a pivotal regulator of several important physiological functions including the inflammatory immune response, proliferation, cell survival and cell invasion. These activities are well-described hallmarks of cancer, and NF-κB activation has been observed in a wide range of tumors (Baud and Karin, 2009; Dolcet et al., 2005; Prasad et al., 2010), leading some to suggest that NF-κB serves as a bridge between inflammation and cancer (Baldwin, 2001; Karin, 2006). Activation of NF-κB in cancer arises either from extrinsic signals in the tumor microenvironment or from intrinsic dysregulation of the pathway within the tumor. In either case, several different components of the NF-κB signaling cascade may contribute to this activity in particular cancers.
Five NF-κB members exist in mammals including RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2). While RelA, RelB, and cRel are synthesized as mature forms, p105 and p100 are synthesized as longer precursor proteins that are cleaved to form the mature p50 and p52 proteins. This processing is necessary for p50 and p52 to function as transcription factors (Dolcet et al., 2005). Distinct NF-κB complexes are formed from combinations of homo- and heterodimers of these family members (Bonizzi and Karin, 2004). NF-κB complexes are retained in the cytoplasm by a family of NF-κB-binding proteins known as the inhibitors of NF-κB (IκBs). A variety of inflammatory stimulants initiate the induction of NF-κB and trigger activation of the IκB Kinase (IKK) complex, which is composed of the catalytic kinases IKKα and IKKβ, and the regulatory NF-κB essential modifier, (NEMO, or IKKγ) (Perkins, 2007). One important function of the IKK complex is to mark IκB for phosphorylation and ubiquitination, which in turn, leads to proteasomal degradation. This activity facilitates the release and accumulation NF-κB dimers in the nucleus where a transcriptional program involving many target genes related to immune response are activated. While the classical NF-κB pathway primarily involves the activation of RelA and p50 and is strictly facilitated by the IKK complex, an alternative pathway is active in B cells where NF-κB inducing kinase (NIK) promotes the activation of RelB and p52/p100 complexes (Ghosh and Hayden, 2008). Genetic experiments have demonstrated that IKKβ is the principal IKK for the canonical NF-κB pathway, whereas IKKα plays a more dominant role in the non-canonical pathway (Bonizzi and Karin, 2004; Pasparakis et al., 2006; Perkins, 2007). In addition to the conventional IKKs, a related pair of non-canonical kinases, IKKΣ (IKKi, encoded by IKBKE) and TBK1 (NAK), have been identified as important mediators of both inflammatory and oncogenic signaling.
IKKε and TBK1 function in inflammation
IKKΣ was simultaneously identified in a subtractive hybridization screen as a LPS-inducible gene and as a PMA-inducible protein with a kinase domain that is 27% identical to IKKα and IKKβ (Peters et al., 2000; Shimada et al., 1999). Concurrently, TBK1, which exhibits 49% identity and 65% similarity to IKKΣ was identified as an interaction partner with the scaffolding molecule, TRAF-associated NF-κB activator (TANK) (Pomerantz and Baltimore, 1999). Both IKKΣ and TBK1 are comprised of an N-terminal kinase domain, an ubiquitin-like domain, a C-terminal LZ and a HLH motif (Figure 1). Despite their similarity in structure, TBK1 and IKKΣ exhibit differential expression patterns. TBK1, like IKKα and IKKβ, is ubiquitously expressed (Shimada et al., 1999). In contrast, IKKΣ expression is restricted to particular tissue compartments, with highest levels detected in lymphoid tissues, peripheral blood lymphocytes and the pancreas (Shimada et al., 1999). Various epithelial derived cell lines also exhibit IKKΣ expression (Bibeau-Poirier et al., 2006; Gravel and Servant, 2005; Honda et al., 2005; Shimada et al., 1999). Mitogenic stimulation with LPS and TNFα can also induce IKKΣ and TBK1 expression in a NF-κB dependent manner (Hemmi et al., 2004; Kravchenko et al., 2003; Shimada et al., 1999). With these partially overlapping characteristics, IKKΣ and TBK1 are functionally more similar to each other than the canonical IKKs (Clement et al., 2008).
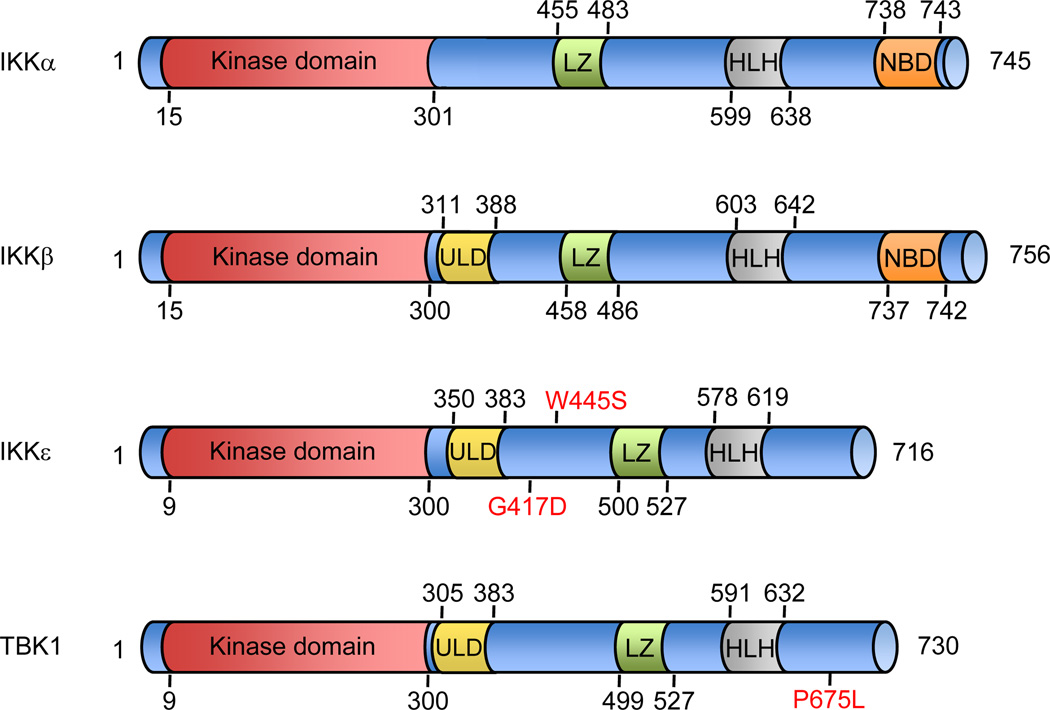
Figure 1. Structural comparison of the classical and non-canonical IKKs.
The major domains of each IKK kinase are depicted with amino-acid numbers that correspond to the human proteins. The kinase domain of IKKε exhibits 27% and 24% identity to IKKα and IKKβ respectively, and TBK1 shares 49% identity and 65% similarity to IKKε. Somatic mutations of IKKε and TBK1 recently identified in lung adenocarcinomas are marked in red. ULD, ubiquitin-like domain; LZ, leucine zipper; HLH, helix-loop-helix; NB, NEMO-binding domain (Hiscott et al., 2006; Kan et al., 2010; May et al., 2004; Perkins, 2007).
The non-canonical IKKs coordinate the interferon response
IKKΣ and TBK1 are critical inducers of interferon signaling in response to viral infection (Fitzgerald et al., 2003; Sharma et al., 2003). Following activation of toll-like receptors (TLR) via viral components, IKKΣ and TBK1 assemble with TRAF3 and TANK to phosphorylate interferon regulatory factors (IRF) 3, 5, and 7 at multiple serine and threonine residues (Caillaud et al., 2005; Cheng et al., 2006; McWhirter et al., 2004; Mori et al., 2004; Pomerantz and Baltimore, 1999). This activity allows for heterodimerization and nuclear translocation of the IRFs and induction of proinflammatory and antiviral genes, including type I interferon (Lin et al., 1998; Sato et al., 2000). TLR-independent mechanisms also activate IKKΣ and TBK1 to induce the interferon response. In this scenario, viral dsRNA and dsDNA initiate signaling through intracellular RNA and DNA sensors such as RIG-I, MDA-5 and DAI (Andrejeva et al., 2004; Kawai et al., 2005; Meylan et al., 2005; Seth et al., 2005; Takaoka et al., 2007; Yoneyama et al., 2004). IFNβ also activates a TLR-independent pathway by stimulating IKKε phosphorylation of STAT1 to facilitate binding with ISGF3, a complex that serves as the transcriptional machinery important for activating a subset of interferon response genes (Tenoever et al., 2007). Moreover, engagement of both TLR-dependent and -independent pathways recruits additional scaffolding molecules including FADD, TRADD, MAVS, NAP1, HSP90, and SINTBAD necessary for IKKΣ and TBK1-mediated interferon activation (Balachandran et al., 2004; Gatot et al., 2007; Guo and Cheng, 2007; Hacker et al., 2006; Michallet et al., 2008; Oganesyan et al., 2006; Rothe et al., 1996; Ryzhakov and Randow, 2007; Yang et al., 2006). Thus, IKKΣ and TBK1 form several protein complexes, the composition of which is dependent on the type of cellular stimuli. Ultimately, these signaling complexes share a role in activating interferon responses required to induce the antiviral response.
The ability of IKKΣ and TBK1 to activate the interferon response is also dependent on posttranslational modifications by proteasome independent Lys-63 linked ubiquitin chains (Figure 2). Both IKKΣ and TBK1 have an ubiquitin like domain (ULD), and Lys-63 ubiquitination of both kinases are promoted by TRAF3, which itself is Lys-63 ubiquitinated. Disruption of this activity by TAXBP1 and the deubiquitinase A20 ablates the interferon response (Ikeda et al., 2007; Parvatiyar et al., 2010). Both TBK1 and IKKΣ also mediate ubiquitination of TANK by an unknown E3 ligase (Gatot et al., 2007). Although TANK further serves as a phosphorylation target of TBK1 and IKKΣ, TANK ubiquitination seems to occur independently of this kinase activity. The mechanism by which TANK ubiquitination contributes the activation of IKKΣ and TBK1 is unknown. Lys -63 ubiquitination also plays a role in the negative regulation of IKKε and TBK1 induced responses. For example, during by RNA viruses, Lys-63 ubiquitination of MAVS is essential for the recruitment of IKKΣ and leads to the inhibition of antiviral and NF-κB induced inflammatory genes (Paz et al., 2009). Ultimately, these modifications will likely dictate a dynamic system of regulating IKKΣ and TBK1-mediated function in both inflammation and cancer.
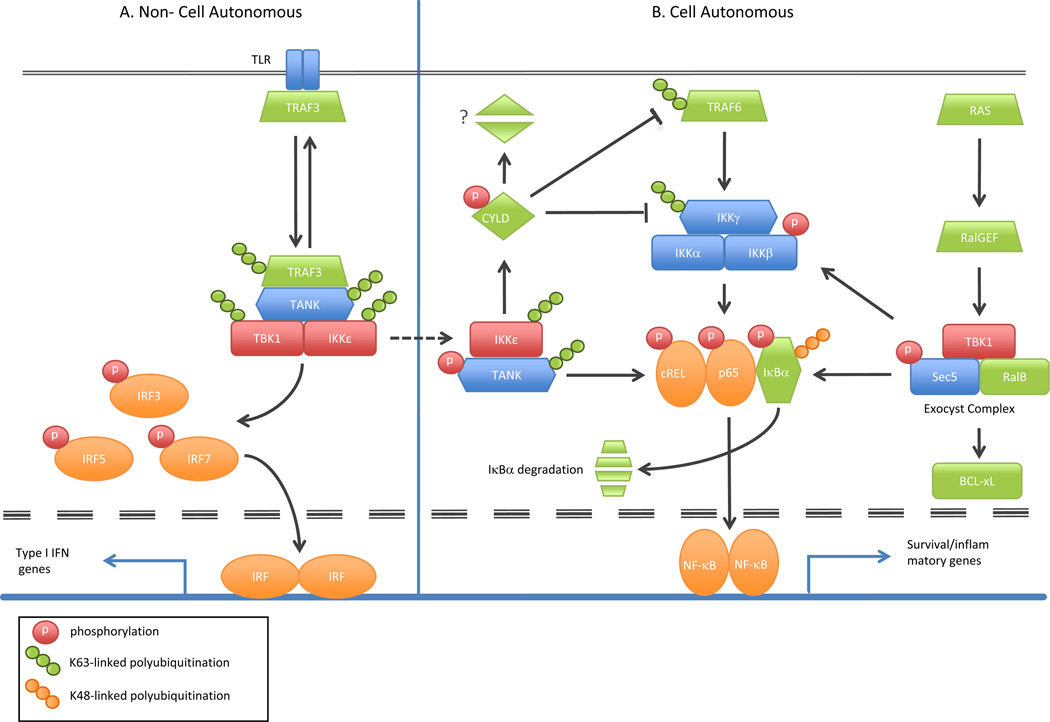
Figure 2. Cell autonomous and non-cell autonomous roles of IKKε and TBK1 in inflammation and cancer.
(A) Engagement of toll-like receptors (TLRs) initiates Lys-63 linked ubiquitination of TRAF3 and recruitment of IKKε and TBK1 through the adaptor molecule TANK. This recruitment promotes Lys-63 linked ubiquitination and activation of IKKε/TBK1. TANK is modified by both ubiquitination and phosphorylation through IKKε/TBK1, although it is unclear how these activities are related. The TANK/IKKε/TBK1 complex can then target interferon response factors (IRF3, IRF5, and IRF7) for phosphorylation and promote translocation to the nucleus where IFN target genes are activated. This cascade primarily defines the non-cell autonomous functions of the IKK-related kinases. In parallel to the interferon pathways, IKKε and TBK1 also phosphorylate many NF-κB effectors such as IκBα, p65 and cREL in response to cellular stimuli. This activity facilitates NF-κB nuclear translocation as a homo- or hetero- dimer and induces numerous inflammatory and survival- related target genes. (B) The IKKε/TBK1 oncogenic pathways function in a cell autonomous manner and are predominantly driven by aberrant levels of IKKε/TBK1. In this context, oncogenic RAS activates RalGEF, which in turn recruits the exocyst complex consisting of TBK1, RalB and Sec5, amongst other secretory proteins. TBK1 mediates phosphorylation of multiple effectors including Sec5, IKKβ, and IκBα that ultimately lead to the activation of both BCL-xL and NF-κB. Similarly, aberrant levels of IKKε in cancer also promote NF-κB activation through the phosphorylation of CYLD. CYLD negatively regulates NF-κB signaling by deubiquitinating Lys-63 linked ubiquitin chains on TRAF6 and IKKγ. It is hypothesized that IKKε-mediated phosphorylation of CYLD leads to either its degradation or sequestration, thereby inactivating its negative role on the NF-kB signaling cascade.
The non-canonical IKKs function as NF-κB effectors
Although TBK1 and IKKΣ are not a part of the classical IKKα/β/γ signaling complex, these kinases were originally characterized as activators of NF-κB and target multiple NF-κB members and effectors. Both IKK-related kinases phosphorylate IκBα at, one of the two serine residues typically targeted on IκBα. Although IKKε phosphorylates Ser36, TBK1 phosphorylates Ser32 (Pomerantz and Baltimore, 1999; Shimada et al., 1999; Tojima et al., 2000). IKKε overexpression reduces IκBα levels suggesting that phosphorylation at a single residue may result in increased IκBα turnover. However, it remains possible that the IKK-related kinases phosphorylate a canonical IKK that in turn leads to IκBα turnover (Boehm et al., 2007; Eddy et al., 2005). The canonical NF-κB, RelA/p65, is another substrate for IKKΣ and TBK1, and phosphorylation of RelA at serine 536 by these kinases occurs at a basal level independently of extracellular stimuli (Buss et al., 2004; Fujita et al., 2003). cRel is also phosphorylated by both IKK-related kinases. This activity is sufficient to promote nuclear translocation; however, it does not modulate downstream NF-κB activity (Harris et al., 2006). TANK plays a similar role in IKKε/TBK1-mediated NF-κB activation as it does in interferon activation and acts as both a substrate and critical adaptor molecule for these kinases. Furthermore, in most cells, autophosphorylation of TBK1 and IKKΣ is readily detected. However, it has also been suggested that other kinases may phosphorylate the IKK-related kinases (Gatot et al., 2007; Ikeda et al., 2007). The significance of these phosphorylation events remains unclear.
Although closely related, TBK1 and IKKΣ also phosphorylate distinct substrates and may activate NF-κB through different mechanisms (Table 1). For example, TBK1 is the only of the two IKK-related kinases to phosphorylate and activate IKKβ, suggesting that TBK1 may act preferentially on canonical NF-κB signaling (Tojima et al., 2000). On the other hand, in stimulated T cells, IKKΣ targets a second residue on p65/RelA, serine 468. Although phosphorylation at this site occurs in addition to the serine 536 residue that is phosphorylated by both IKK-related kinases, it is not clear whether this event occurs in other cellular contexts (Mattioli et al., 2006). IKKΣ also associates with p100/p52 in a ternary complex with p65 following TNF induction and this interaction facilitates transactivation of p52 dependent genes (Wietek et al., 2006). Moreover, recent experiments indicate that IKKΣ can associate with the chromatin of promoters of specific NF-κB target genes (Moreno et al., 2010).
Table 1.
Differential substrates of the non-canonical IKKs
| Substrates | Targeted region | Phosphorylation site | Suggested function | References |
|---|---|---|---|---|
| IKKε specific substrates | ||||
| RelA/p65 | TAD | Ser 468 | NF-kB activation | Matioli et al. |
| p100/p52 | - | not phosphorylated | Transactivation via p65 | Wietek et al. |
| STAT1 | C-Terminal | Ser 708 | ISGF3 stability | Tenover et al. |
| CYLD | Central | Serine 418 | NF-kB activation | Hutti et al. |
| ERα | N-Terminal, AF-1 | Serine 167 | ERα Transactivation | Guo et al. |
| TBK1 specific substrates | ||||
| Sec5 | Ral Binding domain | Unknown | Interferon induction | Chien et al. |
| IKK | Activation loop | Ser 177/181 | IKK activation | Tojima et al. |
| DDX3X | DEAD and helicase domains | Ser 181/183/240/269/429/442/456/520, Thr 438 | Interferon induction | Soulat et al. |
| IR (Insulin Receptor) | C-Terminal | Ser 994 | insulin resistance | Munoz et al. |
Activation of NF-κB by the IKK-related kinases occurs in response to specific stimuli. PMA-induced NF-κB activation is dependent on IKKε (Peters et al., 2000). Similarly, induction of NF-κB via the T cell receptor (TCR) involves TBK1 (Tojima et al., 2000). In contrast, IKKε or TBK1 are not required for TNF- or IL-1-induced NF-κB activation (Peters et al., 2000; Pomerantz and Baltimore, 1999). The role of the IKK-related kinases in NF-κB activation is not well understood and appears to be highly dependent on specific cellular stimuli. This area has been extensively reviewed elsewhere (Chau et al., 2008; Clement et al., 2008; Hacker et al., 2006). Ultimately, the multiple modes of IKKΣ and TBK1-mediated NF-κB activation highlight both independent and synergistic relationships that are likely dictated by cellular and signal-induced contexts.
In consonance with these biochemical observations, genetically engineered mice lacking either Tbk1 or Ikbke exhibit distinct phenotypes. Tbk1 deficient animals are embryonic lethal and die at E14.5 due to extensive fetal liver degeneration, a phenotype that is also observed in IKKγ, IKKβ and RelA deficient animals (Bonnard et al., 2000; Hemmi et al., 2004). By contrast, Ikbke deficient animals are viable, but are impaired in initiating a productive IFN-β response (Bonnard et al., 2000; Hemmi et al., 2004). Interestingly, NF-κB activation in either Tbk1 or Ikbke single and double knockout models is predominantly normal, apart from minimal defects in the induction of select NF-κB target genes. These models demonstrate that although the non-canonical IKKs are sufficient but not essential for NF-κB activation, IKKΣ and TBK1 play a key role in the induction of interferon signaling.
Extrinsic vs. Intrinsic NF-κB activation in Cancer
In addition to a role in facilitating inflammatory responses to foreign agents, the IKK-related kinases were recently recognized as NF-κB effectors that contribute to tumorigenesis and thus represent a link between NF-κB-mediated inflammation and cancer.
The contribution of NF-κB to inflammation-associated cancer has been studied extensively, and inhibition of the canonical pathway in mouse models of inflammation-associated cancer demonstrates that NF-κB is often essential for cancer initiation and progression (Greten et al., 2004; Karin, 2006; Lawrence et al., 2005; Pikarsky et al., 2004). For example, deletion of IKKβ in either epithelial or myeloid cells of a mouse model of colitis-associated cancer dramatically decreases tumor incidence by nearly 80% (Greten et al., 2004). Anti-TNFα treatment and introduction of the NF-κB super repressor in a model of inflammatory hepatocellular carcinoma also induces apoptosis and inhibition of tumor progression (Pikarsky et al., 2004). These observations underscore how malignant cells are critically dependent on the inflammatory microenvironment for their survival. However, it is difficult to assess whether there are additional cell autonomous contributions of NF-κB activation in inflammation-associated cancer models, and it is likely that both cell autonomous and non-cell autonomous functions are involved.
While much focus of NF-κB activation has revolved around inflammation-associated cancer, this pathway plays a direct cell autonomous role in transformation and tumorigenesis. Activation of NF-κB in tumor cells is frequently observed in both solid tumors and hematologic malignancies (Basseres and Baldwin, 2006; Baud and Karin, 2009; Dolcet et al., 2005). Until recently, it was assumed that this activation is the result of immune stimuli originating from the microenvironment. However, somatic mutations of components in the NF-κB pathway were recently described and provide an alternative means of NF-κB activation in tumors. For example, mutations in NF-κB family members NFKB1(encoding p105/p50) and NFKB2 (encoding p100/p52) as well as genes whose protein products regulate the IKK complex such as CIAP1, CIAP2, CYLD, NIK, and TRAF3 are associated with the pathogenesis of multiple myeloma (Annunziata et al., 2007; Baud and Karin, 2009; Keats et al., 2007). The application of targeted sequencing analyses in other lymphoid malignancies including diffuse large B cell lymphoma and marginal zone lymphomas has led to the identification of mutations in additional regulators of the NF-κB pathway including A20, CARD11, TRAF2, TRAF5, TAK1, and RANK (Compagno et al., 2009; Novak et al., 2009). Furthermore, a more recent genomic analysis of somatic copy number alterations across a large subset of 3131 human cancer tissues and cell lines found amplifications of major NF-κB regulators including TRAF6, IKBKB, IKBKG, IRAK1 and RIPK1 (Beroukhim et al., 2010). Importantly, all of these genes were significantly amplified in epithelial cancers, suggesting that alterations of NF-κB effectors are far more frequent than previously appreciated.
In addition to direct mutations in the NF-κB pathway, NF-κB activation is observed in tumors harboring mutations in numerous oncogenes including HRAS, ERB2, PI3K, Bcr-Abl, the viral oncoprotein TAX, Vav-Pim2, BRAF, MUC1, and BMI-1 (Basseres and Baldwin, 2006; Biswas et al., 2004; Finco et al., 1997; Gustin et al., 2004; Hammerman et al., 2004; Ikenoue et al., 2003; Li et al., 2010; Pianetti et al., 2001; Reuther et al., 1998; Xiao et al., 2001) Although activation of the NF-κB pathway has been documented in each of these contexts, the mechanism by which these oncogenes activate NF-κB signaling and the role of such signaling in tumorigenesis remains incompletely understood. However, H-RASV12–induced transformation depends on NF-κB activity (Mayo et al., 1997), and recent work in genetically engineered mouse models demonstrated that NF-κB activity was required for the development of lung adenocarcinomas driven by oncogenic KRAS in the setting of p53 loss (Basseres et al., 2010; Meylan et al., 2009). Other recent studies suggest that the non-canonical IKKs, IKKε and TBK1 may play key roles in the cell autonomous activation of NF-κB in cancer.
IKKΣ in cancer
A potential role for IKKΣ in breast cancer was first shown by Sonenshein and her colleagues who demonstrated that IKKε was constitutively expressed in MDA-MB-231 and Hs578T breast cancer cells lines, 4 of 6 breast patient carcinomas, and carcinogen-induced mouse mammary tumors (Eddy et al., 2005). In this study, tumors and mammary glands derived from a casein kinase 2 catalytic subunit alpha (CK2α) transgenic mouse model of mammary adenocarcinoma exhibit higher levels of IKKε. Likewise, overexpression of CK2 in MCF10F cells also increased IKKε expression. These results suggested that CK2 might play an important role in regulating IKKΣ. In addition, expression of the kinase-inactive form of IKKΣ in breast cancer cells reduced levels of two NF-κB target genes, Cyclin D1 and RelB, as well as anchorage-independent growth and invasion in matrigel (Eddy et al., 2005). Adli et al. also found elevated IKKε expression in prostate and breast cancer cell lines. In this work, IKKε was found to activate NF-κB at a basal level by phosphorylating serine 536, and inhibition of this activity significantly suppressed cancer cell proliferation (Adli and Baldwin, 2006). Together, these studies provided the first evidence of IKKε involvement in breast cancer.
More recently, IKKΣ was identified as a breast oncogene through the intersection of three complementary genomic approaches. First, Boehm et al. developed an experimental model of cell transformation that was dependent on both MEK and AKT signaling. By screening kinases that could replace AKT and induce tumorigenesis, we found that IKKΣ and CSNK1E were able to transform such cells (Boehm et al., 2007). In parallel, loss of function screens to identify kinases that were essential for the proliferation and survival of breast cancer cell lines revealed that both IKKΣ and CSNK1E were required for the survival of breast cancer cell lines. Of these two genes, IKBKE is amplified in 8 of 49 (16.3%) breast cancer cell lines and in over 30% of primary breast tumors (Boehm et al., 2007). FISH analysis further confirmed increased copy number of IKBKE by up to 10 additional copies is the consequence of these amplification events. Taken together, these studies suggested that IKKε was a breast cancer oncogene.
IKKε activates both interferon and NF-κB signaling, and overexpression of IKKε in mammary epithelial cells induced both interferon regulated genes and several NF-κB target genes including MMP9, BCL2, CIAP1 and CIAP2. In agreement with these observations, primary breast carcinomas in which IKKΣ expression was elevated showed nuclear localization of the NF-κB factor cREL. Many cancer cells with increased levels of IKKΣ also exhibit increased levels of serine 536 phosphorylated p65. Strikingly, expression of the NF-κB super-repressor in IKKΣ-transformed cells blocked IKKε– induced cell transformation. In contrast, suppression of IRF3 and IRF7 via shRNA failed to alter the transformation phenotype, indicating that the role of IKKΣ in mediating inflammatory interferon responses is unrelated to its function in cancer (Boehm et al., 2007).
Recent observations demonstrate that IKKΣ also functions to protect cells from DNA damage-induced death, and the underlying mechanism for this response is mediated by IKKΣ sumoylation (Renner et al., 2010). Following genotoxic stress, IKKΣ translocates to the nucleus and induces phosphorylation of the promyelocytic leukemia tumor suppressor PML. This activity results in the sequestration of IKKΣ in PML nuclear bodies. Multiple nuclear targets including p65 are then phosphorylated following critical sumoylation of IKKΣ by the SUMO ligase TOPORS. Ultimately, these events facilitate an NF-κB mediated antiapoptotic response to DNA damage (Renner et al., 2010). These observations identify a novel function for IKKΣ in promoting cell survival and suggest that IKKΣ acts in parallel to the p53 proapoptotic pathway.
Although IKBKE copy number gain does not correlate with estrogen receptor (ER) or HER2/Neu status, Guo et al. demonstrated that IKKε phosphorylates estrogen receptor alpha (ERα). In ER-positive cells, this activity is necessary for activation of Cyclin D1. However, IKKε is also sufficient to activate Cyclin D1 in ER-negative cells. (Boehm et al., 2007; Guo et al., 2010). Furthermore, although overexpression of IKKε promotes tamoxifen resistance, suppression of IKKε sensitizes cells to tamoxifen-induced death (Guo et al., 2010). These findings highlight potential crosstalk between NF-κB dependent and independent pathways in IKKε transformed breast cells.
In addition to breast cancer, IKKε also appears to have a role in ovarian cancer. A recent study showed that IKKε is overexpressed in 63 of 95 ovarian carcinomas, and elevated IKKε levels were associated with poor prognosis (Guo et al., 2009). Notably, 76.5 % of breast cell lines and 47.6% of breast carcinomas exhibit increased expression of IKKΣ in the absence of 1q32 copy number changes. Furthermore, recent deep sequencing of a set of human cancers including, breast, ovarian, lung and prostate tumors identified two IKKε mutations, G417D and W445S, in lung adenocarcinomas (Kan et al., 2010). The function of these mutations, which do not map to any previously described domains of IKKε, remains unclear (Figure 1). Taken together, these observations suggest that IKKΣ expression may be dysregulated through multiple mechanisms in various cancer types.
TBK1 in Cancer
TBK1 is highly expressed in lung, breast and colon cancers and a recent TBK1 mutation, P675L, was recently identified in lung adenocarcinoma (Barbie et al., 2009; Kan et al., 2010; Korherr et al., 2006) (Figure 1). RalB is a key factor that mediates TBK1 activation in cancer (Bodemann and White, 2008). The Ras-like RalB proteins function downstream of RAL-GEF (Ras-like- guanine nucleotide exchange factor) to initiate a variety of regulatory processes associated with oncogenesis (Bodemann and White, 2008). The activation of RalB by Ral-GEF is necessary for RAS-induced transformation (Lim et al., 2006; Rangarajan et al., 2004; Yoneyama et al., 2004). Downstream of Ral-GEF, RalB-mediated activation of TBK1 promotes TBK1 assembly with the exocyst complex, which contains several core secretory proteins, namely Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84. TBK1 is able to participate in the exocyst complex through its direct interaction with Sec5 and further facilitates transformation through the phosphorylation of Sec5 (Camonis and White, 2005; Chien et al., 2006). This cascade is not only a critical function in transformation, but is also essential for mediating the host defense to viral infection. Importantly, suppression of either TBK1 or Sec5 was shown to induce apoptosis in Ras-transformed cells, and expression of oncogenic alleles of KRAS induced cell death in TBK1 deficient murine embryonic fibroblasts (Chien et al., 2006).
In recent work, TBK1 was identified as a gene whose expression was selectively required in KRAS-dependent cancer cell lines (Barbie et al., 2009). Suppression of TBK1 affects the survival of KRAS-dependent cells, while having little to no effect in cells where KRAS is not essential. Transcriptional profiling using gene set enrichment analysis in KRAS-transformed cells revealed the enrichment of several NF-κB activation signatures. Expression of the NF-κB super repressor also selectively induced apoptosis in KRAS-dependent lung cell lines. In these cells, TBK1 promoted degradation of IκBα and induced expression of the survival factor BCL-xL, which in part sustains the survival of KRAS-driven tumors (Barbie et al., 2009).
In addition, a recent study identified another novel function for TBK1 as an inducer of angiogenesis. Korherr et al. screened a genome-wide cDNA library in HEK293 cells for genes that regulate vascularization. Supernatants isolated from growth factor-producing HEK293 cells were evaluated for the ability to induce proliferation of HUVEC endothelial cells. TBK1 was found to act as a trigger in stimulating a secretion program consisting of endothelial growth factors RANTES and IL-8, as well as other proangiogenic factors such as CXCL10, CXCL11, and IFN-β. Moreover, several cancer cell lines with upregulated TBK1 exhibit a similar autocrine makeup (Korherr et al., 2006).
New and old substrates of IKKΣ and TBK1 in cancer
The initial characterization of IKKΣ as a breast cancer oncogene indicated that its intrinsic kinase activity was essential to activate NF-κB and transform cells, but the substrates and effectors responsible for these phenotypes remained undefined. Both TBK1 and IKKΣ appear to have many substrates that modulate NF-κB (Figure 2) in response to extracellular stimuli, namely p65, cREL, and IκBα. However, engagement of these targets by the non-canonical IKKs is different than what has been observed for the other IKKs that lead to NF-κB activation. For example, it is uncertain how phosphorylation of one of the two required sites on IκBα is sufficient to activate NF-κB. Similarly, although IKKΣ and TBK1 can phosphorylate cREL, this modification fails to promote significant NF-κB activation (Harris et al., 2006).
To identify IKKΣ and TBK1 substrates, Hutti and her colleagues developed and used an unbiased peptide screening method coupled with bioinformatic analyses to identify a potential substrate recognition motif. In this approach, a collection of 198 biotinylated peptide libraries was employed to perform simultaneous kinase assays with recombinant IKKε or TBK1 and radiolabeled ATP. Each library contains mixtures of degenerate peptides with two fixed residues, a serine at the central position and another natural amino acid at one additional position neighboring the serine. The relative IKKε or TBK1 preference for each amino acid at each position was determined after capture of the biotinylated peptides on a streptavidin-coated membrane. The resulting IKKε/TBK1 substrate recognition motif was used to identify potential substrates through the proteomic search engine Scansite (Hutti et al., 2009).
The IKKΣ and TBK1 phosphorylation motif consists of a central serine that is surrounded by a hydrophobic residue at the +1 position relative to the phosphorylation site, an aromatic residue at the −2 position and bulky hydrophobic residues at the +3 position (x-Y/F/P-x-pS-L/I-x-Y/W/F-x). Parallel analysis of IKKΣ and TBK1 using the scanning peptide library screen independently identified the same target motif for each kinase (Hutti et al., 2009). In vitro kinase assays demonstrate that IKKΣ efficiently targets peptides with the identified IKKΣ recognition motif. In contrast, the observed activity of IKKΣ toward a peptide representing the IκBα target sequence was much less robust, suggesting that IκBα is unlikely to be a preferred substrate of IKKΣ (Hutti et al., 2009).
Bioinformatic analysis of the IKKΣ recognition motif identified many potential IKKΣ substrates. Interestingly, the top scoring candidates consisted of many NF-κB regulators including the known IKKΣ/TBK1 substrate TANK. Additional putative substrates appear to be involved in ubiquitination. Although further functional validation of these substrates is necessary, these findings suggest that IKKΣ and TBK1 function in a network of pathways that converge to activate NF-κB (Hutti et al., 2009).
The proteomic/ bioinformatic approach taken by Hutti et al. further identified the familial tumor suppressor CYLD as an IKKΣ substrate that was essential for transformation. CYLD is a deubiquitinase that attenuates NF-κB signaling by targeting various effectors in the pathway (Brummelkamp et al., 2003; Kovalenko et al., 2003; Trompouki et al., 2003). IKKΣ phosphorylates CYLD at serine 418 and disruption of this activity substantially hinders both IKKΣ-induced NF-κB activation and tumorigenicity. Although CYLD expression is necessary for IKKΣ–mediated transformation, it is likely that other substrates also facilitate IKKΣ function in cancer (Hutti et al., 2009).
Several other cancer-related substrates have also been described for IKKΣ/TBK1. As previously discussed, IKKΣ phosphorylates estrogen receptor alpha (ERα) through a direct interaction. This activity occurs on serine 167 and promotes ERα transactivation and induction of target genes such as Cyclin D1 (Guo et al., 2010). STAT1 is another substrate of IKKΣ discussed above that typically functions in mediating the interferon response, but may play an additional role in cancer progression (Tenoever et al., 2007). STAT proteins, including STAT1, STAT3 and STAT5, are critical for the regulation of several genes that function in antiviral immunity and also act as oncogenic transcription factors in various malignancies (Bowman et al., 2000). STAT1 expression is constitutively active in many breast cancer cell lines, but also has a paradoxical role in promoting apoptosis in tumor cells (Kim and Lee, 2007; Yarilina et al., 2008). In addition to the STAT proteins, IRF3, IRF5 and IRF7 also behave as tumor suppressors and facilitate anti-tumorigenic effects in various cancers. However, since suppression of these factors in IKKε-transformed cells fails to decrease tumorigenic potential, they likely play a minimal role in the IKKε oncogenic pathway.
Conclusions
IKKΣ and TBK1 appear to behave as integrators of signals induced not only by proinflammatory stimuli but also by oncogenes and tumor suppressors (Figure 2). Through the phosphorylation and modulation of several NF-κB effectors and interferon regulatory factors, the IKK-related kinases primarily function as inflammatory regulators. More recently, IKKε was identified as a breast oncogene that requires NF-κB activation to mediate transformation, and TBK1 was demonstrated to be essential for the survival of KRAS-dependent non-small cell lung cancers. The discovery of IKKε and TBK1 as oncogenic kinases that are intricately associated with Ras–mediated transformation suggests that these non-canonical IKK regulators are subverted in cancer cells.
While recent studies have defined CYLD as an important IKKΣ substrate that contributes to transformation, it is not known whether other targets are involved in the IKKΣ oncogenic pathway. Likewise, it will be important to define the critical substrates for TBK1 in KRAS-dependent lung cancers. It is clear for IKKΣ that kinase activity contributes to its transforming activity.
The finding that both TBK1 and IKKΣ preferentially phosphorylate the same consensus motif, and for the most part share the same substrates, raises the question as to how these kinases act differently in cell transformation (Hutti et al., 2009). One possibility is that IKKΣ and TBK1 may be differentially regulated. Negative feedback mechanisms involving CYLD and A20 have been shown to control both TBK1 and IKKΣ activity (Zhang et al., 2008). However, other negative regulators such as the phosphatase SHP2 and the NOD-like protein NLRC5 primarily affect TBK1 and not IKKε activity (An et al., 2006; Cui et al., 2010; Lin et al., 2006). In addition, the participation of IKKΣ and TBK1 in different protein complexes that are modified by Lys-63 linked ubiquitin chains proposes that the regulation, stability and activation of complex formation could contribute to differential oncogenic signaling. Another explanation for the differential roles of the non-canonical IKKs in cancer is the growing list of substrates that are distinct for IKKε and TBK1 (Table 1). The subtle but disparate regulation and activity of the non-canonical IKKs would likely influence divergent activities of TBK1 and IKKε in cancer.
Current work suggests that IKKΣ and TBK1 participate in both cell autonomous and cell non-autonomous functions in inflammation and cancer (Figure 2). The non-canonical IKKs may augment inflammatory signaling in addition to its intrinsic role of promoting cell survival. Integration of these pathways may explain the high levels of NF-κB activation observed in aggressive inflammatory breast cancer (Lerebours et al., 2008; Van Laere et al., 2006). The recent finding that IKKΣ regulates fat metabolism adds another potential mechanism by which IKKε may contribute to transformation (Chiang et al., 2009). Both IKK-related kinases increase the production of IL-6 and TNF, two inflammatory cytokines associated with insulin resistance and the induction of oncogenic STAT3 signaling (Chiang et al., 2009; Park et al., 2010; Peant et al., 2009). These findings begin to suggest crosstalk between pathways involving inflammation, insulin resistance, and cellular transformation.
Several drug discovery efforts have focused on the development of NF-κB inhibitors for various malignancies (Karin et al., 2004; Lee and Hung, 2008). Although there is some concern that prolonged NF-κB inhibition will lead to unacceptable side effects in inflammatory disease, such inhibitors could be used transiently to treat cancer patients. Surprisingly, IKKβ inhibitors increase the production of IL-1β and other inflammatory cytokines that are normally suppressed by NF-κB, and treatment with these agents results in an adverse exacerbation of inflammation (Greten et al., 2007). The non-canonical IKKs may thus serve as more attractive candidates for therapeutic development in cancer. Although further work is necessary to decipher whether these non-canonical IKKs are therapeutic targets in certain malignancies, the finding that these non-canonical IKKs play important roles in the maintenance of epithelial tumors suggests that targeting the NF-κB pathway may be an attractive strategy in a wide range of epithelial cancers.
Acknowledgements
We thank the members of the Hahn laboratory for advice. This work was supported in part by the U.S. NIH (R01 CA130988).
References
- Adli M, Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell-associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006;281:26976–26984. doi: 10.1074/jbc.M603133200. [DOI] [PubMed] [Google Scholar]
- An H, Zhao W, Hou J, Zhang Y, Xie Y, Zheng Y, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–928. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, et al. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Basseres DS, Ebbs A, Levantini E, Baldwin AS. Requirement of the NF-kappaB subunit p65/RelA for K-Ras-induced lung tumorigenesis. Cancer Res. 2010;70:3537–3546. doi: 10.1158/0008-5472.CAN-09-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibeau-Poirier A, Gravel SP, Clement JF, Rolland S, Rodier G, Coulombe P, et al. Involvement of the IkappaB kinase (IKK)-related kinases tank-binding kinase 1/IKKi and cullin-based ubiquitin ligases in IFN regulatory factor-3 degradation. J Immunol. 2006;177:5059–5067. doi: 10.4049/jimmunol.177.8.5059. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–140. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
- Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bonnard M, Mirtsos C, Suzuki S, Graham K, Huang J, Ng M, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- Caillaud A, Hovanessian AG, Levy DE, Marie IJ. Regulatory serine residues mediate phosphorylation-dependent and phosphorylation-independent activation of interferon regulatory factor 7. J Biol Chem. 2005;280:17671–17677. doi: 10.1074/jbc.M411389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15:327–332. doi: 10.1016/j.tcb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Chau TL, Gioia R, Gatot JS, Patrascu F, Carpentier I, Chapelle JP, et al. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem Sci. 2008;33:171–180. doi: 10.1016/j.tibs.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Cheng TF, Brzostek S, Ando O, Van Scoy S, Kumar KP, Reich NC. Differential activation of IFN regulatory factor (IRF)-3 and IRF-5 transcription factors during viral infection. J Immunol. 2006;176:7462–7470. doi: 10.4049/jimmunol.176.12.7462. [DOI] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Clement JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18:889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Landesman-Bollag E, Seldin DC, et al. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65:11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
- Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ, Baldwin AS., Jr Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Fujita F, Taniguchi Y, Kato T, Narita Y, Furuya A, Ogawa T, et al. Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling. Mol Cell Biol. 2003;23:7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatot JS, Gioia R, Chau TL, Patrascu F, Warnier M, Close P, et al. Lipopolysaccharide-mediated interferon regulatory factor activation involves TBK1-IKKepsilon-dependent Lys(63)-linked polyubiquitination and phosphorylation of TANK/I-TRAF. J Biol Chem. 2007;282:31131–31146. doi: 10.1074/jbc.M701690200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Gravel SP, Servant MJ. Roles of an IkappaB kinase-related pathway in human cytomegalovirus-infected vascular smooth muscle cells: a molecular link in pathogen-induced proatherosclerotic conditions. J Biol Chem. 2005;280:7477–7486. doi: 10.1074/jbc.M410392200. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–11826. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- Guo JP, Shu SK, He L, Lee YC, Kruk PA, Grenman S, et al. Deregulation of IKBKE is associated with tumor progression, poor prognosis, and cisplatin resistance in ovarian cancer. Am J Pathol. 2009;175:324–333. doi: 10.2353/ajpath.2009.080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JP, Shu SK, Esposito NN, Coppola D, Koomen JM, Cheng JQ. IKKepsilon phosphorylation of estrogen receptor alpha Ser-167 and contribution to tamoxifen resistance in breast cancer. J Biol Chem. 2010;285:3676–3684. doi: 10.1074/jbc.M109.078212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, et al. Cell type-specific expression of the IkappaB kinases determines the significance of phosphatidylinositol 3-kinase/Akt signaling to NF-kappa B activation. J Biol Chem. 2004;279:1615–1620. doi: 10.1074/jbc.M306976200. [DOI] [PubMed] [Google Scholar]
- Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- Hammerman PS, Fox CJ, Cinalli RM, Xu A, Wagner JD, Lindsten T, et al. Lymphocyte transformation by Pim-2 is dependent on nuclear factor-kappaB activation. Cancer Res. 2004;64:8341–8348. doi: 10.1158/0008-5472.CAN-04-2284. [DOI] [PubMed] [Google Scholar]
- Harris J, Oliere S, Sharma S, Sun Q, Lin R, Hiscott J, et al. Nuclear accumulation of cRel following C-terminal phosphorylation by TBK1/IKK epsilon. J Immunol. 2006;177:2527–2535. doi: 10.4049/jimmunol.177.4.2527. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Sato S, Yamamoto M, Kaisho T, Sanjo H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–6867. doi: 10.1038/sj.onc.1209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Shen RR, Abbott DW, Zhou AY, Sprott KM, Asara JM, et al. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol Cell. 2009;34:461–472. doi: 10.1016/j.molcel.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Hecker CM, Rozenknop A, Nordmeier RD, Rogov V, Hofmann K, et al. Involvement of the ubiquitin-like domain of TBK1/IKK-i kinases in regulation of IFN-inducible genes. EMBO J. 2007;26:3451–3462. doi: 10.1038/sj.emboj.7601773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Hikiba Y, Kanai F, Tanaka Y, Imamura J, Imamura T, et al. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132–8137. [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal. 2007;19:454–465. doi: 10.1016/j.cellsig.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Korherr C, Gille H, Schafer R, Koenig-Hoffmann K, Dixelius J, Egland KA, et al. Identification of proangiogenic genes and pathways by high-throughput functional genomics: TBK1 and the IRF3 pathway. Proc Natl Acad Sci U S A. 2006;103:4240–4245. doi: 10.1073/pnas.0511319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Kravchenko VV, Mathison JC, Schwamborn K, Mercurio F, Ulevitch RJ. IKKi/IKKepsilon plays a key role in integrating signals induced by pro-inflammatory stimuli. J Biol Chem. 2003;278:26612–26619. doi: 10.1074/jbc.M303001200. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14:5656–5662. doi: 10.1158/1078-0432.CCR-08-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, et al. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gong LY, Song LB, Jiang LL, Liu LP, Wu J, et al. Oncoprotein Bmi-1 renders apoptotic resistance to glioma cells through activation of the IKK-nuclear factor-kappaB Pathway. Am J Pathol. 2010;176:699–709. doi: 10.2353/ajpath.2010.090502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, O'Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, et al. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–2394. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Yang L, Nakhaei P, Sun Q, Sharif-Askari E, Julkunen I, et al. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J Biol Chem. 2006;281:2095–2103. doi: 10.1074/jbc.M510326200. [DOI] [PubMed] [Google Scholar]
- Mattioli I, Geng H, Sebald A, Hodel M, Bucher C, Kracht M, et al. Inducible phosphorylation of NF-kappa B p65 at serine 468 by T cell costimulation is mediated by IKK epsilon. J Biol Chem. 2006;281:6175–6183. doi: 10.1074/jbc.M508045200. [DOI] [PubMed] [Google Scholar]
- May MJ, Larsen SE, Shim JH, Madge LA, Ghosh S. A novel ubiquitin-like domain in IkappaB kinase beta is required for functional activity of the kinase. J Biol Chem. 2004;279:45528–45539. doi: 10.1074/jbc.M408579200. [DOI] [PubMed] [Google Scholar]
- Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci U S A. 2004;101:233–238. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Moreno R, Sobotzik JM, Schultz C, Schmitz ML. Specification of the NF-{kappa}B transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKK{varepsilon} Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Yoneyama M, Ito T, Takahashi K, Inagaki F, Fujita T. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J Biol Chem. 2004;279:9698–9702. doi: 10.1074/jbc.M310616200. [DOI] [PubMed] [Google Scholar]
- Novak U, Rinaldi A, Kwee I, Nandula SV, Rancoita PM, Compagno M, et al. The NF-{kappa}B negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood. 2009;113:4918–4921. doi: 10.1182/blood-2008-08-174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K, Barber GN, Harhaj EW. TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1-IKKi kinases. J Biol Chem. 2010;285:14999–15009. doi: 10.1074/jbc.M110.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- Paz S, Vilasco M, Arguello M, Sun Q, Lacoste J, Nguyen TL, et al. Ubiquitin-regulated recruitment of IkappaB kinase epsilon to the MAVS interferon signaling adapter. Mol Cell Biol. 2009;29:3401–3412. doi: 10.1128/MCB.00880-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peant B, Diallo JS, Dufour F, Le Page C, Delvoye N, Saad F, et al. Over-expression of IkappaB-kinase-epsilon (IKKepsilon/IKKi) induces secretion of inflammatory cytokines in prostate cancer cell lines. Prostate. 2009;69:706–718. doi: 10.1002/pros.20912. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Hong SJ, Gifford A, Weinberg RA. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6:171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Renner F, Moreno R, Schmitz ML. SUMOylation-dependent localization of IKKepsilon in PML nuclear bodies is essential for protection against DNA-damage-triggered cell death. Mol Cell. 2010;37:503–515. doi: 10.1016/j.molcel.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS., Jr A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 1998;12:968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Xiong J, Shu HB, Williamson K, Goddard A, Goeddel DV. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc Natl Acad Sci U S A. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. EMBO J. 2007;26:3180–3190. doi: 10.1038/sj.emboj.7601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Tenoever BR, Ng SL, Chua MA, McWhirter SM, Garcia-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, et al. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Van Laere SJ, Van der Auwera I, Van den Eynden GG, Elst HJ, Weyler J, Harris AL, et al. Nuclear factor-kappaB signature of inflammatory breast cancer by cDNA microarray validated by quantitative real-time reverse transcription-PCR, immunohistochemistry, and nuclear factor-kappaB DNA-binding. Clin Cancer Res. 2006;12:3249–3256. doi: 10.1158/1078-0432.CCR-05-2800. [DOI] [PubMed] [Google Scholar]
- Wietek C, Cleaver CS, Ludbrook V, Wilde J, White J, Bell DJ, et al. IkappaB kinase epsilon interacts with p52 and promotes transactivation via p65. J Biol Chem. 2006;281:34973–34981. doi: 10.1074/jbc.M607018200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, et al. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001;20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Shi H, Qi R, Sun S, Tang Y, Zhang B, et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol Biol Cell. 2006;17:1461–1471. doi: 10.1091/mbc.E05-09-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008;9:378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wu X, Lee AJ, Jin W, Chang M, Wright A, et al. Regulation of IkappaB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J Biol Chem. 2008;283:18621–18626. doi: 10.1074/jbc.M801451200. [DOI] [PMC free article] [PubMed] [Google Scholar]